Partial pore blockage and polymer chain rigidification phenomena in PEO/ZIF-8 mixed matrix membranes synthesized by in situ polymerization☆
2018-05-26XiaoliDingXuLiHongyongZhaoRanWangRunqingZhaoHongLiYuzhongZhang
Xiaoli Ding *,Xu LiHongyong Zhao 4,*,Ran Wang Runqing Zhao Hong LiYuzhong Zhang
1 State Key Laboratory of Separation Membranes and Membrane Processes/National Center for International Joint Research on Separation Membranes,Tianjin Polytechnic University,Tianjin 300387,China
2 Tianjin Key Laboratory of Hollow Fiber Membrane Materials and Processes,Tianjin Polytechnic University,Tianjin 300387,China
3 Institute of Separation Material and Process Control,School of Material Science and Engineering,Tianjin Polytechnic University,Tianjin 300387,China
4 School of Environmental and Chemical Engineering,Tianjin Polytechnic University,Tianjin 300387,China
1.Introduction
Carbon dioxide separation is one of the important processes nowadays,not only for the purpose of environment protection(e.g.removal CO2from flue gas,CO2/N2separation)[1],but also for the purpose of the energy development(e.g.removal CO2from nature gas,CO2/CH4separation)[2]and so on.Therefore,the removal of CO2from the complex mixture is a formidable technological and scientific challenge which has received considerable attention for several years.Generally,CO2can be removed from the mixture by absorption,adsorption,cryogenic separation,membrane separation,etc.[3].Among them,CO2separation by membrane technology has been paid more and more attention due to its inherent advantages including the relatively low energy consumption,low investment and ease of operation[1,4].
Membranes are fabricated mainly by organic polymer material and inorganic material.Organic polymer membranes occupy a main part in the membrane gas separation market with the advantages of easy manufacture and low cost etc.However,pure polymeric membrane separation is generally restricted by the famous trade-off between gas permeability and selectivity,termed Robeson upper bound[5,6].A substantial effort has been devoted to exceed the permeation–separation trade-off line to obtain the excellent performance material with high permeability and high selectivity.Currently,tremendous improvements have been achieved in tailoring organic polymers with special physical and chemical structure,such as facilitated transport membrane materials with carriers facilitating the transport of CO2in the membrane[7],polymers of intrinsic microporosity with special ladder structure contributing to high free volume and high surface areas[8],thermally rearranged polymers with tuned microvoids contributing to performance enhancement in the selective molecular transport[9],solubility selective membrane materials with polar groups enhancing the solubility selectivity[10,11].On the other hand,mixed matrix membrane(MMM)comprising organic polymer with dispersed inorganic filler has been developed to exceed the trade-offline.Inorganic membrane materials show high permeation–separation performance lying far beyond the trade-off line for the organic polymers.However,because of their vast expense and fragile structures,continuous and defect-free membranes are difficult to fabricate on a larger scale[12].MMMs combining the processing versatility of organic polymers with the permeation–separation characteristic of inorganic particles have been developed and considered to be an efficient way to suppress the limit of the trade-off line.
During the last decades,different kinds of inorganic particles have been used as filler in MMMs,including zeolites,carbon molecularsieves,mesoporous material,non-porous nanoparticles,and graphene[13].The main challenge of successful application of MMMs is the poor compatibility between the inorganic filler and organic polymer matrix,especially when rigid glassy polymer is used as the continuous phase[14].The separation performance of MMMs fails due to the interfacial region with nonselective voids between the polymer and inorganic filler.The permeation performance fails due to the partial pore blockage and polymer chain rigidification[15].
In recently years,zeolitic imidazolate frameworks(ZIFs),a new class of porous materials,has received wide attention.The partial organic character of ZIFs,as a consequence of the imidazolate links,makes them more compatible with organic polymer matrices used for the preparation of MMMs,which leads to the defect-free membranes without the need of high temperatures membrane processing or complex compatibilization protocols[16].ZIF-8 with excellent chemical and thermal stability[17],has a finite pore size of 0.34 nm allowing size exclusion of gas molecules due to the narrow size of six membered ring,which makes it as a good candidate for CO2/CH4separation[18,19].The intrinsic gas permeation–separation performance of ZIF-8 is presented in Table 1.While ZIF-8 based mixed matrix membrane also suffers from partial pore blockage and polymer chain rigidification.Shahid et al.[18]observed the polymer chain rigidification in Matrimid/ZIF-8 MMMs,Mueller et al.[20]observed the polymer chain rigidification in 6FDA-DAM/ZIF-8 MMMs,Fang et al.[21]observed the partial pore blockage and polymer chain rigidification in PDMS/ZIF-8 MMMs,and Xu et al.[19]observed the polymer chain rigidification in Pebax/ZIF-8 MMMs.While in mostcases,polymer chain rigidification was beneficialto separation performance,MMMs membranes presented improved permeation performance and separation performance by incorporation of ZIF-8[18,19,21].

Table 1 The permeability and selectivity of the ZIF-8 and the polymer matrix
Poly(ethylene oxide)(PEO)polymer is one of the promising materials for CO2separation which gets a lot of attention[23].In this study,polymer chain rigidification phenomenon in PEO polymer with soft segment was studied.The ZIF-8 based MMMs were synthesized with the multi-armed and star-like cross-linked PEO rubber as matrix,which was reported in previous works of our team[11].Unlike MMMs in the previous reports fabricated by the method of solution blending,MMMs based on ZIF-8 fabricated by in situ polymerization are reported in this work.The intrinsic gas permeation–separation performance is also presented in Table 1.The pre-polymerization solution composed of monomer,cross-linker,initiator and ZIF-8 was polymerized under UV irradiation,which is an efficient way to fabricate membrane with the advantages of short cycle and solvent free,etc.The partial pore blockage and polymer chain rigidification phenomena were studied.Glass transition temperature(Tg),d-spacing(d)and the permeation–separation performance of the MMM were also investigated to confirm the exist of two undesirable phenomena.
2.Experimental
2.1.Materials
Poly(ethylene glycol)methyl ether acrylate(PEGMEA,Aldrich,Mn=480 g·mol−1),pentaerythritol triacrylate(PETA,Alfa Aesar),1-hydroxylcyclohexyl phenyl ketone(HCPK,J&K Chemical)and ZIF-8(PlasmaChem,25 nm)were used as received.The chemical structures
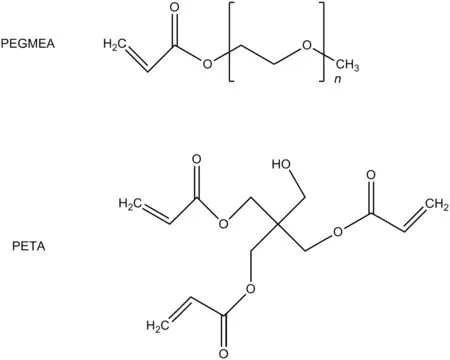
Fig.1.The structures of the monomer and cross-linker.
ofthe monomerand cross-linker are shown in Fig.1.Nitrogen,methane and carbon dioxide(Tianjin Xiqing Liufang high technology gas supply station,purity 99.99%)were used as received.
2.2.Synthesis of cross-linked PEO rubber/ZIF-8 MMMs
Cross-linked PEO/ZIF-8 MMMs were synthesized by in situ polymerization.The pre-polymerization solution of cross-linker PETA with tri-acrylate groups,monomer PEGMEA with mono-acrylate group,dispersed phase ZIF-8 were prepared with 0.1 wt%HCPK relative to the total amount of the cross-linker and monomer.The PETA and PEGMEA were mixed according to the mass rate of 8:2.ZIF-8 nanoparticles were added into the pre-polymerization solution,corresponding to the equation as follow:

where mZIF-8,mmonomerand mcross-linkerare the mass of ZIF-8,monomer and cross-linker in the pre-polymerization solution,respectively.The fabrication process of MMMs,viz.polymerization was carried out under UV irradiation,the details had been described elsewhere previously[11].
2.3.Characterization of cross-linked PEO rubber/ZIF-8 MMMs
Fourier Transform infrared spectroscopy(FTIR)spectra were recorded on a Bruker Vector-22 spectroscope to determine the conversion of acrylate groups and the presence of the ZIF-8.For each measurement,the filmsample was scanned at4 cm−1resolution over400–4000 cm−1range.Scanning electron microscope(SEM,Hitach,Japan)was used to study the surface and cross-section morphologies.
X-ray diffraction(XRD)patterns were obtained by using a Scintag theta–theta diffractometer(Bruker AXS,D8 Advanced,Germany)utilizing CuKαradiation.The generator was operated at 45 kV and 40 mA,at room temperature about 25°C.The samples were measured with a 2θscan from5 to 40°with 0.02°step.The d-spacing,corresponding to the position of the diffraction maximum,was calculated by Bragg's Law as follow:

where λ is the wavelength of CuKαradiation(0.154 nm),and θ is the broad peak maximum(°).
Thermal transitions were determined using a differential scanning calorimeter(DSC200F3,NETZSCH,Germany).Samples were initially quenched to −90 °C and scanned at a heating rate of 20 °C·min−1to 40 °C under a dry N2purge flow rate of 50 ml·min−1.The midpoint of the heat capacity step change was taken as the glass transition temperature.

The density of dry sample was determined by hydrostatic weighing using a precision balance(Model 4,Shaihai Hengping Scientific Instrument Co.,Ltd.,China)with a density determination kit.The density(ρm,g·cm−3)was calculated by using the following equation:where MAis the membrane mass in air(g),MLis the membrane mass in the auxiliary liquid(g),and ρ0is the density of the auxiliary liquid(g·cm−3).Hexane was used as the auxiliary liquid in our study,since poly(ethylene oxide)does not have an affinity for this alkane.
2.4.Permeation–separation performance of cross-linked PEO rubber/ZIF-8 MMMs
Pure gas permeability in MMMs was determined using the time-lag method by a constant-volume/variable-pressure apparatus at 35°C with a feed pressure of 0.2 MPa.The permeability was calculated from the pressure rise in a downstream vessel of known volume.In this study,all samples were partially masked using impermeable aluminum tape on the upstream.The o-ring in the permeation cell was in direct contact with the aluminum tape to avoid the damage of the film.After aluminum tape masking,the surface area of the sample available for gas transport was 3.14 cm2.After a sample was mounted in the system,both upstream and downstream volumes were exposed to vacuum overnight to degas the film.Permeant gas was then introduced into the upstream side,and the permeant pressure on the downstream side was monitored using a MKS-Baratron pressure transducer.The pressure increase in the downstream volume was determined by subtracting the effect of the leak.
Permeability coefficient P(Barrer)was calculated from the steadystate rate of pressure increase in a fixed downstream volume:if xed upstream pressure and under vacuum(Pa·s−1),respectively.The ideal selectivity is defined as follows:


where Vdis the downstream volume(cm3),l is the film thickness(cm),here the membrane thickness is about 0.02 cm,p2is the upstream absolute pressure(Pa),A is the film area available for gas transport(cm2),R is the gas constant(8.34 × 106cm3(STP)·Pa·mol−1·K−1),T is the absolute temperature for test(K),(d p1/d t)ssand(d p1/d t)leakare the steady-state rates of pressure rise in the downstream volume at a
where the subscripts A and B refer to gas A and gas B,respectively.
The apparent diffusion coefficient D(cm2·s−1)was obtained from the time-lag(θ′)as follows:

where θ′(s)is the diffusivity time-lag and l(cm)is the thickness of membrane measured using a digital micrometer(Shanghai Chuanlu Measuring Tools Co.,Ltd.,China).
The apparent solubility coefficient S(cm3(STP)·cm−3·Pa−1)was evaluated as follows:

The diffusivity selectivity of gas A over gas B(SA/SB)is defined as the ratio of diffusion coefficient for gas A over diffusion coefficient for gas B.The solubility selectivity of gas A over gas B(DA/DB)is defined as the ratio of solubility coefficient for gas Aoversolubility coefficient for gas B.
3.Results and Discussion
3.1.Characterization of the MMMs

Fig.2.Appearances of the pure polymer membrane and MMMs.
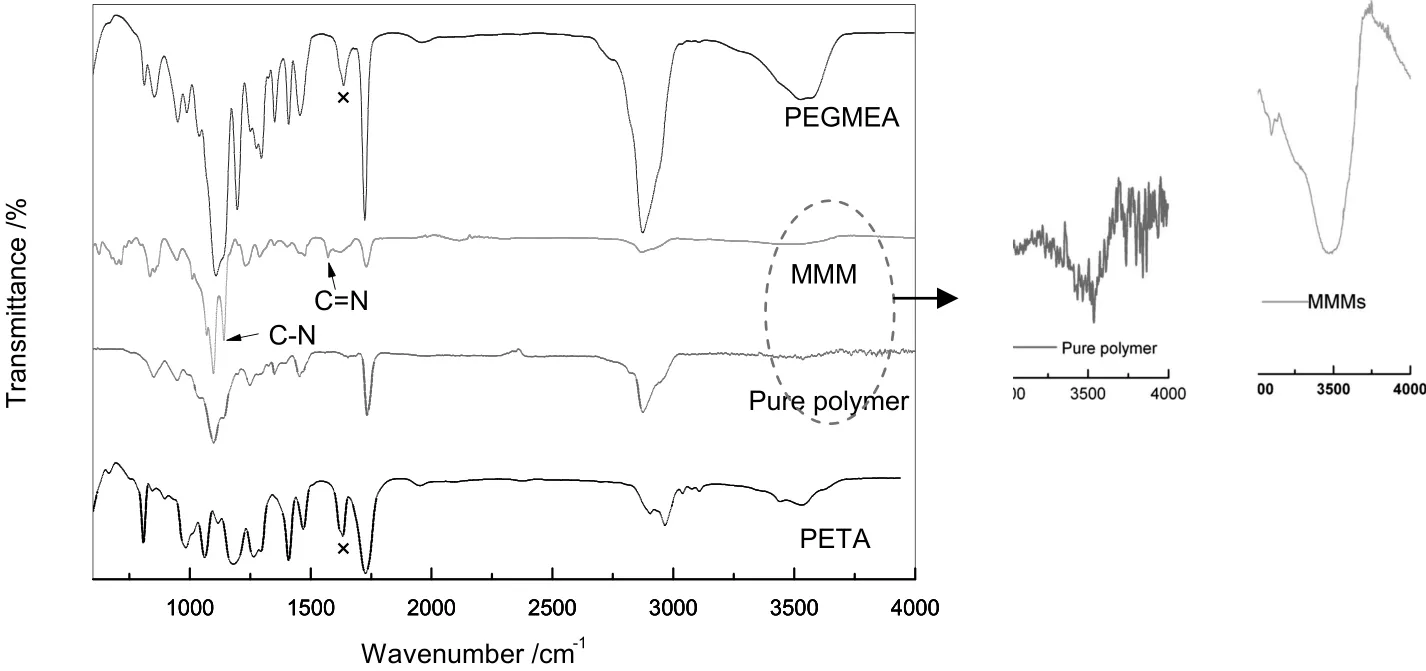
Fig.3.FTIR spectra of PETA,PEGMEA,pure cross-linking polymer membrane and MMM with 7.5 wt%ZIF-8 loading.
The membranes were changed from colorless and transparent to white gradually with the incorporation of ZIF-8 as shown in Fig.2.The white power,ZIF-8,led to the color change.FTIR spectra of PETA,PEGMEA,pure cross-linked polymer membrane and MMM with 7.5 wt%ZIF-8 loading are shown in Fig.3.The characteristic peaks for carbon–carbon double bond(atabout1635 cm−1)practically disappear in the spectra after cross-linking(pure polymer membrane spectrum and MMM spectrum).It indicates that the reaction conversion of PEGMEA and PETA were close to 100%.A strong C–N absorption band atabout1142 cm−1,representative ofZIF-8,appears in MMMspectrum[24].And the peak at 1585 cm−1can be assigned to the C=N stretch mode[25].The peak at 3539 cm−1assigned to–OH stretch variation in pure polymer membrane shifts to 3470 cm−1in MMMs accompanied with increase in peak width,which results from the H-bonding between ZIF-8 and the polymer matrix[26].
The surface morphologies of MMMs are shown in Fig.4,and the cross section morphologies are shown in Fig.5.The images show the incorporation of ZIF-8 particles into the polymer matrix.No visible phase separation can be observed in the surface,while some interface voids are found in cross section images.The surface images indicate the nano-particles disperse uniformly in the horizontal direction due to their increased interaction induced by the large surface area and the inorganic/organic hybrid nature of ZIF-8 nano-particles.Additionally,it is owing to the short fabricating time of UV-irradiation polymerization.While in vertically,we found the agglomeration phenomenon in MMM with 7.5%ZIF-8 loading.In cross section images,it can be found that some ZIF-8 lost,which was induced by the stress in manufacture process of SEM sample.Obvious stress cracking is shown in cross section images for MMMs with more than 1.5 wt%ZIF-8 loading.
In addition to FTIR spectra and FESEM images,the existence of ZIF-8 in MMMs can also be confirmed by XRD,which is commonly used to investigate the presence of crystals of inorganic materials in amorphous matrixes.The effects of incorporated ZIF-8 on the crystallinity are usually considered to be from two aspects.On the one hand,the incorporated ZIF-8 in the polymer matrix may increase the free volume and decrease the crystallinity of membranes.On the other hand,with high ZIF-8 loading,the incorporated ZIF-8 may act as the nucleation site,which enhances the crystallinity of membranes.These two opposing effects determine whether the membrane crystallinity is increased or decreased with an increase in ZIF-8 loadings[25].The peak for the pure ZIF-8 is sharp with intensity due to the crystalline structure as shown in Fig.6(A),while the peak for the pure polymer,which is an amorphous material,is rather broad as shown in Fig.6(B).As observed,there is no obvious change for the XRD patterns of membranes with low ZIF-8 loading.And when a tiny amount of ZIF-8 loading(e.g.0.5 wt%),the peak in XRD is broad as that for the pure amorphous polymer.With the increase in ZIF-8 loading(1 wt%–7.5 wt%),the sharp peaks are observed gradually.The intensity of characteristic peaks of ZIF-8 at about 7.3°representing the plane(011)of ZIF-8 crystalline structure and being usually used as reference for the identification of ZIF-8,is increased with the increase in ZIF-8 loading in MMMs,which indicates that the polymer has not changed the crystallinity of the ZIF-8.The intensity ration(r)of the first and second peak area,at 7.3°and 10.3°respectively,was calculated,which was decreased from 8.3 for ZIF-8 to 1.0–1.5 for MMMs.This decrease can be explained by the penetration of polymer chains into the pore of ZIF-8[27].And this partial blockage exists in the MMMs synthesized by in site polymerization,since the monomer chain is more flexible than polymer.As seen from Fig.6(B),the peak intensity at 26.65°reflecting the ZIF-8 window size(0.34 nm)disappears or appears at high-value zone(26.8°),which also results from the partial blockage or coverage.
In addition,the amorphous nature of the polymer in MMMs is revealed in the corresponding diffractogram,with the broad peak for the polymer centered at 2θ=20.55°being moved to the high-value zone of the reflection peak.The movement is consistent with the strong interaction between continuous and dispersed phases,which reduces the distance between polymer chains.This might imply the tightening of polymer chains,which is consistent with the DSC results as mentioned in the next paragraph.While the decrease of the d-spacing is independent with the ZIF-8 loading amount(Table 2),since ZIF-8 impacts d-spacing in two ways.On the one hand,the strong interaction between continuous and dispersed phases reduces the distance between polymer chains[28,29].On the other hand,the formation of some phases of polymer matrix around the fillers resulted in an increase of d-spacing,because of the capacity of fillers to alter the polymer chain packing[13,30,31].The two opposite effects led to the irregular decrease in d-spacing.Anyway,the d-spacings of MMMs are decreased compared with that of the pure polymer membrane.
The glass transition temperature from DSC(Fig.7)is used to investigate the interaction between the polymer and filling particles.The DSC results of MMMs with different ZIF-8 loadings and pure polymer membrane are presented in Table 2.Single and clear Tgforall MMMs samples are observed,resulting from combination of filling particle and polymer matrix on molecular lever and formation of a new material.The addition of ZIF-8 to polymer increased Tgof material,and which was increased with the increase in the ZIF-8 loading.The increase in Tgis considered to be a result of concentration and interaction between filling particles and polymer matrix.In this study,the increase in Tgis a result of H-bonding between ZIF-8 and the polymer matrix[24,32],which also indicates that the polymer's segmental mobility is decreased.This might imply the tightening of polymer chains,which is consistent with the XRD result as mentioned in the previous paragraph.
3.2.Gas permeation–separation performance of MMMs
The Maxwell equation,which is suitable to predict the physiochemical properties of heterogeneous systems where particles are randomly dispersed in other continuous phases,has been extended to predict gas separation performance of MMMs as follows by many researchers[33]:

Fig.4.SEM images of surface of MMMs.
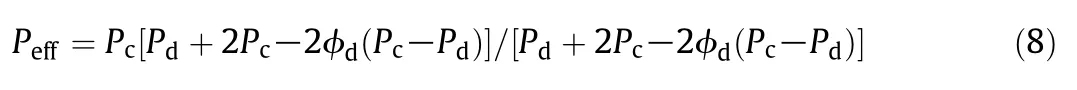
where Peffis the effective permeability of MMMs,Φ is the volume fraction,and the subscripts d and c refer to the dispersed and continuous phases,respectively.The permeabilities of MMMs predicted by the Maxwell equation and tested at 35°C and 0.2 MPa,are presented in Fig.8.It is obvious that the Maxwell equation provides fairly poor predictions in this study.The experimental permeability deviates from the predictions positively or negatively.And the predictions overestimated the ideal selectivity.Although the cross-linked PEO polymer is a lower-Tgrubber with high chain mobility,a polymer rigidification effectnear the polymer-sieve interface might also be significant in MMMs,causing a reduced permeability[33].Tgmeasurements indicating a Tgincrease for MMMs and d-spacing measurements indicating a d-spacing decrease for MMMs(Table 2)support this hypothesis.Since the chain mobility and d-spacing are both decreased with the incorporation of ZIF-8,the free volume fraction of MMMs is decreased.Furthermore,the penetration of polymer chains into the pore of ZIF-8,inducing the partial pore blockage confirmed by XRD result,led to the lower than-predicted permeability.While as shown in Fig.5,interface voids were formed in MMMs,which led to higher-than-predicted permeability.We also consider that the phenomenon of higher-than-predicted permeability is possibly owing to the deviation of intrinsic permeability for ZIF-8 between the tested value from dense film and the estimated value used in this study,which was estimated based on the composite membranes.
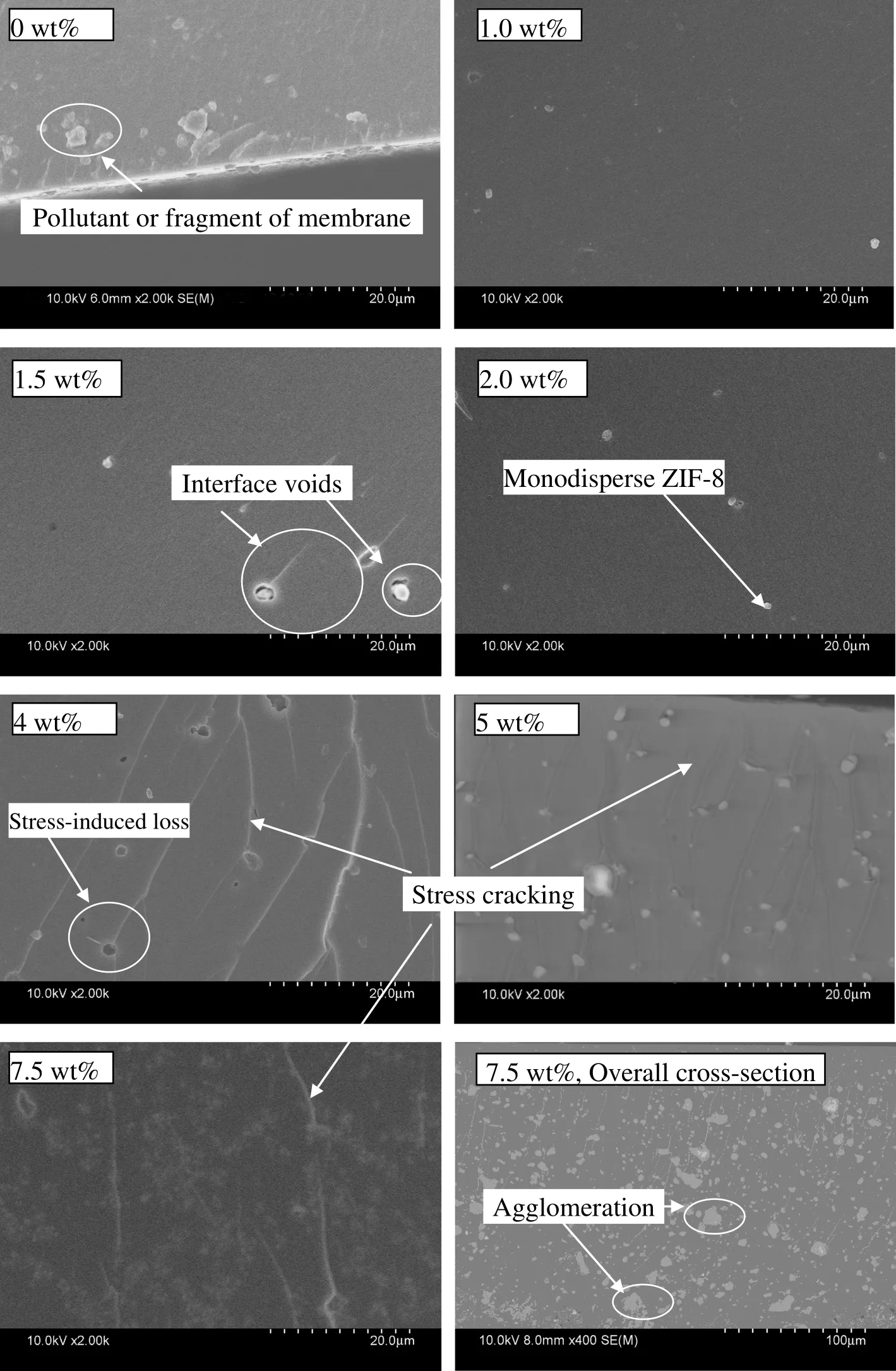
Fig.5.SEM images of cross section of MMMs.
The gas diffusivity coefficient and solubility coefficient of MMMs are summarized in Table 3.It is obvious that the incorporation of ZIF-8 produces a decrease in diffusion for CO2,and an increase for CH4in most cases.Generally,the incorporation of porous ZIF-8 increases the diffusivity coefficient,since ZIF-8 has a much great diffusivity coefficient than polymers[34].However,as mentioned earlier,the incorporation of ZIF-8 reduced the chain mobility and d-spacing,leading to the increase in diffusion resistance and the decrease in diffusivity coefficient,which influences the diffusion of the CO2(small molecule)greatly compared with CH4(large molecule).It is obvious that the latter dominated the changing trend for CO2.In the case of CH4,a fact should be considered that the effective aperture size of ZIF-8 is~0.40 nm,much larger than the crystallo-graphically determined aperture size due to the flopping motion of the ligand[35],which influences the diffusion of the CH4(large molecule)greatly compared with CO2(small molecule).The two opposite effects result in the increase in the diffusivity coefficient of CH4,and the highest diffusivity coefficients for all gases at the 1.5 wt%loading.In addition,the highest diffusivity coefficients are attributed partly to the defect in the polymer-sieve interface as mentioned in previous paragraph as shown in Fig.5.The incorporation of ZIf-8 also leads to two opposite effects on the solubility coefficient.On the one hand,the reduced chain mobility and d-spacing decrease the free volume fraction and then the solubility coefficient.On the other hand,the high sorption of ZIF-8 increases the solubility coefficient.The two opposite effects can be used to explain that the solubility coefficient is more or less independent of the ZIF-8 loading.
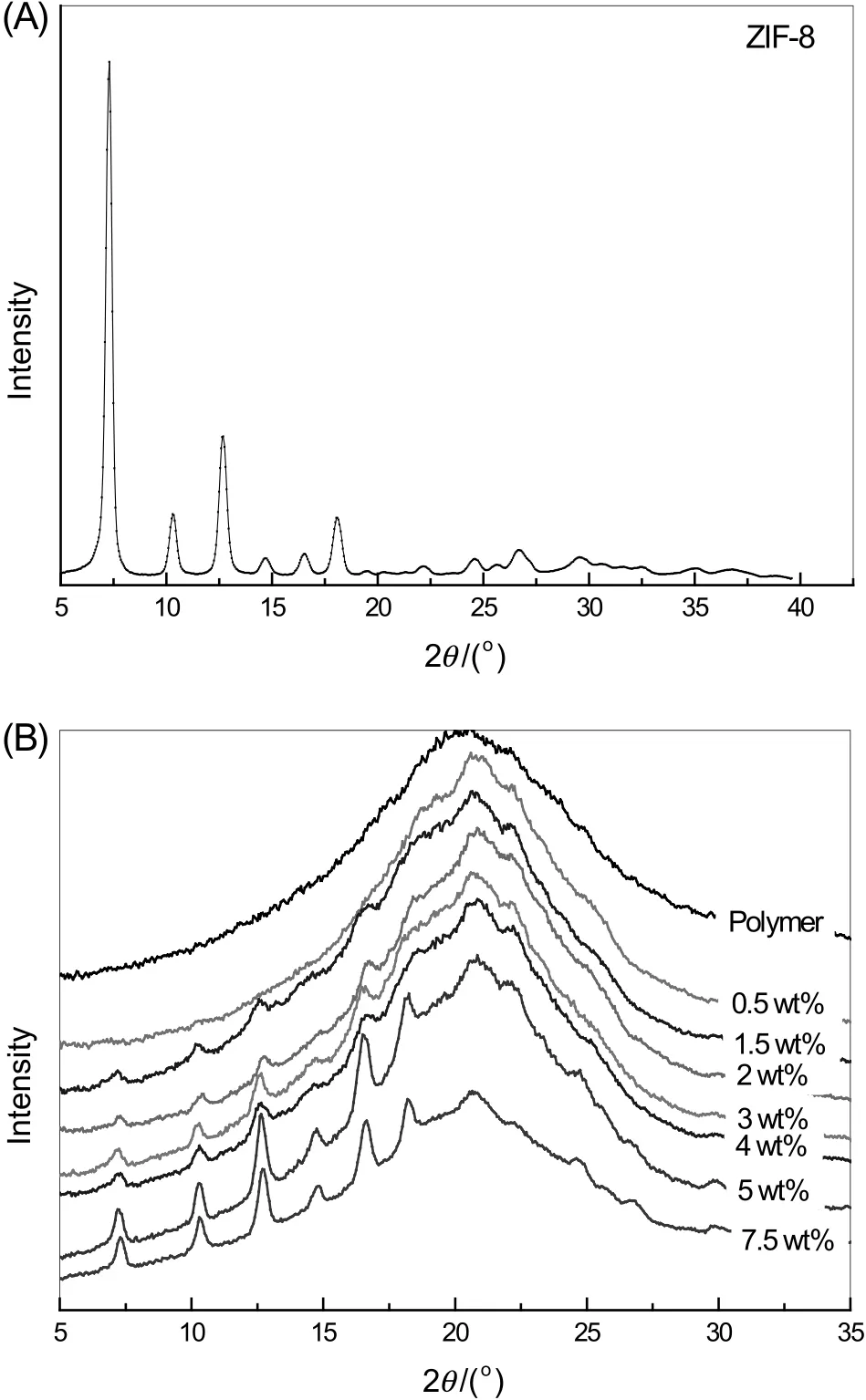
Fig.6.X-ray diffraction patterns of ZIF-8(A),the MMMs with different ZIF-8 loadings and the pure polymer membrane(B)at room temperature.
The diffusivity selectivity and solubility selectivity of MMMs are also presented in Table 3.It can be seen that the ideal selectivity depended on the solubility selectivity for all membranes,which indicates that the incorporation of ZIF-8 does notchange the nature of solubility selectivity.The diffusivity selectivity and solubility selectivity are more or less independent of the ZIF-8 loading.The change in selectivity was mainly caused by the two opposite effects:(1)The decrease in free volume fraction induced by the decrease in chain mobility and d-spacingtermed as polymer rigidification and the partial blockage of ZIF-8 pore increased the diffusion selectivity and solubility selectivity[36];(2)The intrinsically low diffusion selectivity and solubility selectivity for ZIF-8 decrease the diffusion selectivity and solubility selectivity.Fig.9 compares the permeation–separation performance with the 2008 Robeson upper bound.It can be seen that performances of MMMs are moved away from the upper bound compared with that of pure polymer membrane,which is different compared with predicted data.The slight decrease in selectivity is mainly caused by the incorporation of ZIF-8 because of the intrinsically low CO2/CH4selectivity.The change in permeability was mainly caused by the two opposite effects as mentioned above:(1)the decrease in free volume fraction induced by the decrease in chain mobility and d-spacing termed as polymer rigidification and the partial blockage of ZIF-8 pore;(2)the intrinsically high permeability for ZIF-8.Obviously,the former effect dominates the change tendency,that is to say,the partial pore blockage and polymer chain rigidification due to the corporation of ZIF-8 decreases the permeability.
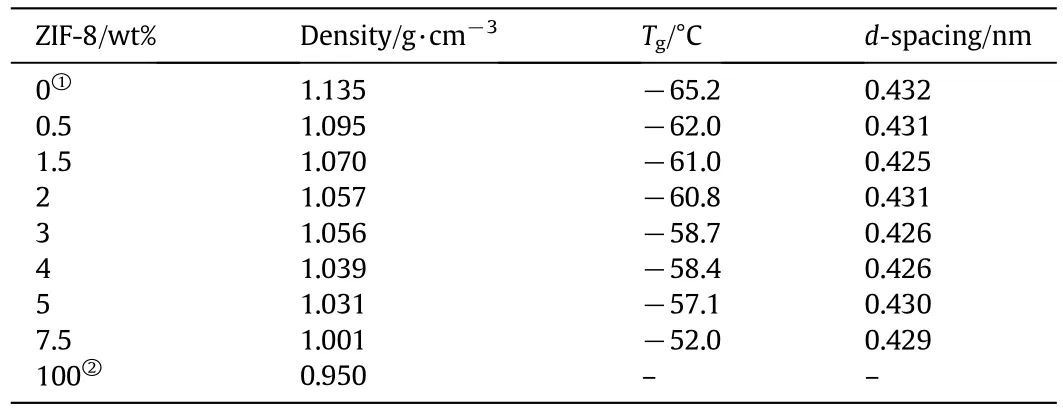
Table 2 The physical properties of MMMs,ZIF-8 and pure polymer membrane
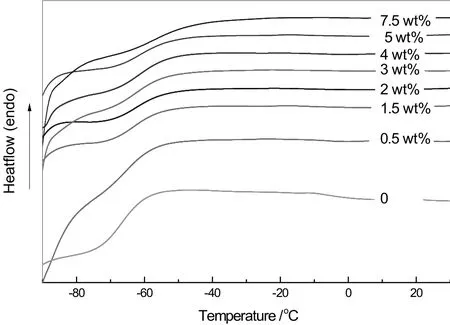
Fig.7.Differential scanning calorimetry thermo-grams of MMMs and the pure polymer membrane.
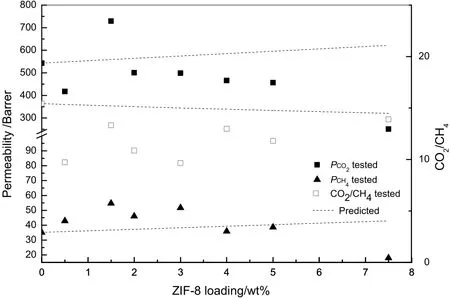
Fig.8.A comparison between the Maxwell prediction and experimental data of pure gas transport performance for MMMs.

Table 3 Gas diffusivity coefficient,solubility coefficient,diffusivity selectivity and solubility selectivity of MMMs

Fig.9.CO2/CH4 permeation–separation performance of pure polymer membrane,ZIF-8 membrane,and MMMs in Robeson upper bound plot.
4.Conclusions
Cross-linked PEO rubber was used as matrix,MMMs incorporating different contents of ZIF-8 nano-particles were synthesized by in situ polymerization for CO2separation.The physical characteristic and permeation–separation performance were investigated.The following conclusion could be obtained from this study:
(1)ZIF-8 nanoparticles were well dispersed in MMMS thought in situ polymerization,accompanied with some agglomeration in vertically;
(2)XRDresults indicated a decrease in the d-spacing for MMMscompared with that of pure polymer.DSC results showed an increase in Tgwith the increasing of ZIF-8 loading,indicating a decrease in chain mobility.Both of them indicated the polymer rigidification effect around the ZIF-8.XRD results also indicated the partial blockage of ZIF-8 pore;
(3)The improvement effort in permeation–separation performance by incorporation of ZIF-8 ended in failure.The polymer rigidification effect and partial pore blockage were significant in PEO rubber/ZIF-8 MMMs.In most cases,the increase in permeability induced by the incorporation of the high-permeability ZIF-8 was offset by the decrease in permeability induced by the polymer rigidification effect and partial pore blockage in this work.
[1]R.Khalilpour,K.Mumford,H.B.Zhai,A.Abbas,G.Stevens,E.S.Rubin,Membrane based carbon capture from flue gas:A review,J.Clean.Prod.103(2015)286–300.
[2]S.Faramawy,T.Zaki,A.A.E.Sakr,Natural gas origin,composition,and processing:A review,J.Nat.Gas Sci.Eng.34(2016)34–54.
[3]K.Osman,C.Coquelet,D.Ramjugernath,Review of carbon dioxide capture and storage with relevance to the South African power sector,S.Afr.J.Sci.110(2014)1–12.
[4]I.Sreedhar,R.Vaidhiswaran,B.M.Kamani,A.Venugopal,Process and engineering trends in membrane based carbon capture,Renew.Sust.Energ.Rev.68(Part 1)(2017)659–684.
[5]L.M.Robeson,Correlation of separation factor versus permeability for polymeric membranes,J.Membr.Sci.62(1991)165–185.
[6]L.M.Robeson,The upper bound revisited,J.Membr.Sci.320(2008)390–400.
[7]H.C.Ferraz,L.T.Duarte,M.Di Luccio,T.L.M.Alves,A.C.Habert,C.P.Borges,Recent achievements in facilitated transport membranes for separation processes,Braz.J.Chem.Eng.24(2007)101–118.
[8]H.Y.Zhao,Q.Xie,X.L.Ding,J.M.Chen,M.M.Hua,X.Y.Tan,Y.Z.Zhang,High performance post-modified polymers of intrinsic microporosity(PIM-1)membranes based on multivalent metal ions for gas separation,J.Membr.Sci.514(2016)305–312.
[9]H.B.Park,S.H.Han,C.H.Jung,Y.M.Lee,A.J.Hill,Thermally rearranged(TR)polymer membranes for CO2separation,J.Membr.Sci.359(2010)11–24.
[10]H.Q.Lin,Solubility selective membrane materials for carbon dioxide removal from mixtures with light gases(Ph.D.Thesis)The University of Texas at Austin,Austin,2005.
[11]H.Y.Zhao,X.L.Ding,P.P.Yang,L.Y.Li,X.Li,Y.Z.Zhang,A novel multi-armed and starlike poly(ethylene oxide)membrane for CO2separation,J.Membr.Sci.489(2015)258–263.
[12]G.Q.Lu,J.C.Diniz da Costa,M.Duke,S.Giessler,R.Socolow,R.H.Williams,T.Kreutz,Inorganic membranes for hydrogen production and purification:A critical review and perspective,J.Colloid Interface Sci.314(2007)589–603.
[13]P.S.Goh,A.F.Ismail,S.M.Sanip,B.C.Ng,M.Aziz,Recent advances of inorganic fillers in mixed matrix membrane for gas separation,Sep.Purif.Technol.81(2011)243–264.
[14]R.Mahajan,R.Burns,M.Schaeffer,W.J.Koros,Challenges in forming successful mixed matrix membranes with rigid polymeric materials,J.Appl.Polym.Sci.86(2002)881–890.
[15]K.Mohammad Gheimasi,T.Mohammadi,O.Bakhtiari,Modification of ideal MMMs permeation prediction models:Effects of partial pore blockage and polymer chain rigidification,J.Membr.Sci.427(2013)399–410.
[16]J.Ahmad,M.B.Hagg,Effect of zeolite preheat treatment and membrane post heat treatment on the performance of polyvinyl acetate/zeolite 4A mixed matrix membrane,Sep.Purif.Technol.115(2013)163–171.
[17]J.Caro,M.Noack,P.Kölsch,R.Schäfer,Zeolite membranes-state of their development and perspective,Microporous Mesoporous Mater.38(2000)3–24.
[18]S.Shahid,K.Nijmeijer,Performance and plasticization behavior of polymer-MOF membranes for gas separation at elevated pressures,J.Membr.Sci.470(2014)166–177.
[19]L.W.Xu,L.Xiang,C.Q.Wang,J.Yu,L.X.Zhang,Y.C.Pan,Enhanced permeation performance of polyether-polyamide block copolymer membranes through incorporating ZIF-8 nanocrystals,Chin.J.Chem.Eng.25(2017)882–891.
[20]R.Mueller,V.Hariharan,C.Zhang,R.Lively,S.Vasenkov,Relationship between mixed and pure gas self-diffusion for ethane and ethene in ZIF-8/6FDA-DAM mixed-matrix membrane by pulsed field gradient NMR,J.Membr.Sci.499(2016)12–19.
[21]M.Q.Fang,C.L.Wu,Z.J.Yang,T.Wang,Y.Xia,J.D.Li,ZIF-8/PDMS mixed matrix membranes for propane/nitrogen mixture separation:Experimental result and permeation model validation,J.Membr.Sci.474(2015)103–113.
[22]L.Hao,P.Li,T.Yang,T.S.Chung,Room temperature ionic liquid/ZIF-8 mixed-matrix membranes for natural gas sweetening and post-combustion CO2capture,J.Membr.Sci.436(2013)221–231.
[23]S.L.Liu,L.Shao,M.L.Chua,C.H.Lau,H.Wang,S.Quan,Recent progress in the design of advanced PEO-containing membranes for CO2removal,Prog.Polym.Sci.38(2013)1089–1120.
[24]S.Hwang,W.S.Chi,S.J.Lee,S.H.Im,J.H.Kim,J.Kim,Hollow ZIF-8 nanoparticles improve the permeability of mixed matrix membranes for CO2/CH4gas separation,J.Membr.Sci.480(2015)11–19.
[25]S.Zhao,X.C.Cao,Z.J.Ma,Z.Wang,Z.H.Qiao,J.X.Wang,S.C.Wang,Mixed-matrix membranes for CO2/N2separation comprising a poly(vinylamine)matrix and metal-organic frameworks,Ind.Eng.Chem.Res.54(2015)5139–5148.
[26]J.H.Hu,X.F.Zheng,Practical Infrared Spectroscopy,Science Press,Beijing,2011.
[27]S.Sorribas,B.Zornoza,C.Téllez,J.Coronas,Mixed matrix membranes comprising silica-(ZIF-8)core-shell spheres with ordered meso-microporosity for natural-and bio-gas upgrading,J.Membr.Sci.452(2014)184–192.
[28]B.Zornoza,B.Seoane,J.M.Zamaro,C.Tellez,J.Coronas,Combination of MOFs and zeolites for mixed-matrix membranes,ChemPhysChem 12(2011)2781–2785.
[29]B.Zornoza,C.Téllez,J.Coronas,Mixed matrix membranes comprising glassy polymers and dispersed mesoporous silica spheres for gas separation,J.Membr.Sci.368(2011)100–109.
[30]M.J.C.Ordoñez,K.J.Balkus Jr.,J.P.Ferraris,I.H.Musselman,Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes,J.Membr.Sci.361(2010)28–37.
[31]S.Basu,A.Cano-Odena,I.F.J.Vankelecom,Asymmetric Matrimid®/[Cu3(BTC)2]mixed-matrix membranes for gas separations,J.Membr.Sci.362(2010)478–487.
[32]V.Na fisi,M.-B.Hagg,Gas separation properties of ZIF-8/6FDA-durene diamine mixed matrix membrane,Sep.Purif.Technol.128(2014)31–38.
[33]D.Q.Vu,W.J.Koros,S.J.Miller,Mixed matrix membranes using carbon molecular sieves:II.Modeling permeation behavior,J.Membr.Sci.211(2003)335–348.
[34]Y.C.Pan,W.Liu,Y.J.Zhao,C.Q.Wang,Z.P.Lai,Improved ZIF-8 membrane:Effect of activation procedure and determination of diffusivities of light hydrocarbons,J.Membr.Sci.493(2015)88–96.
[35]H.Li,L.H.Tuo,K.Yang,H.-K.Jeong,Y.Dai,G.Y.He,W.Zhao,Simultaneous enhancement of mechanical properties and CO2selectivity of ZIF-8 mixed matrix membranes:Interfacial toughening effect of ionic liquid,J.Membr.Sci.511(2016)130–142.
[36]V.I.Bondar,B.D.Freeman,I.Pinnau,Gas sorption and characterization of poly(etherb-amide)segmented block copolymers,J.Polym.Sci.B Polym.Phys.37(1999)2463–2475.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
