Benzene selective hydrogenation over supported Ni(nano-)particles catalysts:Catalytic and kinetics studies
2018-05-26PeyroviParsafardMohammadian
M.H.Peyrovi*,N.Parsafard *,Z.Mohammadian
1 Faculty of Chemistry Science and Petroleum,Department of Physical Chemistry,University of Shahid Beheshti,Tehran 1983963113,Iran
2 Kosar University of Bojnord,Department of Applied Chemistry,North Khorasan,Iran
1.Introduction
In recent years,strict environmental regulations have forced the petrochemical industry to produce cleaner fuels for use in the internal combustion engines.Arenes are the most threatening materials to life especially due to their carcinogenic effects.On this basis,the hydrogenation reaction is of significant importance to saturate these contents in the petroleum industry[1–5].However,the stabilization of aromatic rings by resonance makes it difficult to saturation[6].With increasing demand for hydrogenation of aromatics,the development of effective hydrogenation catalysts is of critical importance[1–5].Benzene is one of the aromatic compounds that is used as a reaction model to study the performance of the hydrogenation catalysts[1,3].So far a lot of materials have been used for the hydrogenation of benzene.According to a reported category[5,7],these materials can be expressed as nickel-[8],platinum-[6,9],palladium-[10],rhodium-[11,12],iridium-[13],ruthenium-[14,15],iron-[5,16]and cobalt-based catalysts[17].Various published researches reported many affecting parameters on the catalytic activity of metal-based catalysts in hydrogenation reaction such as the nature of support,acidity,support's interaction with an active metal,metal particle size,etc.[18,19].
The importance for benzene hydrogenation catalyst includes high activity,selectivity,stability and coke resistance.Among the various metal-based catalysts that were used for this reaction,nickel supported catalysts have been considered more than other catalysts because of nickel's high activity in hydrogenation reaction and especially its low price for commercial uses[3,5,19].Accordingly,the main goal of this contribution is to determine the effect of different natures of supports such as Al2O3,SiO2,HMS,HZSM-5 and HZSM5/HMS.Herein,the catalytic behavior of 25 wt%Ni supported catalysts was taken into account for benzene hydrogenation reaction.The catalytic features(activity and selectivity)and the reaction kinetics,the effects of reaction temperature and residence time on the catalytic activity and stability for the hydrogenation reaction of benzene(Bz),toluene(Tu)and benzene-toluene mixture in a fixed-bed reactor have been compared.A detailed kinetic study of this reaction over prepared catalysts was undertaken in order to understand the reaction mechanism.The power law is the first mathematical model that was used in this work.The second model that was evaluated and compared to the power law model is the Langmuir–Hinshelwood model.
2.Experimental
2.1.Catalyst preparation
The synthesis methods for various used supports in present work have been described brie fly as follows.
Theγ-alumina was synthesized by adding drops ofaqueous ammonia(13.3 mol·L−1)to 1.7 mol·L−1solution of aluminum nitrate(Analytical grade)at room temperature until pH of this solution reaches 6.After the formation of alumina gel,it was filtered and washed by distilled water.The obtained gel was placed in oven at 110°C for 24 h and then calcined in air at 600°C for 8 h.
For preparation of silica,6%H2SO4(Merck)was added drop by drop to diluted sodium silicate(Aldrich)until pH=4–5 and a cloudy colloidal precipitate was formed.The obtained white precipitate was filtered,washed and dried in oven at 100°C for more than 24 h,and calcined in air at 600°C for 6 h.
The HZSM-5(Si/Al mole ratio=14)was prepared by hydrothermal method[20].The synthesized material was washed in distilled water and calcined in air at 600°C for 12 h.Then decationization of Na-ZSM-5 powder was carried out by ion exchange four times using ammonium chloride solution followed by drying and calcination at 600°C for 8 h.
The HMS was synthesized by sol–gel method similar to our reported work[21]using tetraethyl ortho silicate(Merck)and dodecyl amine(Merck).The solid product was separated by filtration and dried at 110 °C overnight and calcined at 600 °C for 6 h in the flowing air.
To synthesize the HZSM-5/HMS(denoted as ZH)composite material,1 g of HZSM-5 was added to a preformed HMS colloidal precursor[21].The synthesized method of this composite is similar to the reported procedure[22].After preparing this composite material,the solid product was filtered and dried at 110 °C overnight and calcined at 600 °C for 6 h in air.
A solution of Ni(NO3)2·6H2O(Merck)with appropriate concentration for 25 wt%loading of nickel was used for the impregnation of each support.The catalysts were mixed at room temperature with this solution and evaporated at 85°C.The impregnated materials were dried at 110 °C and calcined in flowing air at 300 °C for 4 h.
2.2.Catalyst characterization
The textural properties of the calcined materials were studied by means of various methods as follows.
The chemical composition of the catalysts was determined by means of the X-ray fluorescence(XRF)method using an XRF-8410 Rh apparatus and a voltage of 60 kV.The X-ray diffraction(XRD)measurements were performed on an X-PERT diffractometer equipped with a Nifilter and graphite monochromator,using CuKαradiation(0.15406 nm)and operating at 45 kV and 50 mA.The samples were scanned between 1°<2θ< 80°,with a step size 0.06°and time/step of 1 s.Hydrogen pulse chemisorption measurements were carried out on TPD/TPR analyzer(2900 Micromeritics)equipped with a thermal conductivity detector(TCD).Approximately 0.2 g of each catalyst was reduced in H2stream(14 ml·min−1)at 450 °C for 2 h,followed by outgassing at 500 °C for 1 h.Then,the catalyst was cooled down to 25°C.The mono-layeruptake of hydrogen over the surface of each catalyst was calculated by extrapolating the hydrogen adsorption isotherms to zero pressure.Assuming the complete reduction of Ni2+ions and the spherical forms for Ni particles,the number of nickel atom on the surface was calculated according to Ni/H=1 chemisorption stoichiometry.Fourier transform infrared spectra(FT-IR)were recorded on a BOMEM FT-IR spectrophotometer model Arid-Zone TM,MB series.The samples were mixed with KBr(Sample:KBr=1:100,mass ratio)and pressed into pellets.All the samples were recorded in the wave number range of 400–4000 cm−1,and KBr was used as background.Diffuse-Reflectance UV–vis spectra were measured on a Shimadzu UV-2100 spectrometer,equipped with a diffuse-reflectance sphere and using BaSO4as a reference.The recorded range of these spectra is 200–800 nm at room temperature.The absorption intensity was calculated with the Schuster–Kubelka–Munk equation,F(R∞)=(1 − R∞)2/2.R∞,where R∞is the diffuse reflectance from a semi-in finite layer and F(R∞)is proportional to the absorption coefficient.The specific surface area of the catalysts was determined by nitrogen adsorption isotherms(BET isotherms)at the temperature of liquid nitrogen(−196 °C)and in the p/p0range 0.05–0.3 using an ASAP-2010 Micromeritics(USA)analyzer.The catalysts were out gassed at 300°C for 3 h before being subjected to N2adsorption.The average pore diameter(dp)and pore volume(Vp)were determined using the Barrett–Joyner–Halenda(BJH)method.The acidity of the prepared catalysts was monitored by temperature-programmed desorption of ammonia(NH3-TPD)using a TPD/TPR analyzer(2900 Micromeritics)equipped with a thermal conductivity detector.The catalysts(0.2 g)were first pre-treated in pure helium from 25 to 800°C at a heating rate of10°C·min−1for 1 h.Aftercooling to room temperature,the samples were saturated with ammonia.TPD profiles for ammonia desorption are obtained by raising the sample temperature from 25 to 550°C at a heating rate of 10 °C·min−1.Field emission scanning electron microscope(FESEM)of the coated catalysts with gold was measured on a HITACHI S-4160 instrument operating at an accelerating voltage of 30 kV.The deposition of coke and thermal stability of prepared catalysts were measured by thermo gravimetric/differential thermal analysis(TG/DTA)method by a STA503 M instrument under flowing of gas mixture(5 vol%O2/N2,60 ml·min−1)in 25–800 °C with 10 °C·min−1heating rate.
2.3.Reactor test
The benzene hydrogenation in the presence of toluene was performed in the vapor phase and in a Pyrex fixed-bed reactor equipped with a thermocouple.1 g of each catalyst was separately placed in the reactor and was reduced in a pure H2with 40 ml·min−1flow rate at 350°C for 2 h.A gas mixture containing the aromatic hydrocarbon(6 vol%of benzene and/or 8 vol%of toluene dissolved in an excess of liquid n-heptane)and H2was prepared by passage of an H2stream(40 ml·min−1flow rate)through a benzene or toluene(Merck,>99%)system vaporizer via a syringe pump with 2 ml·h−1flow rate.The catalytic test was carried out at various temperatures(130–190°C)on each catalyst.The sample was heated at a rate of 20 °C·h−1.The reaction products were analyzed each 1 h using an Agilent Technologies 7890A online gas chromatograph,operated at a programmed temperature and with a flame ionization detector.
To investigate the stability of prepared catalysts against the carbonaceous formation over the catalyst's surfaces in this reaction,the catalytic performance was carried out at a constant temperature(150°C)for72 h of time on stream.The reaction products were also analyzed by GC in the same conditions similar to catalytic activity.
Two kinetic models were used to investigate the kinetic of reaction on our prepared catalysts.The partialorders of reaction and other kinetic parameters were calculated based on the simplest model(power law model).The kinetics evaluation of the benzene hydrogenation reaction was carried out to determine the reaction order and activation energy for these synthesized catalysts.The reaction orders with respect to benzene concentration[(2–8)vol%]and hydrogen pressure(2.6–5.9 Pa)were estimated at various temperatures ranging from 130 to 190°C.In this method,the partial pressure of hydrogen and benzene were changed respectively forevaluating the order of benzene and hydrogen.
3.Results and Discussions
3.1.Physico-chemical properties
The Ni contents of the catalysts were determined by XRF analysis.The results show in close agreement with the expected theoretical ones(Table 1).
The XRDspectra of Nicatalysts aftercalcination were shown in Fig.1.The main peaks that appeared at 37.2°,43.2°,62.7°,75.2°and 79.4°show Ni phase(NiO)which are consistent with NiO fingerprints reported by Therdthianwong et al.[22].The color of these catalysts after calcination(lightgray)confirms the oxidation of nickel.According to the XRD spectra of the Ni supported catalysts(not shown here)after reduction by hydrogen flow,this NiO phase was completely converted to Ni metal(XRD Bragg's angle of nickel:44.2,51.7 and 76.1)[22].In the Ni/Al2O3catalyst,these peaks are almost completely overlappedwith some diffraction peaks.The peaks at 2θ of 37.6°,46°and 68°(with low intensity)are attributed toγ–Al2O3[22,23].The XRD pattern of 25%Ni supported on silica exhibits the intensive diffraction peaks at 2θ=1°–2°and 2θ =2°–4°.According to literature[24],the peaks at 2θ =1°–2°,2.3°and 2.7°are characteristics of 100,110 and 200 planes of silica.Also this XRD pattern exhibits a sharp peak on the broad underlying peaks characteristic of the amorphous silica at2θ~23°[25].The XRD spectra of the Ni/HMS,Ni/HZSM5 and Ni/ZH catalysts are similar to the crystal structures that we reported in our previous work[21].

Table 1 Physicochemical properties of prepared catalysts

Fig.1.The XRD patterns for Ni supported catalysts after calcination.
The primary crystallite sizes of the Ni particles were determined by the Scherer equation[26],

where CNiis the crystallite size of Ni,0.9 is the value in radians when B2θis the full width athalfmaximum(FWHM)ofthe nickel(1 1 1)re flection,λ is the X-ray wavelength corresponding to CuKαradiation(0.15406 nm)and θmaxis the angle of diffraction corresponding to peak broadening.
To check the vibrational properties of our prepared catalysts,FT-IR analysis was performed.Fig.2 shows the FT-IR spectra of these Ni supported catalysts.In all spectra,the absorption peak at around 3400 cm−1and ~1629 cm−1corresponded to the stretching and bending modes ofOHgroup,respectively.In Ni/γ-Al2O3,the peaks located at around 1700(very low intense),1380 and 1200 cm−1could be ascribed as stretching and bending vibrations of intra-molecular interactions in the polymeric species,such as Al13O4(OH)and[AlO4Al12(OH)24(H2O)12]7+.The bending vibrations peaks of Al--OH and Al--O--Al are observed at 1041 and 945 cm−1,respectively.The stretching and the bending modes of Ni(Al)--O and Ni(Al)--O--H are also observed at around 800,461 and below 400 cm−1[27–29].The absorption bands characteristic of Ni/SiO2appeared at near 1078,890,795 and 470 cm−1,which can be assigned to asymmetric and symmetric stretching vibration of Ni(Si)--O bond,the stretching vibration of Ni(Si)--OH and the bending vibration of cyclic Ni(Si)-O,respectively[25,30].The FT-IR spectra of Ni/HMS,Ni/HZSM5 and Ni/ZH are similar to the vibrational bands reported in our previous work[21].So we don't explain these spectra here.

Fig.2.FT-IR spectra for different powder catalysts.
In order to investigate the chemical environment and the symmetry and coordination of nickel species,the UV–vis DR spectra of prepared samples were carried out,as shown in Fig.3.The supports do not show any evident absorption at the region used for this figure(not shown here).But about nickel supported catalysts,the UV–vis spectra exhibit some diffuse reflectance(DR)bands.In all spectra,an absorption band is observed in the UV range about 230–300 nm(with low intensity about Ni/SiO2)which is an absorption associated to a O2−(2p) ˗Ni2+(3d)charge transfer transition(CT)in octahedral symmetry in a NiO lattice and the shoulder at above 300 nm is associated to the d-d transitions of Ni2+.In the case ofNi/SiO2catalyst,the doublet absorption band due to electron transitions related to Ni2+in tetrahedral coordination in the bulk NiO/Al2O3lattice is appeared at 590–630 cm−1[31–33].In contrast,this band is completely disappeared for the other catalysts indicating the absence of the tetrahedral Ni2+(Td)ions in these catalysts,which is in agreement with the observation from XRD.In general,these UV–vis spectra reveal the formation of NiO with less interaction with the supports.

Fig.3.UV–vis DRS patterns of the calcined Ni supported catalysts.
Nitrogen adsorption–desorption isotherms of all catalysts were shown in Fig.4.The isotherms for Ni/HMS and Ni/ZH catalysts show a type IV profile and H1 hysteresis loops,characteristic of mesoporous materials with highly uniform cylindrical pores.Two steps of capillary condensation are shown in these isotherms.The first step at p/p0=0.3 due to intra particles mesoporosity inside the Ni/HMS and Ni/ZH.Secondly,at higher partial pressure of 0.9,a small hysteresis loop that was attributed to inter particle textural porosity,which indirectly represents the size of particles,i.e.a higher partial pressure was associated with a smaller particle size[34].About Ni/SiO2catalyst,the observed isotherm is type IV.The appearance of a type H1 hysteresis loop(p/p0range of 0.55–0.8)may indicate the mesoporous material consisted of agglomerates of approximately spherical particles with fairly uniform size and distribution.The isotherms of Ni/γ–Al2O3had a plateau above a certain relative partial pressure,characteristic of purely mesoporous materials.The hysteresis loops of this catalyst can be classified as type H2(IUPAC classification),and suggest the presence of approximately tubular mesopores with a relatively narrow pore size distribution.The results of this analysis were also summarized in Table 1.The BET surface area of Ni/HMS to Ni/γ–Al2O3show a decreasing from 763 to 158 m2·g−1with ascending trend of pore size from 3.62 to 11.93 nm which can partially evidence the inverse relationship between pore size and surface area in inorganic porous oxides.Loading of Ni is due to a reduction in the surface area,which provides complementary evidence of pores being blocked by nickel oxide particles.
The acidity of the supports was studied by temperature programmed desorption of ammonia that was presented in Table 1 and Fig.5.It can be seen that all of the samples have one/two strong desorption peaks in the temperature region of 100–550 °C,interpreting as the desorption peak of the weak and medium acid,and the desorption peaks area of Ni/Al2O3sample is the largest in this region(296 μmol·g−1).The summarized results are evolving by the following order:Ni/Al2O3>Ni/ZH>Ni/HZSM-5>Ni/SiO2>Ni/HMS.
3.2.Catalytic activity
3.2.1.Hydrogenation of benzene and toluene

Fig.4.N2 adsorption–desorption isotherms at−196 °C for the prepared catalysts.

Fig.5.NH3-TPD profiles of Ni supported catalysts.
The effect of temperature on conversion in the vaporphase hydrogenation of benzene and toluene over various supported catalysts is shown in Fig.6.Under our experimental conditions,the only detectable products for hydrogenation of benzene and toluene were cyclohexane and methyl cyclohexane,respectively.In the hydrogenation of toluene,small amounts(<3%)of ethyl cyclopentane and dimethyl cyclopentane were also observed.As can be seen in this figure,the catalysts presented high activity in this reaction as a function of reaction temperature[Fig.6(a)].The maximum conversion in benzene hydrogenation(100%)was achieved for Ni/HMS at 150°C,while Ni/SiO2,Ni/HZSM5 and Ni/ZH shown 95%,79%and 76%maximum conversions at 150°C and the maximum activity(99%)for Ni/Al2O3at 130°C.In toluene hydrogenation,the maximum amount of conversion(100%)was also observed for Ni/HMS,Ni/ZH(at all temperatures)and Ni/Al2O3(at 170 °C and 190 °C).This amount is 72%(at 170 °C)and 32%(at 150°C)for Ni/HZSM5 and Ni/SiO2catalysts,respectively.
Also,the selective hydrogenation of benzene in benzene-toluene mixture(3:4 molar ratios)was investigated for each catalyst at four temperatures:130,150,170 and 190°C[Fig.6(b)].Overall aromatics conversion(Ovconv(%))was calculated by the following expression,

In this expression,m and conv are the molar ratio and conversion in benzene-toluene mixture,respectively.
The highest value obtained for overall aromatics conversion(100%)was observed for Ni/HMS catalyst at 150°C.
Fig.6(c)shows the dependences of the catalysts selectivity in benzene hydrogenation on the overall aromatics conversion.Selectivity toward benzene hydrogenation(SBz(%))was calculated by the following equation,

The results show that Ni/SiO2catalysthas the bestselectivity(75%at 130°C)toward benzene hydrogenation in aromatic mixture.This implies that Ni/SiO2catalyst has a tendency to preferably hydrogenate benzene.
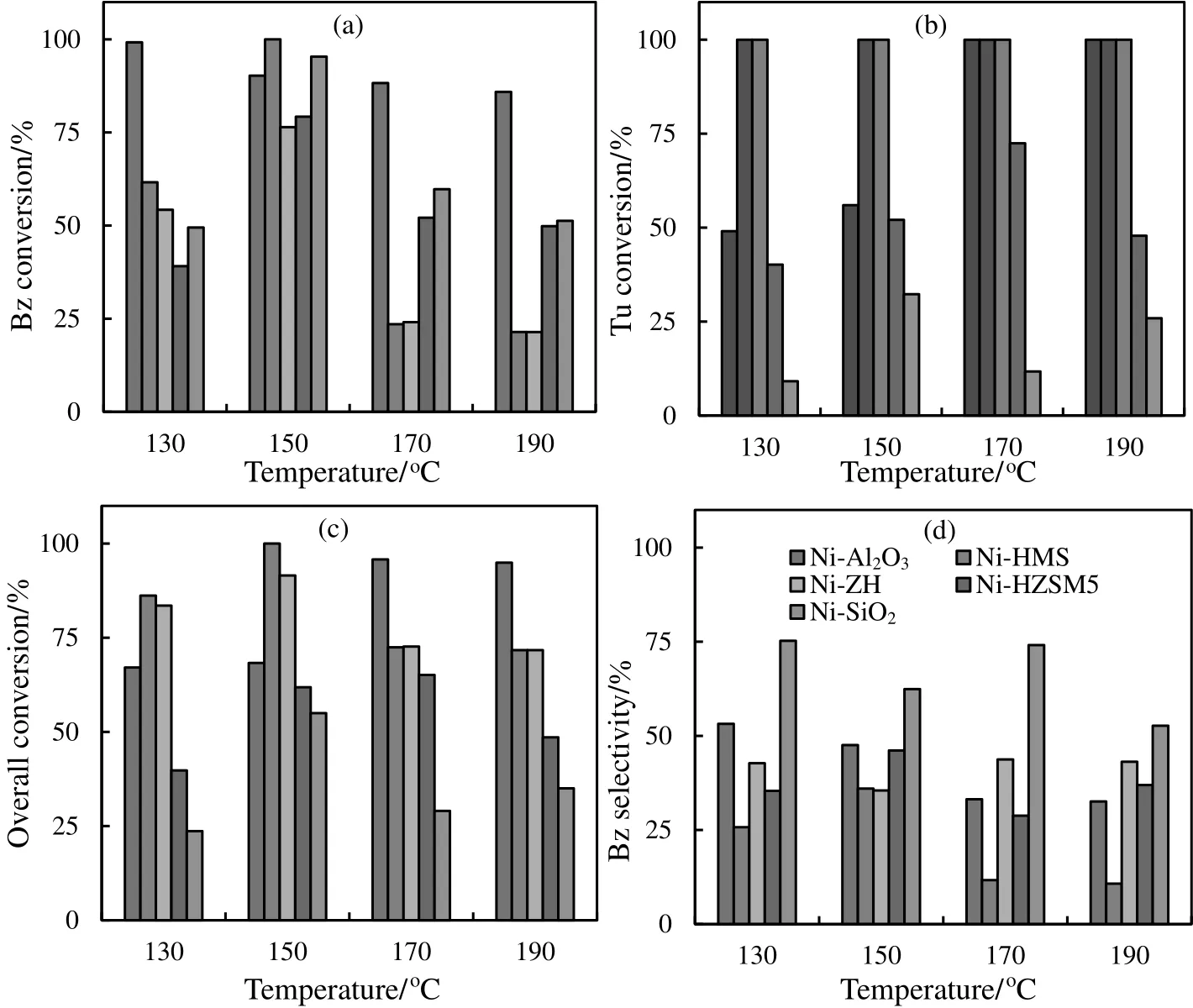
Fig.6.Dependence of conversion on temperature in(a)benzene and(b)toluene hydrogenation,(c)overall conversion on temperature and(d)selectivity in benzene hydrogenation on temperature for various catalysts.

Fig.7.(a)Bz conversion,(b)Tu conversion and(c)overall conversion as a function of stream time(72 h)and(d)coke deposition at 150°C over prepared catalysts.
In order to understand the deactivation of prepared catalysts and the coke formation during the reaction,the activity with respect to stream time(72 h)at 150 °C was examined and shown in Fig.7(a–c).The activity of the above mentioned catalysts has the maximum decrease in the first 10 h.It seems that the greatest amount of coke deposition is formed in this time.According to the occurred changes in the catalysts activities and the amount of coke deposition,Ni/HZSM5 catalyst showed better stability than other catalysts in this reaction without coke content.However,Ni/HMS catalyst even after 72 h on stream has more activity than other catalysts.The results of TG/DT analysis show that the amount of coke deposited on various catalysts is close together and very low[Fig.7(d)].Since all five catalysts reveal almost similar on stream stability with a loss in conversion after 72 h,the deactivation during the constant temperature reaction test is not significant.
3.2.2.Kinetic study
3.2.2.1.Power law model(PL).Power law model was chosen as a simplest kinetic equation.The reaction orders of benzene and hydrogen were determined in separate experiments.These experiments were performed according to the procedure described above and the reaction products were analyzed by GC in the same conditions as for the test of benzene hydrogenation.
To obtain the reaction order of benzene,the catalysts were examined at temperature range of 130–190 °C for a series of reaction mixtures containing various concentrations of benzene[(2–8)vol%with 2 ml·min−1flow rate]while maintaining constant the H2flow rate at 40 ml·min−1.After each reaction,catalysts were cleaned in a flow of H2while heating at 350 °C before cooling to 130 °C and continuing the other step of reaction.In the same step,the reaction order of H2was obtained.In this step,the flow rate of H2was varied between 20 and 45 ml·min−1while maintaining the benzene at 6 vol%.In this condition and at a constant temperature,the logarithmic representations of reaction rate against hydrogen or benzene pressures are mostly linear and their slopes express the order of reaction for the component with varying pressure.In kinetic studies,the reaction rate was defined as follows,

Fig.8(a)shows the dependence of the reaction rate on a partial pressure of hydrogen and a partial pressure of benzene.The partial reaction orders were calculated with respect to the empirical kinetic equation,

where n and m are the partial orders of hydrogen and benzene respectively and k is the rate constant.
The reaction orders for benzene on prepared metal catalysts at a constant temperature were measured zero to 0.5 orders while for hydrogen were found 0.3 to 2.0(Table 2).The zero order for benzene indicates its saturation coverage on the surface.As can be seen in this table,the reaction orders ofH2and benzene increase with reaction temperature,probably due to a substantial decrease in the coverage of each reactant.
The Arrhenius plot was shown in Fig.8(b)for measuring the apparent activation energy() .The correlation coefficients(R2)for the Arrhenius plots and the reaction orders are above 0.9 that show a good fit to the experimental data.
The temperature dependence of the reaction rate constant(k)was evaluated according to the logarithmic form of the Arrhenius equation,

It should be noted that the activation energies for our prepared catalysts in this reaction was determined at similar condition for conversion levels less than 10%where the best linear correlation was observed between the logarithmic rate constant(ln k)and the inverse temperature(1/T).
The results of apparent activation energies(Table 2)calculated for the impregnated Ni catalysts are in good agreement with that reported in the literatures for nickel loaded on various supports[3,19].The kinetics study indicates that the activation energy in the benzene hydrogenation carried out on Ni/HMS(1.5 kJ·mol−1)and also Ni/SiO2(2.6 kJ·mol−1)are lower than that described in the literature[3,19]for nickel loaded on various supports depending on both natures of a support and Ni loading.This low amount of activation energy calculated in this work for Ni/HMS confirms that mesoporous molecular sieves are very attractive supports for nickel active in the hydrogenation reaction.

Fig.8.(a)Double-log plots of the hydrogenation reaction rates versus the partial pressures of Bz and H2,(b)Arrhenius plot,(c)estimated data by power law model and(d)estimated data Langmuir–Hinshelwood model.

Table 2 Kinetic parameters and activation energies for benzene hydrogenation
3.2.2.2.Langmuir–Hinshelwood model(LH).Arising from the abovedescribed experimental data,a Langmuir–Hinshelwood mechanism is proposed for the hydrogenation of benzene over Nisupported catalysts.According to this model,the hydrogenation reaction of benzene is generally regarded to proceed following steps,

In this mechanism has been assumed that one molecule of benzene and three dissociated molecules of hydrogen are chemisorbed on the same type of separated active sites at the surface.Then these preadsorbed molecules simultaneously react as step 9(rate determining step)in many consecutive mini-steps.According to this step,the theoretical rate equation would be as follows,

where k is the rate constant,KHand KBzare the chemisorption equilibrium constants(Ki=ki/k−i)and pHand pBzare the hydrogen and benzene partial pressures,respectively.
To confirm the final equation[Eq.(11)]resulting from the proposed mechanism,the linear transformation of Eq.(11),i.e.plotting 1/r as a function of(pHbeing constant),was evaluated.The plots of our experimental results(not shown here)are close to a straight line starting in origo.It was found experimentally that this mechanism supports our reported results for benzene hydrogenation reaction.
In this equation[Eq.(11)],it was assumed that the adsorption constants(kHand kBz)follow a Van't Hoff type equation.and also the rate constant follows an Arrhenius type equation.

Eqs.(12)and(13)can expand the expression of Eq.(11).
The same set of experiments was done as the experiments of the PL model and the kinetic parameters of the expanded Eq.(11)have been estimated and listed in Table 2.
These results show that the activation energies calculated via the LH model are almostlower than the activation energies measured in the PL model.This observation is probably due to the simplicity of PL model and the lack of consideration of the H2and Bz adsorptions in the PL model.
The data in Table 2 show negative values for the adsorption heats of Bz and H2that are based on the exothermic nature of the Bz hydrogenation reaction.The pre-exponential factor of adsorption for Bz(ABz)is higher than thatofthe H2(AH2).This probably implies a faster and stronger adsorption of Bz over the prepared catalysts.
The parameters obtained were used to simulate the selectivity to Bz products for different prepared catalysts.The results of calculated Bz selectivity vs.experimental Bz selectivity were presented in Fig.8(d).The calculated data of LH model present a same trend of experimental data,but the accuracy at low conversions is slightly bad.
According to the results obtained of these two models,the LHmodel presents the less accuracy than the PL model[Fig.8(c)&(d)].This is probably because the power law has a simpler expression versus numerical adjustment.
A question arises as to whether competitive or noncompetitive adsorption of benzene or hydrogen occurs during this catalysis reaction.
The observed increase in both hydrogen and benzene orders with increasing reaction temperature is described on the basis of a noncompetitive adsorption of two components.However,in some literature[3],competitive adsorption wasreported.Asa result,both reactant molecules(benzene and hydrogen)are adsorbed on the surface sites during catalysis reaction that their strength of adsorption effects on reaction orders.
4.Conclusions
In this study,the various porous supports were synthesized and their physicochemical properties were characterized by XRF,XRD,H2chemisorption,FT-IR,UV–vis DRS,NH3-TPD,nitrogen adsorption–desorption and TGA techniques.A detailed structural/catalytic/kinetics study was carried out in the benzene hydrogenation.Among the synthesized catalysts,the maximum benzene conversion(99.19%)in the Bz cut was achieved over Ni/Al2O3at 130°C.For the Tu cut,the conversion of 100%was attained for Ni/Al2O3,Ni/HMS and Ni/ZH in most of temperatures.According to the results,it could be inferred that in the mixture Bz+Tu cut,Ni/SiO2catalyst has high selectivity of benzene(75.26%)at130°C.Since the importance is no change in octane number of gasoline,as a result,the bestcatalystis a catalyst that has the foremost selectivity to benzene in the hydrogenation of Bz+Tu mixture.Based on the results of coke deposition and kinetics study,Ni/SiO2would be an appropriate catalyst in benzene hydrogenation reaction in industrial uses.
Additionally,a series of kinetic tests performed with these catalysts under various operating conditions showed that the Ni/SiO2catalyst could perform the Bz hydrogenation reaction with appropriate rate.Furthermore,two kinetic models(power law and Langmuir–Hinshelwood)were tested in the kinetic study by first estimating kinetic parameters and then simulating selectivity data to be compared to experimental data,in order to validate the model.The obtained results show that the hydrogenation of Bz is good described by a Langmuir–Hinshelwood model,suggesting is as the reaction mechanism,which has never been shown for these prepared catalysts.
[1]N.A.Bakar,M.M.Bettahar,M.A.Bakar,S.Monteverdi,J.Ismail,Low temperature activation of Pt/Ni supported MCM-41 catalysts for hydrogenation of benzene,J.Mol.Catal.A Chem.333(1)(2010)11–19.
[2]J.Mahmoudi,M.N.Lotfollahi,A.H.Asl,Comparison of synthesized H-Al-MCM-41 with different Si/Al ratios for benzene reduction in gasoline with propylene,J.Ind.Eng.Chem.24(2015)113–120.
[3]R.Wojcieszak,S.Monteverdi,M.Mercy,I.Nowak,M.Ziolek,M.M.Bettahar,Nickel containing MCM-41 and AlMCM-41 mesoporous molecular sieves characteristics and activity in the hydrogenation of benzene,Appl.Catal.A Gen.268(2004)241–253.
[4]Y.Ma,Y.Huang,Y.Cheng,L.Wang,X.Li,Biosynthesized ruthenium nanoparticles supported on carbon nanotubes as efficient catalysts for hydrogenation of benzene to cyclohexane:An eco-friendly and economical bioreduction method,Appl.Catal.A Gen.484(2014)154–160.
[5]S.Lu,W.W.Lonergan,J.P.Bosco,S.Wang,Y.Zhu,Y.Xie,J.G.Chen,Low temperature hydrogenation of benzene and cyclohexene:a comparative study between γ-Al2O3supported PtCo and PtNi bimetallic catalysts,J.Catal.259(2)(2008)260–268.
[6]F.Domínguez,J.Sánchez,G.Arteaga,E.Choren,Gallia as support of Pt in benzene hydrogenation reaction,J.Mol.Catal.A Chem.228(1)(2005)319–324.
[7]L.Zhu,H.Sun,H.Fu,J.Zheng,N.Zhang,Y.Li,B.H.Chen,Effect of ruthenium nickel bimetallic composition on the catalytic performance for benzene hydrogenation to cyclohexane,Appl.Catal.A Gen.499(2015)124–132.
[8]R.J.White,R.Luque,V.L.Budarin,J.H.Clark,D.J.Macquarrie,Supported metal nanoparticles on porous materials.Methods and applications,Chem.Soc.Rev.38(2)(2009)481–494.
[9]T.Jiang,L.Lu,X.Yang,Q.Zhao,T.Tao,H.Yin,K.Chen,Synthesis and characterization of mesoporous molecular sieve nanoparticles,J.Porous.Mater.15(1)(2008)67–73.
[10]O.Domí,S.Martí,Y.Henrí,L.D'Ornelas,H.Krentzien,J.Osuna,Silica-supported palladium nanoparticles show remarkable hydrogenation catalytic activity,J.Mol.Catal.A Chem.197(1)(2003)185–191.
[11]A.Gual,C.Godard,S.Castillón,C.Claver,Soluble transition-metal nanoparticlescatalysed hydrogenation of arenes,Dalton Trans.39(2010)11499–11512.
[12]C.Hubert,E.G.Bilé,A.Denicourt-Nowicki,A.Roucoux,Rh(0)colloids supported on TiO2:a highly active and pertinent tandem in neat water for the hydrogenation of aromatics,Green Chem.13(2011)1766–1771.
[13]Y.Tonbul,M.Zahmakiran,S.Özkar,Iridium(0)nanoparticles dispersed in zeolite framework:a highly active and long-lived green nanocatalyst for the hydrogenation of neat aromatics at room temperature,Appl.Catal.B Environ.148(2014)466–472.
[14]K.X.Yao,X.Liu,Z.Li,C.C.Li,H.C.Zeng,Y.Han,Preparation of a Ru-nanoparticles/defective-graphene composite as a highly efficient arene-hydrogenation catalyst,ChemCatChem 4(12)(2012)1938–1942.
[15]S.Niembro,S.Donnici,A.Sha fir,A.Vallribera,M.L.Buil,M.A.Esteruelas,C.Larramona,Per fluoro-tagged rhodium and ruthenium nanoparticles immobilized on silica gel as highly active catalysts for hydrogenation of arenes under mild conditions,New J.Chem.37(2)(2013)278–282.
[16]J.W.Da-Silva,A.J.G.Cobo,The role of the titania and silica supports in Ru-Fe catalysts to partial hydrogenation of benzene,Appl.Catal.A Gen.252(1)(2003)9–16.
[17]L.J.Simon,P.J.Kooyman,J.G.van Ommen,J.A.Lercher,Effect of Co and Ni on benzene hydrogenation and sulfur tolerance of Pt/H-MOR,Appl.Catal.A Gen.252(2)(2003)283–293.
[18]K.Y.Tsai,I.Wang,T.C.Tsai,Zeolite supported platinum catalysts for benzene hydrogenation and naphthene isomerization,Catal.Today 166(1)(2011)73–78.
[19]A.Lewandowska,S.Monteverdi,M.Bettahar,M.Ziolek,MCM-41 mesoporous molecular sieves supported nickel-physico-chemical properties and catalytic activity in hydrogenation of benzene,J.Mol.Catal.A Chem.188(1)(2002)85–95.
[20]M.Rostamizadeh,A.Taeb,Synthesis and reactivity in inorganic,Metal-Org.nano-Met.Chem.46(2016)665–671.
[21]N.Parsafard,M.H.Peyrovi,M.Rashidzadeh,n-Heptane isomerization on a new kind of micro/mesoporous catalyst:Pt supported on HZSM-5/HMS,Microporous Mesoporous Mater.200(2014)190–198.
[22]S.Therdthianwong,C.Siangchin,A.Therdthianwong,Improvement of coke resistance of Ni/Al2O3catalyst in CH4/CO2reforming by ZrO2addition,Fuel Process.Technol.89(2)(2008)160–168.
[23]Y.Gao,F.Meng,K.Ji,Y.Song,Z.Li,Slurry phase methanation of carbon monoxide over nanosized Ni–Al2O3catalysts prepared by microwave-assisted solution combustion,Appl.Catal.A Gen.510(2016)74–83.
[24]F.Huang,R.Wang,C.Yang,H.Driss,W.Chu,H.Zhang,Catalytic performances of Ni/mesoporous SiO2catalysts for dry reforming of methane to hydrogen,J.Energy Chem.25(2016)709–719.
[25]A.Khojastehnezhad,F.Moeinpou,M.Vafaei,Molybdenum oxide supported on silica(moo3/sio2):an efficient and reusable catalyst for the synthesis of 1,8 dioxo decahydro acridines under solvent-free conditions,J.Mex.Chem.Soc.59(2015)29–35.
[26]B.D.Cullity,Elements of X-ray Diffraction,second ed.Addison-Wesley,Reading,MA,1978.
[27]Y.J.Asencios,M.R.Sun-Kou,Synthesis of high-surface-area γ-Al2O3from aluminum scrap and its use for the adsorption of metals:Pb(II),Cd(II)and Zn(II),Appl.Surf.Sci.258(24)(2012)10002–10011.
[28]N.Firdous,N.K.Janjua,I.Qazi,M.H.S.Wattoo,N.Firdous,N.K.Janjua,I.Qazi,M.H.S.Wattoo,Optimal Co-Ir bimetallic catalysts supported on γ-Al2O3for hydrogen generation from hydrous hydrazine,Int.J.Hydrog.Energy 41(2)(2016)984–995.
[29]K.V.Manukyan,A.J.Cross,A.V.Yeghishyan,S.Rouvimov,J.J.Miller,A.S.Mukasyan,E.E.Wolf,Highly stable Ni-Al2O3catalyst prepared from a Ni-Al layered double hydroxide for ethanol decomposition toward hydrogen,Appl.Catal.A Gen.508(2015)37–44.
[30]Y.Z.Wang,F.M.Li,H.M.Cheng,L.Y.Fan,Y.X.Zhao,A comparative study on the catalytic properties of high Ni-loading Ni/SiO2and low Ni-loading Ni-Ce/SiO2for CO methanation,J.Fuel Chem.Technol.41(8)(2013)972–977.
[31]C.Anjaneyulu,S.N.Kumar,V.V.Kumar,G.Naresh,S.K.Bhargava,K.V.R.Chary,A.Venugopal,In fluence of La on reduction behaviour and Ni metal surface area of Ni-Al2O3catalysts for COxfree H2by catalytic decomposition of methane,Int.J.Hydrog.Energy 40(9)(2015)3633–3641.
[32]S.Sepehri,M.Rezaei,G.Garbarino,G.Busca,Facile synthesis of a mesoporous alumina and its application as a support of Ni-based autothermal reforming catalysts,Int.J.Hydrog.Energy 41(2016)3456–3461.
[33]A.Fouskas,M.Kollia,A.Kambolis,C.Papadopoulou,H.Matralis,Boron-modified Ni/Al2O3catalysts for reduced carbon deposition during dry reforming of methane,Appl.Catal.A Gen.474(2014)125–134.
[34]H.Y.Kim,H.M.Lee,J.N.Park,Bifunctional mechanism of CO2methanation on Pd-MgO/SiO2catalyst:Independent roles of MgO and Pd on CO2methanation,J.Phys.Chem.C 114(15)(2010)7128–7131.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
