Indirect photocatalytic reduction of arsenate to arsenite in aqueous solution with TiO2 in the presence of hole scavengers
2018-05-26AbdusSamadShamimAhsanIkkiTateishiMaiFurukawaHideyukiKatsumataTohruSuzukiSatoshiKaneco
Abdus Samad *,Shamim Ahsan ,Ikki Tateishi,Mai Furukawa ,Hideyuki Katsumata ,Tohru Suzuki,Satoshi Kaneco ,,*
1 Department of Chemistry,Jagannath University,Dhaka–1100,Bangladesh
2 Department of Earth and Atmospheric Sciences,Metropolitan State University of Denver,CO 80204,USA
3 Department of Chemistry for Materials,Graduate School of Engineering,Mie University,Tsu 514-8507,Mie,Japan
4 Mie Global Environment Center for Education&Research,Mie University,Tsu,Mie 514-8507,Japan
1.Introduction
The toxicity of arsenic(As)is well-known.Among the As compounds in the environment,As(III)is 25–60 times more toxic than As(V)[1].However,the arsenite compounds also have been applied into various industries.For instance,recently,copper arsenite can be available to the purification of copper electrolytes[2,3].Nicolis et al.[4]have reported the works on the stability and bioavailability of nutritional supplements and medicinal drugs,such as arsenite,and have reviewed the arsenite medicinal use.
In the aqueous systems,inorganic As species exists primarily in two oxidation states:As(V)and As(III).Extensive researches have focused on the photocatalytic oxidation ofarsenite to arsenate.Titaniumdioxide(TiO2)is one of the most studied photocatalyst for the photocatalytic oxidation of As(III)in the aqueous solution[5–9].When TiO2is irradiated by the light with photons of sufficient energy,electrons in the conduction band(eCB−)and hole in the valence band(hVB+)are produced as follows[10]:

Arsenite can be oxidized to arsenate by•OH,which is produced by hole in the valence band from water.While the redox level offor TiO2is 2.9 V vs.NHE[11],the value of reduction potential of the•OH/H2O couple is ~2.7 V vs.NHE[12]and that of As(IV)/As(III)couple is~2.4 V vs.NHE[13].Note that all potential in the present paper are standard value vs.NHE.

On the other hand,the direct reduction of arsenate to arsenite by electrons in the conduction band(eCB−)is thermodynamically impossible[5],because the value of reduction potential of As(V)/As(IV)couple is−1.2 V[13]in relation with−0.3 Vfor the reduction level of eCB−[11].However,an indirectmechanism is possible in the presence ofsacrificial electron donors to produce strongly reductive radicals.The generation of strong reducing hydroxymethyl radicals(•CH2OH)from methanol by hydroxyl radicals is thermodynamically possible[14],since it was reported that the redox levels of•CH2OH/CH3OH are 1.45 V[15].Because the oxidation potential of•CH2OH to formaldehyde(about−0.90~−1.18 V[12]is slightly more positive than the value of reduction potential of As(V)/As(IV)couple,the reduction process forarsenate cannotproceed.However,the redox potentialfor•CH2OH/CH2Omay be more negative on the TiO2surface at pH 3[14].

As(IV)is easily reduced to As(III)by the electrons in the conduction band(eCB−)or trapped electrons with •CH2OH.
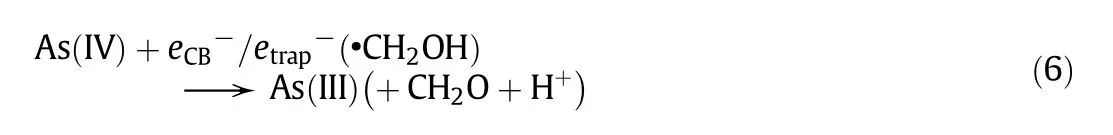
Therefore,the indirect photocatalytic reduction of As(V)in aqueous solution could occur in the presence of methanol[5,14].However,there is little information on the indirect photocatalytic reduction of arsenate to arsenite in water using various hole scavengers.
In the present work,the indirect photocatalytic reduction of arsenate to arsenite in aqueous solution using various hole scavengers has been presented.
2.Experimental
2.1.Chemicals
TiO2(Degussa P25,anatase 75%,rutile 25%,particle size 25 nm,specific surface area 48 m2·g−1)was used as received.Potassium arsenite(KAsO2,90%)and potassium arsenate(KH2AsO4,extra pure)were purchased from Nakarai Tesque,Inc.and were used for the preparation of As(III)and As(V)stock solution,respectively.Ammonium molybdate((NH4)6Mo7O24•4H2O,99%),L-ascorbic acid(C6H8O6,99%),sulfuric acid(H2SO4,98%),methanol(CH3OH,99%),ethanol(CH3CH2OH,99%),isopropanol((CH3)2CHOH,99.7%),acetic acid(CH3COOH,99.7%),formic acid(HCOOH,98%),acetone(CH3COCH3,99.5%)and formaldehyde(HCHO,36%)were purchased from Nakarai Tesque,Inc.Antimony potassium tartrate(C4H4KO7Sb•1/2H2O,99.8%)and potassium permanganate(KMnO4,99.3%)were purchased from Wako Pure Chemical Industries,Ltd.Dilute solutions were prepared daily before the experiment.Pure water was further purified with an ultrapure water system(Advantec MFS,Inc.,Tokyo,Japan)having resistivity>18 MΩ·cm.
2.2.Indirect photocatalytic reduction
The UV irradiation was carried out in a 50 ml cylindrical glass photoreactor cell.As(V)solution(30 ml)with an initial concentration of 10 mg·L−1containing 0.4 mol·L−1hole scavenger was added into the reactor.Seven hole scavengers,such as methanol,ethanol,isopropanol,formic acid,acetic acid,formaldehyde and acetone,were evaluated for the indirect photocatalytic reduction of As(V)in aqueous solution.TiO2catalyst with 20 mg was then put into the solution.The pH was set to 3.The reactor with TiO2suspension was positioned on the magnetic stirrer for agitation.The suspension was stirred in the dark for 30 min to ensure the adsorption equilibrium prior to illumination.The sample solution was irradiated with a black light(Toshiba Lighting&Technology Co., fluorescent type,peak wavelength~352 nm).The lamp was switched on and stabilized for 30 min before irradiation.After the treatment,the catalyst was separated from the solution by filtration through 0.45 μm Advantec membrane filter,and the solution was subsequently analyzed for the determination of the individual arsenic species concentrations.Each experiment was performed more than twice.
2.3.Analysis of As(V)and As(III)
The concentration of arsenate was measured by UV–vis spectrophotometry using arseno–molybdate method[16,17].The concentration of As(III)was determined from the difference between[As]totaland[As(V)].Total As concentration in the solution was evaluated by a modification of the arseno–molybdate method.In order to guarantee total As oxidation,the As(III)in sample solution was firstly oxidized to As(V)with the excess KMnO4oxidant,followed by the determination of total As with UV–Visible spectrophotometry with the arseno–molybdate method.The detailed information was described in the previous paper[16,17].
3.Results and Discussion
3.1.Reduction of As(V)to As(III)
First,the reduction of As(V)to As(III)by photolysis was tested without the photocatalyst.As a consequence,the reduction process of As(V)to As(III)could not occur under the illumination of UV light.Moreover,the photocatalytic reduction of arsenate to arsenite in aqueous solution with TiO2was tested in the absence of methanol.It was confirmed that As(V)could not be directly reduced to As(III)in the absence of methanol hole scavenger by TiO2photocatalysts.On the other hand,we could observe the photocatalytic reduction of arsenate in water with TiO2in the presence of methanol,as shown in Fig.S2.
Next,the effect of oxygen on the photocatalytic reduction of As(V)with TiO2in the presence of methanol was investigated.The results of photocatalytic arsenate reduction with the bubbling of N2gas were compared with those obtained under air atmosphere(Fig.S1).The reduction efficiency of As(V)in the strictly controlled anoxic conditions was larger relative to that obtained under air conditions,owing to the oxygen consumption of As species.
Seven hole scavengers was studied for the indirect photocatalytic reduction of As(V)in aqueous solution with TiO2photocatalysts under anoxic atmosphere at pH 3.The concentration of hole scavenger was constant(0.4 mol·L−1).The results are illustrated in Figs.1 and S2.The photocatalytic reaction kinetics of many compounds has been modeled with Langmuir–Hinshewood(L–H)equation,which also covers the adsorption properties of the substrate on the photocatalyst surface.This model was developed by Turchi and Ollis[18]and expressed as Eq(7):

where r0is the degradation rate ofthe reactant,k is the reaction rate constant and K and C0are the adsorption equilibrium constant and concentration for the reactant,respectively.If the concentration of substrate is very low,i.e.,KC<<1,the L–H equation(Eq.(7))simplifies to a pseudo- first-order kinetic law(Eq.(8))where kobsis being the apparent pseudo- first-order rate constant.

The primary reduction reaction is estimated to follow a pseudo- firstorder kinetic law,according to the Eq.(8).In order to confirm the speculation,−ln(C/C0)was plotted as a function of illumination time(Fig.2).Since the linearplots were observed in Fig.2 as expected,the reduction kinetics in the As(V)solution followed the first–order reduction curve which was consistent to the L–H model resulting from the low coverage in the experimental concentration range(10 mg·L−1).The photocatalytic degradation kinetic parameters such as pseudo- firstorder rate constant,correlation coefficient and substrate half-life are shown in Table 1.Maximum pseudo- first-order rate constant in the photocatalytic reduction was observed in the presence of ethanol.

Fig.1.Typical indirect photocatalytic reduction of As(V)in aqueous solution with TiO2.Concentration of hole scavenger;0.4 mol·L−1 of a)ethanol and b)isopropanol.TiO2 suspension concentration;20 mg in 30 ml solution.As(V)initial concentration;10 mg·L−1.Rhombus;As(V),square;As(III).
3.2.Photocatalytic reduction of As(III)
The photocatalytic reduction of arsenite in aqueous solution containing 0.4 mol·L−1ethanol on TiO2under anoxic atmosphere was investigated at pH 3 and 10.The results for the direct reduction efficiency of As(III)at pH 3 are illustrated in Fig.3.The efficiency for the photocatalytic reduction of arsenite is notso large,since the adsorption of As(III)onto the surface of TiO2was poor.The facts that the photocatalytic reduction efficiency of As(III)in aqueous solution including ethanol was relatively low are the same as those obtained with methanol hole scavenger.It was found in the aqueous methanol solution that the photocatalytic conversion of As(V)to As(III)could efficiently occur,however the conversion of As(III)to metalAs could not almost proceed.On the contrary,at pH 10,the photocatalytic oxidation of arsenite into arsenate could occur rather than the reduction of arsenite.Also,these phenomena were observed in the case of the reduction using methanol[14].

Fig.2.ln(C/C0)versus irradiation time.C:concentration ofAs(V)atthe irradiation time.C0:initial concentration of As(V)(10 mg·L−1).a:ethanol;b formic acid;c:methanol;d:formaldehyde;e:isopropanol;f:acetone;g:acetic acid.
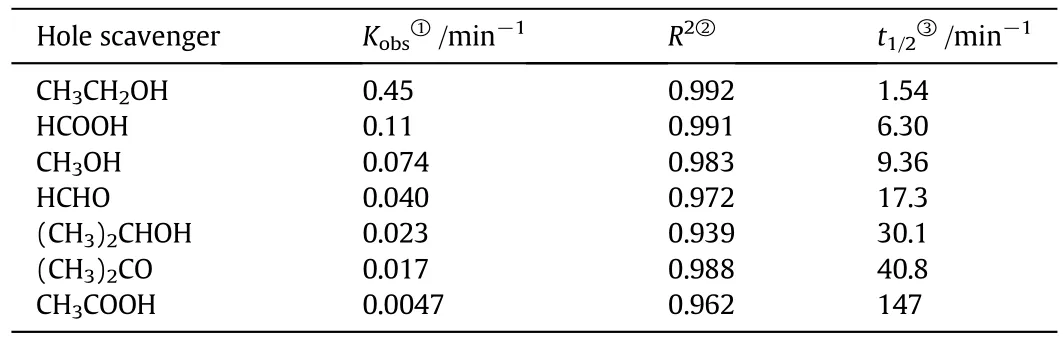
Table 1 Indirect photoreduction kinetic parameters(pseudo- first-order rate constant,correlation coefficient,and substrate half-life)
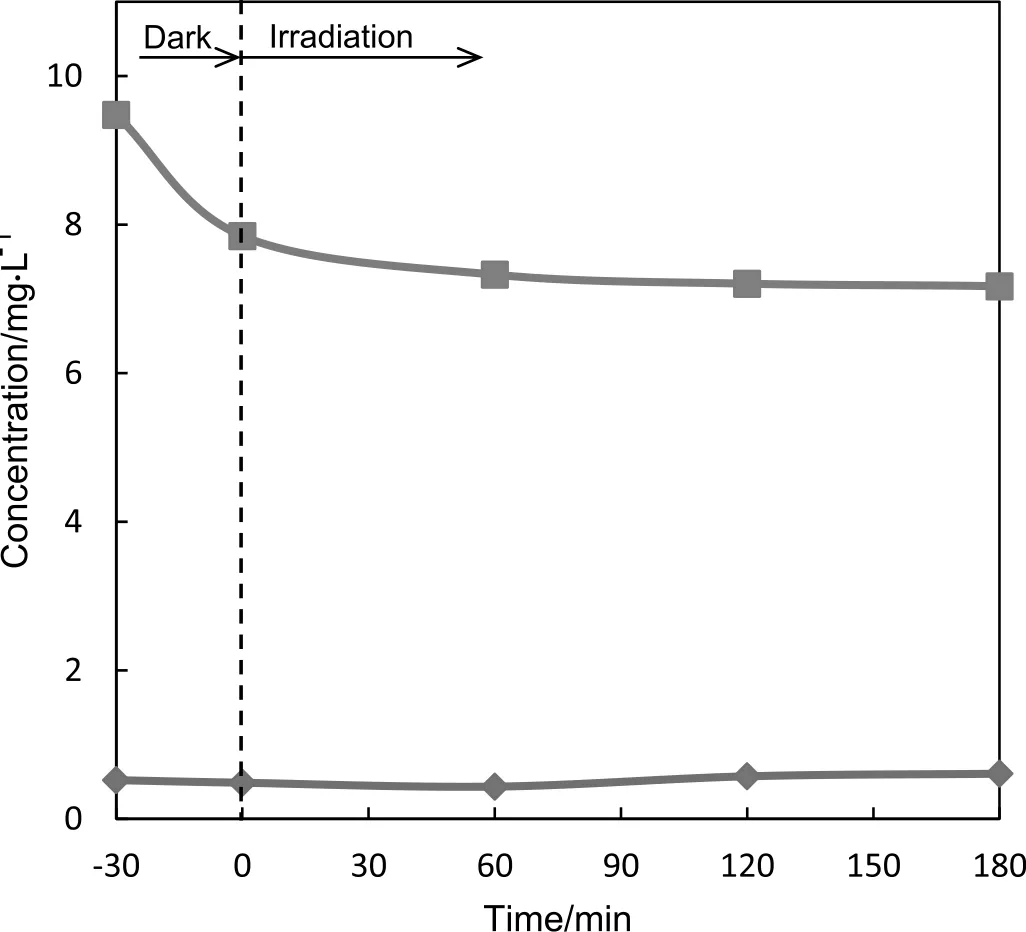
Fig.3.Photocatalytic reduction of As(III)in aqueous solution containing 0.4 mol·L−1 ethanol with TiO2.TiO2 suspension concentration;20 mg in 30 ml solution.As(III)initial concentration;10 mg·L−1.Rhombus;As(V),square;As(III).
3.3.Reaction mechanism
As mentioned before,the directphotocatalytic reduction of arsenate by electrons in the conduction band(eCB−)is not possible,however the indirect reductive mechanism would be possible in the presence of electron donors.For the reduction potential of HR/•R couples,the following values were reported:0.97 V[19]at pH 3 and 1.45 V[15]for methanol,1.15 V[19]for ethanol at pH 3,1.3 V[19]for 2-propanol atpH 3 and 1.3 V[19]for formic acid at pH 2.3.Therefore,the formation of strong reducing radicals by the attack of•OHis possible for methanol,ethanol,2-propanol and formic acid.
As mentioned in the Introduction section,because the oxidation potentialof•CH2OHto CH2O(about−0.90 V to−1.18 V[12])is slightly more positive compared with that of the reduction potential of As(V)/As(IV)couple,the reduction process for arsenate cannot occur.However,the redox potential for•CH2OH/CH2O may be more negative on the TiO2surface at pH 3[14].Hence,the indirect reduction reaction may be possible.
The oxidation potentials of CH3•CHOH to CH3CHO is approximately−1.1 V to−1.25 V[12].Since the value might become more negative on the photocatalyst surface at pH 3,the indirect photocatalytic arsenate reduction may occur[14].Because the redox potentials of(CH3)2•COH to acetone is in the range about from −1.39 V to −1.80 V[12],the indirect reduction of As(V)can proceed.
According to Eq.(5),the oxidation of•CH2OH can give formaldehyde.CH2O can be transformed to formic acid.Formic acid generates a much stronger reducing agent,•CO2−(E0(CO2/•CO2−))= ~−2.0 V[12],which is a better current doubling agent and can contribute to the reducing process of arsenate.

In the photocatalytic degradation of acetone with TiO2,the degradation products were acetaldehyde,formaldehyde,acetic acid and formic acid[20].Therefore,indirectreduction ofAs(V)can proceed in the presence of acetone.
The photocatalytic decarboxylation of acetic acid in the aqueous solution has been reported as shown below[21]:

The active•CH3radicals react with H2O to release CH4,or with another•CH3radical to produce C2H6.On the contrary,methanol is formed by the reaction of the•CH3radicals with hydroxyl radicals.Consequently,the indirect photocatalytic reduction of As(V)could be possible in the presence of methanol,ethanol,2-propanol,formic acid,formaldehyde,acetone and acetic acid.
In order to explore the in fluence of hole scavengers on the indirect reduction rates for arsenate with TiO2,we tried to estimate the relation between the reduction rate of arsenate and radical reactivity.The reaction characters of two radicals such as(1)reactivities of hydroxyl radicals with hole scavengers and(2)reactivities of the radicals which are formed by the reaction of•OH with hole scavengers were postulated to be associated with the reduction rate of As(V).The rate constants for reactions of hydroxyl radicals with various hole scavengers in aqueous solution are as follows:ethanol 1.9 × 109L·mol−1·s−1[22],formic acid 3.2 × 109L·mol−1·s−1[22],methanol 0.97 × 109L·mol−1·s−1[22],formaldehyde ~1.0 × 109L·mol−1·s−1[22],isopropanol 1.9 ×109L·mol−1·s−1[22],acetone 0.11 × 109L·mol−1·s−1[22]and acetic acid 0.016 × 109L·mol−1·s−1[22].The reactivities for the radicals M•which are produced by the reaction of•OH with hole scavengers are reported in the following:6.6 × 109L·mol−1·s−1for •CH2CH2OH[23];4.0 × 109L·mol−1·s−1for •CO2−[24];4.9 × 109L·mol−1·s−1for•CH2OH[25];5.6 × 109L·mol−1·s−1for •CHO[25];3.92 ×109L·mol−1·s−1for CH3•C(OH)CH3[26];3.1 × 109L·mol−1·s−1for CH3CO•CH2[27];1.7 × 109L·mol−1·s−1for•CH2COOH[28].Therefore,three types of plots for the data are prepared as shown in Figs.4–6.The correlation coefficient for three figures was not so small(R2>0.60).Therefore,it could be concluded from the figures that the indirect photocatalytic reduction rate of As(V)may be related with both 1)reaction rate constants of reaction of hydroxyl radicals with hole scavenger and 2)the reactivities forthe radicals M•which are produced by the reaction of•OH with hole scavenger.
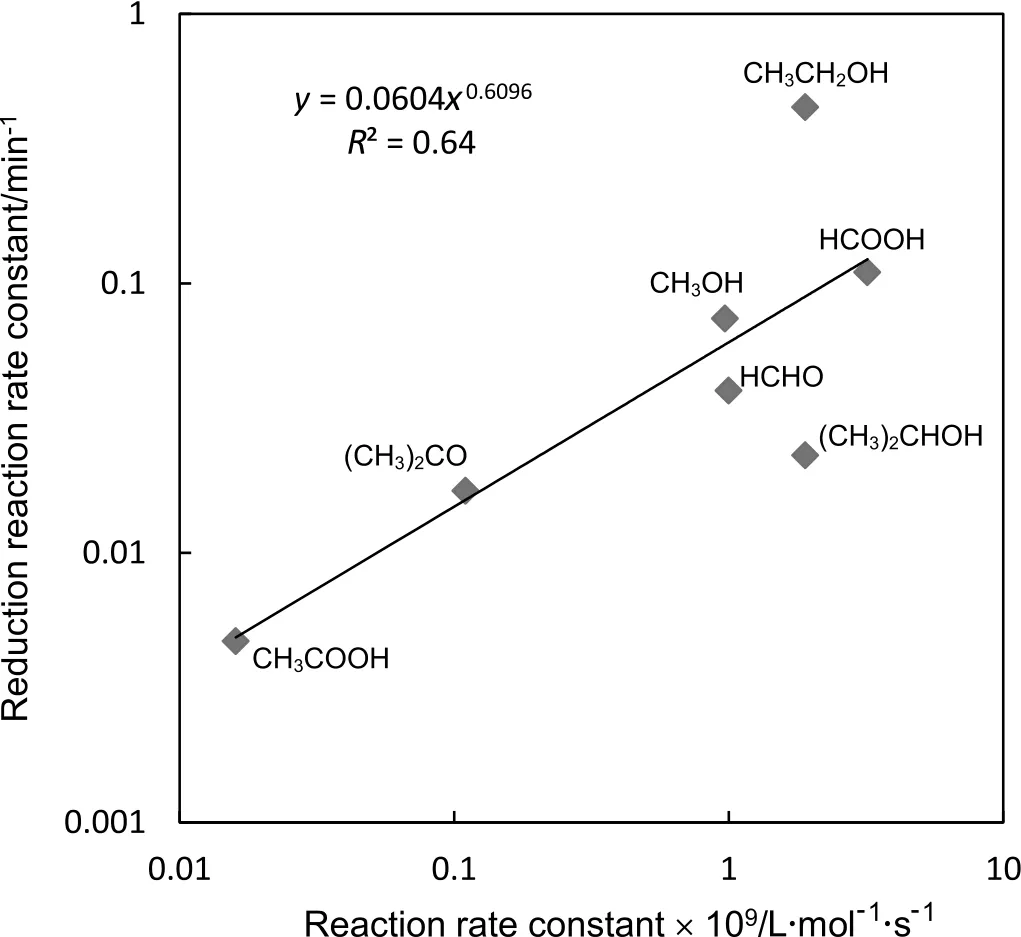
Fig.4.Relation between the indirect photocatalytic reduction rate constant of As(V)and the reaction rate constants of reaction of hydroxyl radicals with hole scavenger.Both axes are log scale.

Fig.5.Relation between the indirect photocatalytic reduction rate constant of As(V)and the reactivities of the radicals which are formed by the reaction of•OH with hole scavenger.The reactivities of radicals which are produced by the reaction of•OH with hole scavenger are used in the case of reaction with oxygen.Both axes are log scale.
4.Conclusions
The indirect photocatalytic reduction of arsenate to arsenite in aqueous solution was studied using TiO2in the presence of various hole scavengers such as methanol,ethanol,2-propanol,formic acid,formaldehyde,acetone and acetic acid.Though the directphotocatalytic reduction of arsenate to arsenite with TiO2was impossible,an indirect reduction of As(V)was possible in the presence of sacrificial electron donors.The addition of ethanol was very effective for the indirect photocatalytic reduction of As(V)with TiO2.The indirect photocatalytic reduction rate of As(V)may be related with both the reaction rate constants of reaction of hydroxyl radicals with hole scavenger and the reactivities for the radicals M•which are produced by the reaction of•OH with hole scavenger.
Supplementary Material
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2017.05.019.
[1]Y.Li,X.Cai,J.Zhou,P.Na,Fe/Ti co-pillared clay for enhanced arsenite removal and photo oxidation under UV irradiation,Appl.Surf.Sci.324(2015)179–187.
[2]Y.-J.Zheng,F.-X.Xiao,Y.Wang,C.-H.Li,W.Xu,H.-S.Jian,Y.-T.Ma,Industrial experiment of copper electrolyte purification by copper arsenite,J.Cent.South Univ.Technol.15(2008)204–208.
[3]X.Xiao,J.Mao,D.Cao,X.Shen,A.A.Volinsky,The role of trivalent arsenic in removal of antimony and bismuth impurities from copper electrolytes,Hydrometallurgy 125-126(2012)76–80.
[4]I.Nicolis,E.Curis,P.Deschamps,S.Bénazeth,Arsenite medicinal use,metabolism,pharmacokinetics and monitoring in human hair,Biochimie 91(2009)1260–1267.
[5]H.Yang,W.-Y.Lin,W.-Y.K.Rajeshwar,Homogeneous and heterogeneous photocatalytic reactions involving As(III)and As(V)species in aqueous media,J.Photochem.Photobiol.A Chem.123(1999)137–143.
[6]Z.Xu,X.Meng,Size effects of nanocrystalline TiO2on As(V)and As(III)adsorption and As(III)photooxidation,J.Hazard.Mater.168(2009)747–752.
[7]M.I.Litter,M.E.Morgada,J.Bundschuh,Possible treatments for arsenic removal in Latin American waters for human consumption,Environ.Pollut.158(2010)1105–1118.
[8]W.Choi,J.Yeo,J.Ryu,T.Tachikawa,T.Majima,Photocatalytic oxidation mechanism of As(III)on TiO2:Unique role ofAs(III)as a charge recombinantspecies,Environ.Sci.Technol.44(2010)9099–9104.
[9]H.Fei,W.Leng,X.Li,X.Cheng’,Y.Xu,J.Zhang,C.Cao,Photocatalytic oxidation of arsenite over TiO2:is superoxide the main oxidant in normal air-saturated aqueous solutions?Environ.Sci.Technol.45(2011)4532–4539.
[10]M.R.Hoffmann,S.T.Martin,W.Choi,D.W.Bahnemann,Environmental applications of semiconductor photocatalysis,Chem.Rev.95(1995)69–96.
[11]S.T.Martin,H.Herrmann,W.Choi,M.R.Hoffmann,Time-resolved microwave conductivity.Part 1.—TiO2photoreactivity and size quantization,J.Chem.Soc.Faraday Trans.90(1994)3315–3322.
[12]P.J.Wardman,Reduction potentials of one-electron couples involving free radicals in aqueous solution,J.Phys.Chem.Ref.Data 18(1989)1637–1755.
[13]U.K.Kläning,B.H.J.Bielski,K.Sehesteds,Arsenic(IV).A pulse-radiolysis study,Inorg.Chem.28(1989)2717–2724.
[14]I.K.Levy,M.Mizrah,G.Ruano,G.Zampieri,F.G.Requejo,M.I.Litter,TiO2-photocatalytic reduction of pentavalent and trivalent arsenic:production of elemental arsenic and arsine,Environ.Sci.Technol.46(2012)2299–2308.
[15]C.Wang,R.Pagel,D.W.Bahnemann,J.K.Dohrmann,Quantum yield of formaldehyde formation in the presence of colloidal TiO2–based photocatalysts:effect of intermittent illumination,platinization,and deoxygenation,J.Phys.Chem.B 108(2004)14082–14092.
[16]V.Lenoble,V.Deluchat,B.Serpaud,J.C.Bollinger,Arsenite oxidation and arsenate determination by the molybdene blue method,Talanta 61(2003)267–276.
[17]A.Samad,M.Furukawa,H.Katsumata,T.Suzuki,S.Kaneco,Photocatalytic oxidation and simultaneous removal of arsenite with CuO/ZnO photocatalyst,J.Photochem.Photobiol.A Chem.325(2016)97–103.
[18]C.S.Turchi,D.F.Ollis,Photocatalytic degradation of organic water contaminants:mechanisms involving hydroxyl radical attack,J.Catal.122(1990)178–192.
[19]J.Lilie,G.Beck,A.Henglein,Pulse radiolysis and polarography.Half-wave potentials for the oxidation and reduction of short-lived organic radicals at the mercury electrode,Ber.Bunsenges.Phys.Chem.75(1971)458–465.
[20]C.L.Bianchi,S.Gatto,C.Pirola,A.Naldoni,A.D.Michele,G.Cerrato,V.Crocellà,V.Capucci,Photocatalytic degradation of acetone,acetaldehyde and toluene in gasphase:comparison between nano and micro-sized TiO2,Appl.Catal.B Environ.146(2014)123–130.
[21]X.Zheng,L.Wei,Z.Zhang,Q.Jiang,Y.Wei,B.Xie,M.Wei,Research on photocatalytic H2production from acetic acid solution by Pt/TiO2nanoparticles under UV irradiation,Int.J.Hydrog.Energy 34(2009)9033–9041.
[22]G.V.Buxton,C.L.Greenstock,W.P.Helman,A.B.Ross,Critical review of rate constants for reactions of hydrated electrons,hydrogen atoms and hydroxyl radicals(•OH/•O−)in aqueous solution,J.Phys.Chem.Ref.Data 17(1988)513–886.
[23]C.F.Cullis,J.M.Francis,Y.Raef,A.J.Swallow,Studies of radiation-induced reactions of ethylene in aqueous solution.II.Reactions in the presence of oxygen as studied by pulse radiolysis and γ–irradiation techniques,Proc.R.Soc.Lond.Ser.A 300(1967)443–454.
[24]M.G.Simic,Pulse radiolysis in study of oxygen radicals,Methods Enzymol.186(1990)89–100.
[25]K.Shibuya,T.Ebata,K.Obi,I.Tanaka,Rate constant measurements for the reactions of HCO with NO and O2in the gas phase,J.Phys.Chem.81(1977)2292–2294.
[26]B.Maillard,K.U.Ingold,J.C.Scaiano,Rate constants for the reactions of free radicals with oxygen in solution,J.Am.Chem.Soc.105(1983)5095–5099.
[27]H.Zegota,M.N.Schuchmann,C.von Sonntag,Elucidation of the mechanisms of peroxyl radical reactions in aqueous solutions using the pulse radiolysis technique,Radioanal.Nuclearchem.101(1986)199–207.
[28]K.Sehested,H.Cor fitzen,J.Holcman,E.J.Hart,Decomposition of ozone in aqueous acetic acid solutions(pH 0–4),J.Phys.Chem.96(1992)1005–1009.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
