氧化石墨烯复合材料吸附铀的研究进展
2018-05-161南华大学核科学技术学院湖南衡阳4210012南华大学铀矿冶生物技术国防重点学科实验室湖南衡阳4210013南华大学污染控制与资源化技术湖南省重点实验室湖南衡阳421001
(1南华大学 核科学技术学院,湖南 衡阳421001;2南华大学 铀矿冶生物技术国防重点学科实验室,湖南 衡阳421001;3南华大学 污染控制与资源化技术湖南省重点实验室,湖南 衡阳 421001)
铀矿冶产生的大量低浓度含铀放射性废水对生态环境构成严重威胁[1-2]。铀作为一种重金属,不仅具有放射性,且具有动态的生物毒性和化学毒性[3-4]。我国的污水综合排放标准规定铀的最高允许排放浓度不超过0.05mg/L,而铀矿冶等生产排放的废水中铀的质量浓度一般在5mg/L左右[5]。因此,开展含铀废水处理技术的研究,使废水达标排放对于核工业可持续发展及环境保护具有重要的意义和应用前景。废水中铀的传统去除方法有化学沉淀法、离子交换法、吸附法、膜分离等[6-7]。吸附法以其操作简单、吸附速率快而被广泛关注,在低浓度含铀废水处理领域得到了很快的发展,是目前该领域最有前景的处理技术之一[8-11]。吸附法的关键是选择合适的吸附剂,目前研究者们致力于探索价廉高效的吸附剂,如黏土矿物[12-14]、二氧化硅[15-18]及沸石[19-22]等。石墨烯(graphene,GN)是一种由单原子层的碳原子通过sp2杂化,组成六角形呈蜂巢晶格状的二维材料[23],其理论比表面积高达2630m2/g,具有非常强的吸附能力,成为纳米吸附剂研究中的热点。氧化石墨烯(graphene oxide, GO)是石墨烯的氧化物,其表面有诸多含氧官能团[24],使其具有亲水的特性,吸附性能更佳。Romanchuk等[25]将石墨烯氧化物原子薄片用于快速吸收受污染水中的放射性废物,实验发现GO吸附U(VI) 的能力比传统的吸附剂膨润土和活性炭要高很多。王云等[26]采用化学方法将多壁碳纳米管打开得到富含氧基团的氧化石墨烯纳米带,其对铀的最大吸附量可达394mg/g。GO还可通过化学改性或与一些化学材料复合来接枝特定吸附功能基团,来提高吸附效果和吸附选择性[27-33]。学者们开展大量氧化石墨烯复合材料吸附铀的研究,并取得一定的成果,但氧化石墨烯复合材料对铀的吸附性能及作用机制的综述仍然很少[31]。本文综述了氧化石墨烯复合材料对铀的吸附性能、吸附影响因素及吸附机理,并对它们在铀废水处理中的应用前景和发展趋势做了展望,以期为后续相关研究及实际应用提供参考依据。
1 氧化石墨烯复合材料对铀的吸附性能
石墨烯的高比表面积使其成为理想的吸附材料[25,31],然而,完整结构的二维石墨烯晶体化学稳定性高,呈惰性,另外其相邻片层之间的π-π作用使石墨烯容易产生团聚或者重新堆积成石墨,从而妨碍了石墨烯在吸附方面的研究及应用,学者们通常利用石墨烯的衍生物——GO作为吸附材料[32-33]。石墨烯(图1)[23]与GO的结构(图2)[24]大致相同,只是在二维基面上连有一些官能团如羟基,环氧基,羧基,羰基等,这些含氧官能团能赋予了GO一些新的特性如分散性、亲水性及对聚合物的兼容性等,使GO成为一种优良的支撑材料,可以结合化学功能基团或复合其他材料,官能团还可提供活性吸附位点吸附铀等环境污染物,进而有效分离废水中的铀等污染物[31-33]。目前学者们对氧化石墨烯复合材料吸附铀的性能进行了一些研究,表1汇总了氧化石墨烯材料对铀吸附性能的主要参数[34-59],这些研究发现该材料能有效吸附水中的铀,等温吸附模型能较好地符合Langmiur模型,吸附热力学研究发现石墨烯基复合材料对铀的吸附为自发放热的过程。
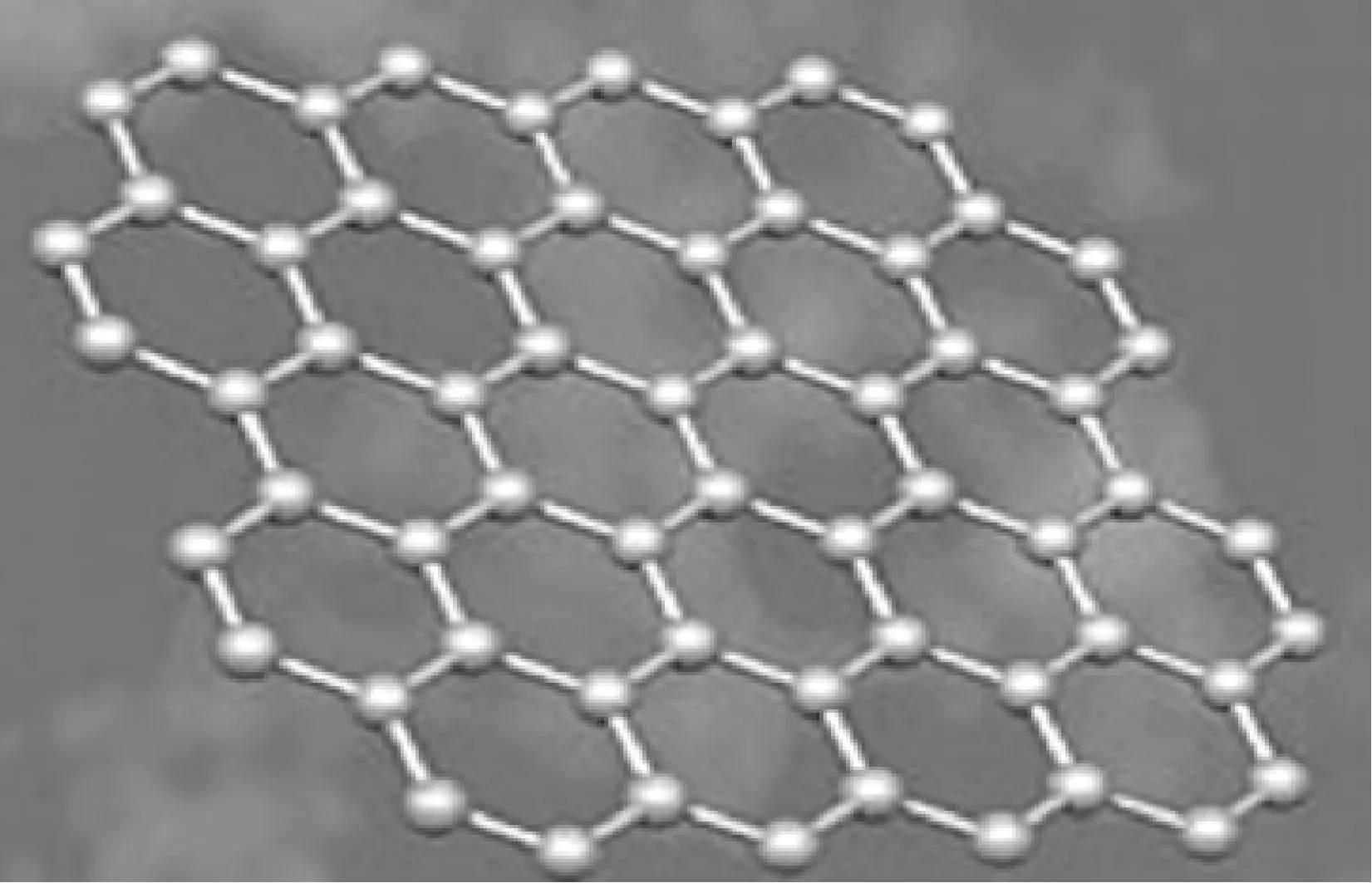
图1 石墨烯结构示意图[23]Fig.1 Basic structure of graphene[23]
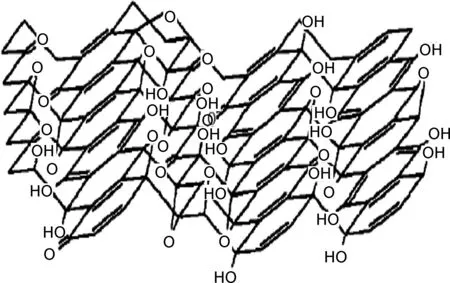
图2 氧化石墨烯的 Dékán 结构模型[24]Fig.2 Dékán structural model of graphene oxide[24]
2 氧化石墨烯复合材料吸附铀的影响因素分析
氧化石墨烯复合材料对铀的吸附效果受到很多因素的影响,如溶液pH值、吸附温度、离子强度、接触时间和吸附剂用量等,因此,不同因素下氧化石墨烯复合材料对铀离子的吸附性能往往存在一定的差异,且不同的氧化石墨烯材料的吸附能力受这些因素的影响各不相同。
2.1 溶液pH值对吸附的影响
2.2 温度对吸附的影响
温度是影响吸附的重要环境因子之一,对重金属的吸附-解吸、沉淀-溶解、氧化-还原等一系列化学和物理过程都有不同程度的影响。因此,温度的变化也可能导致吸附量的变化。Cheng等[37]研究发现温度对氧化石墨烯/海泡石复合材料(GO@sepiolite)吸附铀的影响较大,随着温度升高,GO@sepiolite对铀的吸附效果提高,当温度从298K升高至338K时,GO@sepiolite对铀的吸附量约增加到原来的2.2倍。Zhang等[45]研究了288.15~323.15K温度范围内磺化氧化石墨烯(GOS)对铀的吸附,结果表明,温度越高越有利铀的吸附。其他学者的相关研究也得到了一致的实验结论,发现氧化石墨烯复合材料对铀的吸附是一个放热自发的过程,温度升高能促进吸附反应的进行(见表1)[34-39,41-42,44-46,48-51]。这种现象可能是因为水的溶剂化作用,在水溶液中铀离子以水合离子状态存在,要吸附至石墨烯基材料表面上需要脱去铀离子表面的水合鞘,这个脱水过程需要消耗能量,是吸热过程,而离子吸附到吸附剂表面的过程是放热过程[48,63],当铀离子脱水的能量超过铀离子吸附于氧化石墨烯材料放出的能量时,整个吸附过程表现为吸热反应,温度越高铀离子脱水越容易,因此越有利于氧化石墨烯材料对铀离子的吸附[34]。
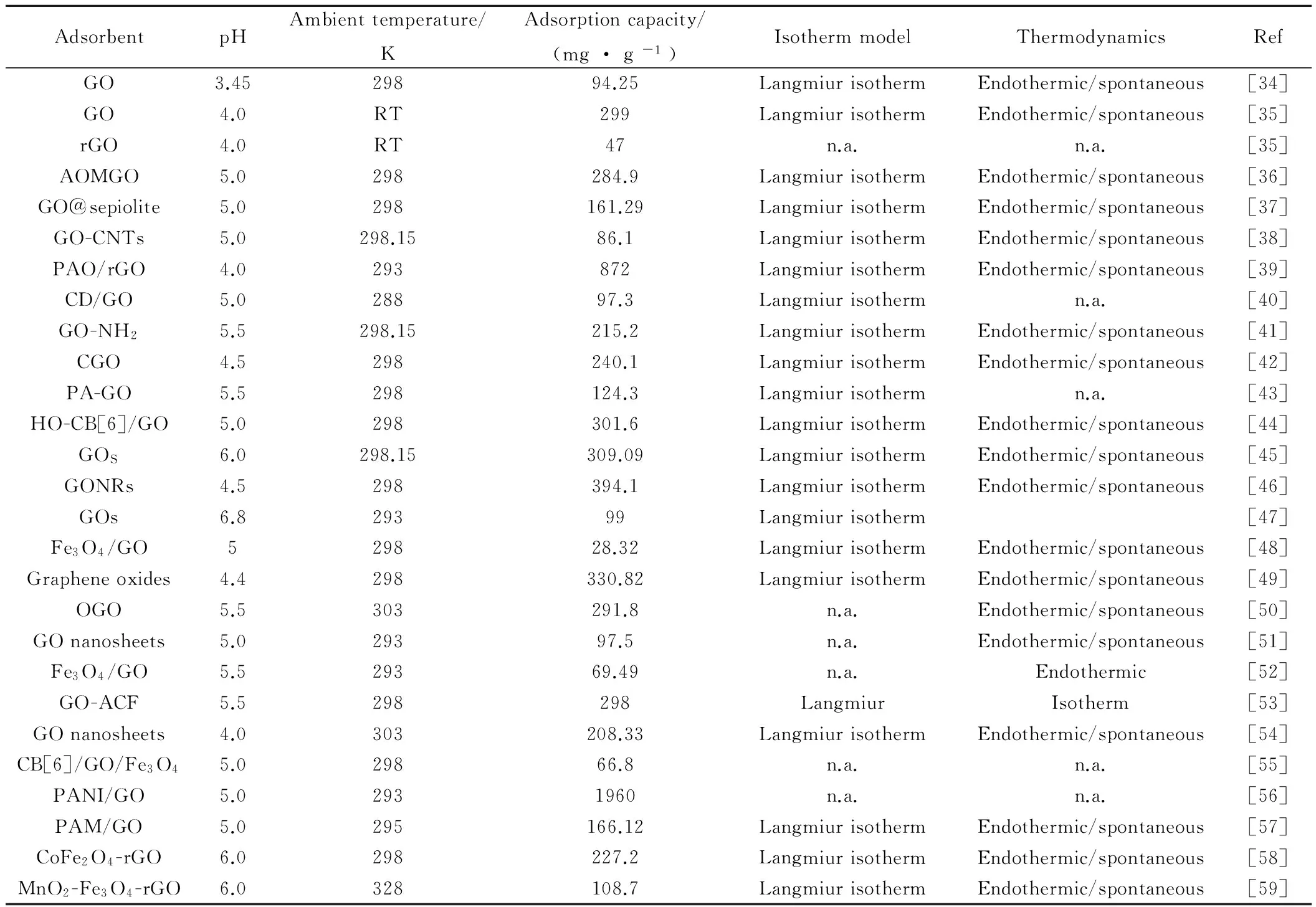
表1 石墨烯基复合材料对铀的吸附容量及主要参数Table 1 Adsorption capacities and mainly parameters of graphene-based composite materials for uranium
Note:RT-room temperature; n.a.-not application.
2.3 离子强度对吸附的影响
溶液的离子强度会影响铀离子存在形态,又能影响氧化石墨烯材料双静电层厚度而改变吸附剂的结合位点数,从而影响其对铀离子的吸附[64]。不同氧化石墨烯材料吸附铀的效果受离子强度影响的情况不尽相同,在一些实际的吸附研究中,它们吸附铀的效果随离子强度变化敏感[40,46]或不敏感的情况均有发生[36,45,47,49-52,54]。Zhang等[45]研究发现离子强度对氧化石墨烯和磺化氧化石墨烯吸附铀的影响不显著。Zhao等[36]研究发现在实验的pH值范围内偕胺肟磁性氧化石墨烯(AOMGO)对U(VI)的吸附效率几乎不受离子强度的影响,这主要是由于U(VI)与AOMGO表面偕胺肟基及其他含氧官能团形成了内层表面络合物,而不是形成外层表面络合物或者发生离子交换[65]。Sun等[66]报道了pH值>4时离子强度对U(VI)吸附于氧化石墨烯和功能化氧化石墨烯上的影响甚微,同理,也是由于铀吸附于材料是通过内层表面络合,且内层表面络合吸附机制通过常用于确定吸附剂与金属离子之间的相互作用机制[67-68]的X射线吸收精细结构谱(EXAFS)得以证实。而有些文献则得出了不一样的结论,Wang等[46]报道了氧化石墨烯纳米带 (GONRs)吸附铀的能力对溶液离子强度的变化敏感。Song等[40]研究发现NaClO4浓度的增加会导致环糊精/氧化石墨烯(CD/GO)对铀的吸附能力下降,因为存在于高离子浓度溶液中的铀离子,其活性会严重下降,从而抑制其转移到CD/GO表面,此外,高离子强度能够减少CD/GO材料之间的静电斥力,从而导致CD/GO产生团聚和吸附能力降低。
2.4 接触时间对吸附的影响
达到吸附平衡所需要的反应时间是影响吸附剂对铀吸附能力的又一重要因素,吸附量随吸附时间的变化情况也是吸附剂吸附动力学的一个重要特征。在实际应用中,吸附时间的长短及处理周期会影响经济效益,氧化石墨烯及其复合材料非常大的比表面积及表面丰富的官能团将有利于提高其吸附铀离子的速率,从而能较快地达到吸附平衡状态。Shao等[44]研究发现HO-CB[6]/GO对铀的吸附非常迅速,仅在5min内就能完成90%铀的吸附,并能够在20min内达到吸附平衡,HO-CB[6]/GO对铀的快速吸附有利于其实际应用于去除大体积溶液中的铀。Li等[35]利用Hummers方法制备得到的单层氧化石墨烯(GO)对铀的吸附过程迅速,1h内能达到吸附平衡状态。Gu等[38]研究发现氧化石墨烯-碳纳米管(GO-CNTs)在开始2h对U(VI)的吸附量增加较快,然后在9h内逐渐达到平衡。Liu等[41]研究了接触时间对GO和GO-NH2吸附U(VI)的影响,结果发现在接触开始吸附速率迅速增加,这归因在这一阶段吸附剂上有大量的空置吸附位点可用。随着时间的推移,空置的吸附位点逐渐被铀酰离子填充,吸附变慢;对于GO-NH2,吸附量缓慢增加的时间段为80~240min之间,这是因为该阶段为内扩散阶段,动力学吸附时间更依赖于内扩散速度,可能需要较长的时间来达到平衡。GO和GO-NH2对U(VI)的吸附平衡时间分别为60min和240min[41]。通常,吸附动力学包括两个阶段:初始阶段,该阶段吸附迅速,对吸附平衡贡献很重要,它为瞬时吸附或外表面吸附阶段,此阶段吸附剂上有大量可用的吸附位点数;第二阶段,吸附过程较慢,为逐渐吸附阶段,吸附速率受颗粒内扩散控制,一直到吸附达到平衡[41,69 ]。为了研究动力学吸附机理,准一级动力学模型和准二级动力学模型常用于研究氧化石墨烯材料对铀(VI)的吸附动力学。大量文献[34,36,38,41-43,45,48]报道的石墨烯基材料对铀(VI)的吸附符合准二级动力学模型,说明这些材料对铀(VI)的吸附过程主要受化学作用的控制。
2.5 吸附剂用量对吸附的影响
吸附剂用量也是影响吸附的重要参数,在实际应用中是一个重要的考察指标。吸附剂投加量直接影响氧化石墨烯材料与U(Ⅵ)的结合位点数目,进而直接影响 U(Ⅵ)的吸附效率[70]。张伟强等[71]在铀离子浓度为50mg/L,pH值为5.0的酸性条件下,考察了氨基三亚甲基膦酸改性石墨烯海绵材料(ATMP-GS)用量与铀离子吸附的关系,结果表明当吸附剂 ATMP-GS用量为7mg时,其对铀离子的吸附量最大为96mg/g,随后随着ATMP-GS用量的增大吸附量出现下降,并在15mg以后不再变化。孙兆勇等[72]研究发现吸附剂用量在0.01~0.03g范围内,吸附率随着吸附剂用量的增加而增加,当吸附剂用量由0.01g增加到0.04g时,吸附率增加较明显,达到35.14%,再增加吸附剂用量时,吸附率增加变得缓慢,仅为0.39%;而吸附量随吸附剂用量的增加不断降低。武里鹏等[73]通过实验研究了在吸附剂用量为0.01~0.03g范围内,磁性石墨烯对铀的吸附容量及去除率随吸附剂用量的变化,结果表明去除率随吸附剂用量的增加而增大,在吸附剂用量为0.03g时去除率达到了90%以上;而吸附容量与吸附剂用量的关系与前者恰好相反。Song等[40]发现吸附剂用量的增加会提高CD/GO对U(VI)的吸附效率,因为随着吸附剂用量增加,参与U(VI)吸附的功能位点也随之增加。本课题组[60]研究发现随着GOS投加量的增加,U(Ⅵ)吸附率逐渐上升,吸附容量逐渐降低,这是由于随着GOS用量的增加,GOS与U(Ⅵ)结合位点数目增多,从而使U(Ⅵ)的吸附率上升;但另一方面GOS投加量的增加导致GOS片层之间相互团聚的概率增大,降低了有效结合位点数目,比表面积也随之减少,导致单位质量吸附剂吸附U(Ⅵ)的结合位点数目减少,所以吸附容量随之降低。
氧化石墨烯材料吸附铀的效果受吸附剂用量的影响,通常表现为高吸附剂用量使吸附效率提高,因为U(Ⅵ)结合位点数目增多,而低吸附剂用量使吸附量更大,因其结合位点和表面积能够得到有效利用[32,59]。因此,在实际应用中,为保证吸附效果且使吸附剂能被充分利用,建议采用适当的吸附剂用量。
3 氧化石墨烯复合材料对铀的吸附机理
目前已有大量关于铀与GO基材料相互作用的研究,这些研究通过批量实验研究了不同因素对吸附作用的影响,进行了吸附动力学和吸附热力学分析,并且采用表面络合模型、光谱分析和理论计算等手段和方法,从实验数据和理论模拟、宏观和微观等角度阐述了GO基材料对铀的吸附机制[28,50-58]。一些批量吸附实验的数据结果可以解释铀与GO基材料相互作用的机理,比如由吸附过程对离子强度敏感而对pH不敏感可以推导出吸附作用主要是通过外表面络合或离子交换,而吸附过程对pH敏感而对离子强度不敏感主要是由于内表面络合作用[28,35,36]。表面络合模型可以展示材料表面上铀络合物的构成情况,从而可根据铀络合物的种类阐述吸附作用机理[54,66]。而通过扩展X射线吸收精细结构光谱(Extended X-ray Absorptionfine Structure Spectroscopy, EXAFS),X射线光电子能谱(X-ray Photoelectric Spectroscopy, XPS),X射线衍射(X-ray Diffraction, XRD),拉曼光谱(Raman Spectroscopy, RS),荧光时间衰减光谱(Time Resolved Laser Fluorescence Spectroscopy, TRLFS),傅里叶变换红外光谱法(Fourier Transformed Infrared Spectroscopy, FTIR)等光谱手段可以分析材料表面官能团与铀结合的微观情况以及从分子水平揭示铀的形态和微观结构[27-29]。理论计算如密度泛函理论是评价核素与固体材料之间物理化学作用的一种非常有用的手段[29,74-77],例如,通过理论计算铀与不同官能团之间的结合能,可以确定物理吸附或化学吸附等的吸附性能,从而得到铀与材料间的相互作用机理。不同实验条件下的研究结果表明石墨烯基材料对铀有很强的吸附能力,主要是由于其表面或边缘部位充斥着大量的含氧官能团[47,57,77]。
3.1 表面络合模型对吸附机理的研究

3.2 光谱技术分析
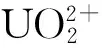
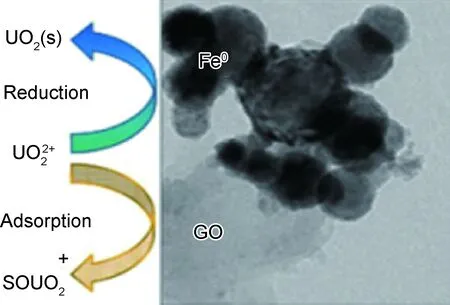
图3 铀在NZVI/rGO上的吸附与还原[79]Fig.3 Simultaneous adsorption and reduction of U(VI) onNZVI/rGO[79]
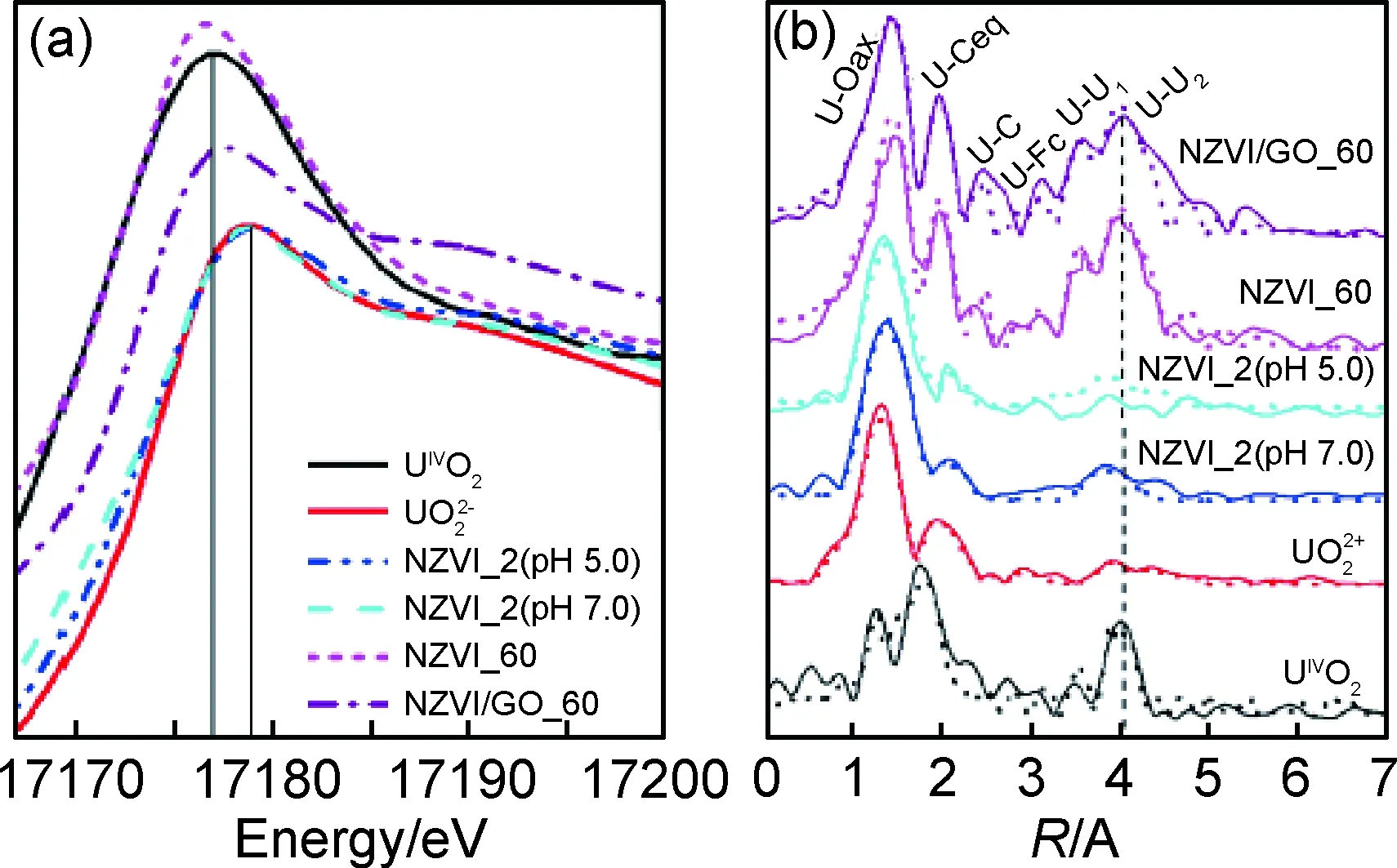
图4 NZVI 、NZVI/GO、U(VI)作用后的NZVI 和NZVI/GO以及参考样品的XANES光谱(a)和 EXAFS光谱(b)(T=(25±1)℃,I=0.01mol/L NaClO4,pH 5.0)[79]Fig.4 XANES spectra (a) and Fourier transform (FT) of EXAFSspectra (b) for reference samples and U(VI)-reacted NZVI andNZVI/GO(T=(25±1)℃, I=0.01mol/L NaClO4, pH 5.0)[79]
3.3 理论计算对吸附机理的研究
计算化学研究能从分子水平获得GO与放射性核素之间所形成配合物的电子结构、形态分布、配位性质和热力学性质等数据,对阐明放射性核素与GO之间的固液界面作用机理起着重要作用[81-82]。利用理论计算研究得到的放射性核素与GO相互作用数据与实验结果进行比较和相互验证,可以使实验研究更具说服力和可靠性。而对于一些比较难开展的实验,如所有的锕系元素,特别是具有放射性和毒性的超铀元素,阻碍了实验研究开展,理论计算可以提供一条有效的途径来补充有关锕系元素配合物的电子结构及性质等[76,81]。密度泛函理论(Density Functional Theory,DFT)是理论计算的重要工具之一,它能以有效的方式计算相关能量和形态等基础数据而在计算化学研究领域得到非常好的应用[83-84]。DFT常用于阐述局部相互作用表面分子的吸收,结合TRLFS,EXAFS,XPS等光谱分析结果能在分子水平上更好地阐明GO等材料与放射性核素的相互作用机制,为评价放射性核素在环境中的物理化学行为提供重要的参考价值。
近年来,一些研究者通过理论计算研究了铀在石墨烯基材料表面的吸附行为。Wu等[76]利用DFT结合相对论小芯赝势优化了铀酰离子和GO之间的22种配合物,研究的含氧官能团有羟基、羧基、氨基和二甲基甲酰胺等,研究结果表明铀原子和GO上氧原子之间的距离(U-OG)在阴离子GO配合物(铀酰/GO-/2-)中要短于在中性GO配合物(铀酰/GO)中,铀酰/GO-/2-配合物中氢键的形成可以提高带负电的GO对铀酰离子的结合能力,此外,热力学计算表明,铀酰离子更容易与羟基和羧基功能化的阴离子GO配合物发生络合反应,同时几何结构和热力学能量都表明,由羟基和羧基改性的GO对铀酰离子的结合能力比氨基和二甲基甲酰胺基改性的GO要强得多。偕胺肟基和羧基经常用于修饰石墨烯材料,Wang等[77]采用量子化学计算模拟了铀与一系列通过烷基链嫁接偕胺肟基和羧基等基团的吸附剂之间的相互作用,并研究了不同官能团及其组合对配位结构与萃取稳定性等产生的影响。
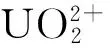
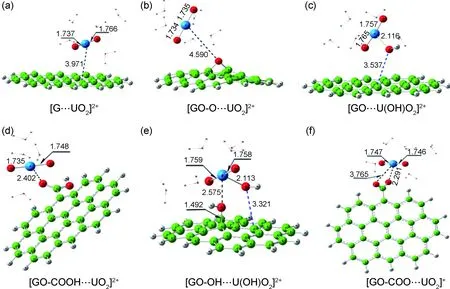
图5 rGOs-铀酰配合物和GOs-铀酰配合物的DFT优化几何结构[66]Fig.5 DFT-optimized geometries of the rGOs-uranyl complexes and GOs-uranyl complexes[66]
4 结束语
氧化石墨烯复合材料由于其巨大的表面积及丰富的表面功能基团对铀能实现高效的吸附。从目前国内外的研究现状来看,石墨烯基复合材料对铀的吸附机理的研究主要通过采用批量吸附实验研究不同因素对其吸附能力的影响,吸附动力学以及吸附热力学研究,以及利用表面络合模型、光谱分析技术与理论计算等方法来实现。然而,由于氧化石墨烯含氧官能团的多样性(如羟基、羧基、羰基、环氧基等),以及表面功能化修饰引入一些新的官能团,而且铀在环境中的化学形态变化复杂,因此尽管已有不少有关石墨烯基材料吸附铀的研究工作,还需深入地开展石墨烯基材料对铀的吸附行为及其开发应用的研究,可在下述几个方面进行研究工作:
(1)将实验、模型模拟及理论计算,宏观的批量实验与微观的光谱技术结合起来运用于研究石墨烯基材料对铀的吸附行为,将实验与理论,宏观与微观的研究结论互相补充互相验证,以便更准确深入地揭示石墨烯基材料与铀的作用机理。
(2)目前研究发现GO基材料对铀的高效吸附主要归因于其表面或边缘的功能官能团,但由于GO基材料表面或边缘存在不同种类的官能团,不同官能团对吸附铀的发挥作用大小却缺乏深层次的理论和实验研究。
(3)关于氧化石墨烯材料脱附铀的研究报道不多,应研究温度、脱附剂种类、离子强度和初始铀浓度等对脱附效果的影响并探讨相关脱附机理,为氧化石墨烯材料在铀污染处理方面的重复利用提供理论基础。
(4)随着氧化石墨烯材料在多学科领域的大量应用,部分GO基材料不可避免会被释放到自然环境中而成为环境污染物。GO基材料在水中具有高分散性、高吸附性以及高化学活性,其有可能对水环境生态和水生生物造成不利影响[85-88],因此,为保证GO基材料更安全和环保的应用,未来的研究需要进一步明确GO基材料的生态效应及其环境行为,正确评估其环境风险。
参考文献
[1] 王劲松,邹晓亮,贾亮,等. α-酮戊二酸改性壳聚糖对低浓度U(Ⅵ)的吸附性能[J].原子能科学技术,2015, 49 (2): 255-262.
WANG J S,ZOU X L, JIA L, et al. Adsorption performance of low-strength U (Ⅵ) on α-ketoglutaric acid modified chitosan[J]. Atomic Energy Science and Technology, 2015, 49 (2): 255-262.
[2] 史冬峰, 唐振平, 黄华勇,等. 磁性氧化石墨烯/β-环糊精复合材料对U(Ⅵ)的吸附性能及机理[J].原子能科学技术, 2016, 50 (9): 1556-1564.
SHI D F, TANG Z P, HUANG H Y, et al. Adsorption characteristic and mechanism of Uranium(Ⅵ) on magnetism graphene oxide/β-cyclodextrin composite[J]. Atomic Energy Science and Technology, 2016, 50 (9): 1556-1564.
[3] 商照荣.贫化铀的环境污染影响及其对人体健康的危害[J].辐射防护, 2005(1): 56-61.
SHANG Z R. Impact of depleted uranium on environmental and human health[J].Radiation Protection, 2005(1): 56-61.
[4] 周书葵,娄涛,庞朝晖. 放射性废水处理技术[M].北京: 化学工业出版社, 2012: 7.
ZHOU S K, LOU T, PANG C H. Radioactive waste water treatment technology[M]. Beijing: Chemical Industry Press,2012:7.
[5] WANG J S, BAO Z L, CHEN S G, et al. Removal of uranium from aqueous solution by chitosan and ferrous ions[J]. International Conference on Nuclear Engineering, 2010, 133 (8): 735-740.
[6] MELISA S, GUSTAVO A, ROSA M. Uranium uptake by montmorillonite-biomass complexes[J]. Industrial & Engineering Chemistry Research, 2013, 52(6) : 2273-2279.
[7] YUAN L Y, LIU Y L, SHI W Q, et al. High performance of phosphonate-functionalized mesoporous silica for U(VI) adsorption from aqueous solution[J]. Dalton Trans, 2011, 40: 7446-7453.
[8] ZHANG X F, JIAO C S, WANG J, et al. Removal of uranium(VI) from aqueous solutions by magnetic Schiff base: kinetic and thermodynamic investigation[J]. Chemical Engineering Journal, 2012, 198: 412-419.
[9] LI X Y, ZHANG M, LIU Y B, et al. Removal of U(VI) in aqueous solution by nanoscale zero-valent iron(nZVI)[J]. Exposure and Health, 2013, 5(1): 31-40.
[10] ZHAO D L, WANG X B, YANG S T, et al. Impact of water quality parameters on the sorption of U(VI) onto hematite[J]. Journal of Environmental Radioactivity,2012,103 (1): 20-29.
[11] BELLONI F, KUTAHYALI C, RONDINELLA V V, et al. Can carbon nanotubes play a role in the field of nuclear waste management?[J]. Environment Science & Technology, 2009, 43: 1250-1255.
[12] LIU H J, XIE S B, XIA L S, et al. Study on adsorptive property of bentonite for cesium[J]. Environment Earth Science, 2016, 75(2): 148.
[13] SCHINDLER M, LEGRAND C A, JR M F H. Alteration, adsorption and nucleation processes on clay-water interfaces: mechanisms for the retention of uranium by altered clay surfaces on the nanometer scale[J]. Geochimica Et Cosmochimica Acta, 2015, 153: 15-36.
[14] VILLA-ALFAGEME M, HURTADO S, MRABET S E, et al. Uranium immobilization by FEBEX bentonite and steel barriers in hydrothermal conditions[J]. Chemical Engineering Journal, 2015, 269: 279-287.
[15] GUNATHILAKE C, GORKA J, DAI S, et al. Amidoxime-modified mesoporous silica for uranium adsorption under seawater conditions[J]. Journal of Materials Chemistry A, 2015, 3 (21): 11650-11659.
[16] LIU H J, JING P F, LIU, et al. Synthesis of β-cyclodextrin functionalized silica gel and its application for adsorption of uranium(VI)[J]. Journal of Radioanalytical & Nuclear Chemistry, 2016, 310(1): 1-8.
[17] ZHOU L, ZOU H, WANG Y, et al. Adsorption of uranium(VI) from aqueous solution using phosphonic acid-functionalized silica magnetic microspheres[J]. Journal of Radioanalytical & Nuclear Chemistry, 2016, 310(3): 1-9.
[18] HUMELNICU D, BLEGESCU C, GANJU D. Removal of uranium(VI) and thorium(IV) ions from aqueous solutions by functionalized silica: kinetic and thermodynamic studies[J]. Journal of Radioanalytical & Nuclear Chemistry, 2014, 299 (3): 1183-1190.
[19] BARKAT M, NIBOU D, AMOKRANE S, et al. Uranium (VI) adsorption on synthesized 4A and P1 zeolites: equilibrium, kinetic, and thermodynamic studies[J]. Comptes Rendus Chimie, 2015, 18(3): 261-269.
[20] SHARIFIPOUR F, HOJATI S, LANDI A, et al. Kinetics and thermodynamics of lead adsorption from aqueous solutions onto iranian sepiolite and zeolite[J]. International Journal of Environmental Research, 2015, 9(3): 1001-1010.
[21] SHAKUR H R, SARAEE K R E, ABDI M R, et al. Selective removal of uranium ions from contaminated waters using modified-X nanozeolite[J]. Applied Radiation & Isotopes, 2016, 118: 43-55.
[22] BAKATULA E N, MOLAUDZI R, NEKHUNGUNI P, et al. The removal of arsenic and uranium from aqueous solutions by sorption onto iron oxide-coated zeolite[J]. Water Air & Soil Pollution, 2017, 228(1):5.
[23] VAN N R. Moving towards a graphene world[J]. Nature, 2006, 442(7100): 228-229.
[24] SZABO T, BERKESI O, FORGO P, et al. Evolution of surface functional groups in a series of progressively oxidized graphite oxides[J]. Chemistry of Materials, 2006, 18 (11): 2740-2749.
[25] ROMANCHUK A Y, SLESAREV A S, KALMYKOV S N, et al. Graphene oxide for effective radionuclide removal[J]. Physical Chemistry Chemical Physics, 2013, 15 (7): 2321-2327.
[26] 王云, 顾泽兴, 王正上, 等. 氧化石墨烯纳米带的可控制备及其对铀的吸附研究[C]∥第十三届全国核化学与放射化学学术研讨会. 大理: 中国化学会, 中国核学会, 2014: 38.
WANG Y, GU Z X, WANG Z S, et al. Controllable preparation of graphene oxide nanoribbons and their adsorption to uranium[C]//The Thirteenth National Conference on nuclear chemistry and radiation chemistry.Dali: Chinese Chemical Society, Chinese Nuclear Society, 2014: 38.
[27] JIN Z X, WANG X X, WANG X K, et al. Sequestration of 4-nonylphenol and bisphenol-A on magnetic reduced graphene oxides: a combined experimental and theoretical studies[J]. Environment Science & Technology, 2015, 49: 9168-9175.
[28] WANG X X, YU S J, JIN J, et al. Application of graphene oxides and graphene oxide-based nanomaterials in radionuclide removal from aqueous solutions [J]. Science Bulletin, 2016, 61(20): 1583-1593.
[29] WANG X X, YANG S B, WANG X K, et al. Different interaction mechanisms of Eu(III) and243Am(III) with carbon nanotubes studied by batch,spectroscopy technique and theoretical calculation[J]. Environment Science & Technology, 2015, 49: 11721-11728.
[30] CHEN Y T, ZHANG W, WANG X K, et al. Understanding the adsorption mechanism of Ni(II) on graphene oxides by batch experiments and density functional theory studies[J]. Science China Chemistry, 2016, 59: 412-419.
[31] YU S J, WANG X X, WANG X K, et al. Sorption of radionuclides from aqueous systems onto graphene oxide-based materials: a review[J]. Inorganic Chemistry Frontiers, 2015, 46 (32): 593-612.
[32] 曹明莉,张会霞,张聪,等. 石墨烯及其复合材料在重金属离子吸附方面的应用[J]. 功能材料, 2016, 47 (8): 08001-08007.
CAO M L, ZHANG H X, ZHANG C, et al. Graphene-containing composite materials for heavy metal ions adsorption[J]. Journal of Functional Materials, 2016, 47 (8): 08001-08007.
[33] 吕生华,朱琳琳,李莹,等. 氧化石墨烯复合材料的研究现状及进展[J]. 材料工程, 2016, 44 (12): 107-117.
LYU S H, ZHU L L, LI Y, et al. Current situation and progress of graphene oxide composites[J]. Journal of Materials Engineering, 2016, 44 (12): 107-117.
[34] WANG C L, LI Y, LIU C L. Sorption of uranium from aqueous solutions with graphene oxide[J]. Journal of Radioanalytical and Nuclear Chemistry, 2015, 304 (3): 1017-1025.
[35] LI Z J, CHEN F, YUAN L Y, et al. Uranium(VI) adsorption on graphene oxide nanosheets from aqueous solutions[J]. Chemical Engineering Journal, 2012, 210: 539-546.
[36] ZHAO Y G, LI J X, ZHANG S W, et al. Efficient enrichment of uranium(VI) on amidoximated magnetite/graphene oxide composites[J]. RSC Advances, 2013, 3: 18952-18959.
[37] CHENG H X, ZENG K F, YU J T. Adsorption of uranium from aqueous solution by graphene oxide nanosheets supported on sepiolite[J]. Journal of Radioanalytical and Nuclear Chemistry, 2013, 298(1): 599-603.
[38] GU Z X, WANG Y, TANG J, et al. The removal of uranium(VI) from aqueous solution by graphene oxide-carbon nanotubes hybrid aerogels[J]. Journal of Radioanalytical and Nuclear Chemistry, 2015, 303(3):1835-1842.
[39] SHAO D D, LI J X, WANG X K. Poly(amidoxime)-reduced graphene oxide composites as adsorbents for the enrichment of uranium from seawater[J]. Science China Chemistry, 2014, 57(11): 1449-1458.
[40] SONG W C, SHAO D D, LU S S, et al. Simultaneous removal of uranium and humic acid by cyclodextrin modified graphene oxide nanosheets[J]. Science China Chemistry, 2014, 57(9): 1291-1299.
[41] LIU S J, LI S, ZhANG H X, et al. Removal of uranium(VI) from aqueous solution using graphene oxide and its amine-functionalized composite[J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 309(2): 607-614.
[42] WANG Z S, WANG Y, LIAO J L, et al. Improving the adsorption ability of graphene sheets to uranium through chemical oxidation, electrolysis and ball-milling[J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 308 (3): 1095-1102.
[43] LIU S J, MA J G, ZHANG W Q, et al. Three-dimensional graphene oxide/phytic acid composite for uranium(VI) sorption [J]. Journal of Radioanalytical and Nuclear Chemistry, 2015, 306(2): 507-514.
[44] SHAO L, ZHONG J R, REN Y M, et al. Perhydroxy-CB[6] decorated graphene oxide composite for uranium(VI) removal [J]. Journal of Radioanalytical and Nuclear Chemistry, 2017, 311 (1):627-635.
[45] ZHANG Z B, QIU Y F, DAI Y, et al. Synthesis and application of sulfonated graphene oxide for the adsorption of uranium(VI) from aqueous solutions [J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 310(2): 547-557.
[46] WANG Y, WANG Z S, GU Z X, et al. Uranium(VI) sorption on graphene oxide nanoribbons derived from unzipping of multiwalled carbon nanotubes [J]. Journal of Radioanalytical and Nuclear Chemistry, 2015, 304(3): 1329-1337.
[47] WANG X X, CHEN Z S, WANG X K. Graphene oxides for simultaneous highly efficient removal of trace level radionuclides from aqueous solutions [J]. Science China Chemistry, 2015, 58(11): 1766-1773.
[48] ZHAO D L, CHEN L L, SUN M, et al. Preparation and application of magnetic graphene oxide composite for the highly efficient immobilization of U(VI) from aqueous solutions[J]. Journal of Radioanalytical and Nuclear Chemistry, 2015, 306(1): 221-229.
[49] TAO X Q, YAO X B, LU S S, et al. Efficient removal of radionuclide U(VI) from aqueous solutions by using graphene oxide nanosheets [J]. Journal of Radioanalytical and Nuclear Chemistry, 2015, 303 (1): 245-253.
[50] LIU X, WANG X X, LI J X, et al. Ozonated graphene oxides as high efficient sorbents for Sr(II) and U(VI) removal from aqueous solutions [J]. Science China Chemistry, 2016, 59 (7): 869-877.
[51] ZHAO G X,WEN T,YANG X, et al. Preconcentration of U(VI) ions on few-layered graphene oxide nanosheets from aqueous solutions [J]. Dalton Transactions, 2012, 41 (20): 6182-6188.
[52] ZONG P F, WANG S F, ZHAO Y L, et al. Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions[J]. Chemical Engineering Journal, 2013, 220: 45-52.
[53] CHEN S P, HONG J X, YANG H X, et al. Adsorption of uranium(VI) from aqueous solution using a novel graphene oxide-activated carbon felt composite [J]. Journal of Environmental Radioactivity, 2013, 126(4): 253-258.
[54] DING C C, CHENG W C, SUN Y B, et al. Determination of chemical affinity of graphene oxide nanosheets with radionuclides investigated by macroscopic, spectroscopic and modeling techniques[J]. Dalton Transactions, 2014, 43: 3888-3896.
[55] SHAO L, WANG X F, REN Y M, et al. Facile fabrication of magnetic cucurbit[6] uril/graphene oxide composite and application for uranium removal [J]. Chemical Engineering Journal, 2016, 286: 311-319.
[56] SHAO D D, HOU G S, LI J X, et al. PANI/GO as a super adsorbent for the selective adsorption of uranium(VI) [J]. Chemical Engineering Journal, 2014, 255: 604-612.
[57] SONG W C, WANG X X , WANG Q, et al. Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides [J]. Physical Chemistry Chemical Physics, 2015, 17: 398-406.
[58] TAN L C, LIU Q, SONG D L, et al. Uranium extraction using a magnetic CoFe2O4-graphene nanocomposite: kinetics and thermodynamics studies [J]. New Journal of Chemistry, 2015, 39: 2832-2838.
[59] TAN L C, WANG J, LIU Q, et al. The synthesis of a manganese dioxide-iron oxide-graphene magnetic nanocomposite for enhanced uranium(VI) removal[J]. New Journal of Chemistry, 2015, 39(2): 868-876.
[60] 王亮,谢水波,杨金辉,等. 氧化石墨烯/二氧化硅复合材料对铀(Ⅵ)的吸附性能[J]. 中国有色金属学报, 2016, 26 (6): 1264-1271.
WANG L, XIE S B, YANG J H, et al. Adsorption properties of graphene oxide/silica composite materials for uranium(VI)[J]. The Chinese Journal of Nonferrous Metals, 2016, 26 (6): 1264-1271.
[61] SCHIERZ A, ZNKER H. Aqueous suspensions of carbon nanotubes: surface oxidation, colloidal stability and uranium sorption [J]. Environmental Pollution, 2009, 157(4): 1088-1094.
[62] SONG J, KONG H, JANG J. Adsorption of heavy metal ions from aqueous solution by polyrhodanine-encapsulated magnetic nanoparticles [J]. Journal of Colloid & Interface Science, 2011, 359(2): 505-511.
[63] TAHIR S S, RAUF N. Thermodynamic studies of Ni(II) adsorption onto bentonite from aqueous solution [J]. Journal of Chemical Thermodynamics, 2003, 35: 2003-2009.
[64] HU R, SHAO D D, WANG X K. Graphene oxide/polypyrrole composites for highly selective enrichment of U(VI) from aqueous solutions [J]. Polymer Chemistry, 2014, 5(21): 6207-6215.
[65] YANG S T, ZONG P F, REN X M, et al. Rapid and highly efficient preconcentration of Eu(III) by core-shell structured Fe3O4@humic acid magnetic nanoparticles [J]. Acs Applied Materials & Interfaces, 2012, 4 (12): 6891-6900.
[66] SUN Y B, YANG S B, CHEN Y, et al. Adsorption and desorption of U (VI) on functionalized graphene oxides: a combined experimental and theoretical study [J]. Environ Science Technology, 2015, 49: 4255-4262.
[67] SHENG G D, ALSAEDI A, SHAMMAKH W, et al. Enhanced sequestration of selenite in water by nanoscale zero valent iron immobilization on carbon nanotubes by a combined batch, XPS and XAFS investigation [J]. Carbon, 2016, 99: 123-130.
[68] SHENG G D, HU J, LI H, et al. Enhanced sequestration of Cr(VI) by nanoscale zero-valent iron supported on layered double hydroxide by batch and XAFS study [J]. Chemosphere, 2016, 148: 227-232.
[69] HAN R P, ZOU W H, WANG Y, et al. Removal of uranium(VI) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect [J]. Journal of Environment Radioactivity, 2007, 93:127-143.
[70] WANG H, YUAN X Z, WU Y, et al. Adsorption characteristics and behaviors of graphene oxide for Zn(Ⅱ) removal from aqueous solution[J]. Applied Surface Science, 2013, 279: 432-440.
[71] 张伟强,马建国,刘淑娟,等. 改性石墨烯海绵材料对铀的吸附研究[J]. 东华理工大学学报(自然科学版), 2014, 37(2): 230-235.
ZHANG W Q, MA J G, LIU S J, et al. Uranium adsorption on functionalized graphene sponge[J]. Journal of East China Institute of Technology ( Natural Science), 2014, 37(2): 230-235.
[72] 孙兆勇.石墨烯与复合物的制备以及铀吸附性能研究[D]. 哈尔滨:哈尔滨工程大学, 2013.
SUN Z Y. Synthesis and uranium adsorption characterization of graphene and graphene-based materials[D].Harbin: Harbin Engineering University, 2013.
[73] 武里鹏,毛燕妮,刘淑娟,等. 磁性石墨烯对铀的吸附性能研究[J]. 江西化工, 2015(2): 99-103.
WU L P, MAO Y N, LIU S J, et al. Study on adsorption of uranium of magnetic graphene[J]. Jiang Xi Hua Gong, 2015(2): 99-103.
[74] XIAO C L, WU Q Y, SHI W Q, et al. Quantum chemistry study of U(VI), Np(V) and Pu(IV, VI) complexes with preorganized tetradentate phenanthrolineamide ligands [J]. Inorganic Chemistry, 2014, 53: 10846-10853.
[75] LAN J H, SHI W Q, CHAI Z F, et al. Recent advances in computational modeling and simulations on the An(III)/Ln(III) separation process [J]. Coordination Chemistry Reviews, 2012, 256: 1406-1417.
[76] WU Q Y, LAN J H, SHI W Q, et al. Understanding the bonding nature of uranyl ion and functionalized graphene: a theoretical study [J]. Journal of Physical Chemistry A , 2014, 118: 2149-2158.
[77] WANG C Z, LAN J H, SHI W Q, et al. Theoretical insights on the interaction of uranium with amidoxime and carboxyl groups [J]. Inorganic Chemistry, 2014, 53: 9466-9476.
[78] YANG S T, ZONG P F, SHENG G D, et al. New insight into Eu(Ⅲ)sorption mechanism at alumina/water interface by batch technique and EXAFS analysis[J]. Radiochimica Acta, 2014, 102(1/2): 143-153.
[79] SUN Y B, DING C C,WANG X K, et al. Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron [J]. Journal of Hazardous Materials, 2014, 280: 399-408.
[80] SUN Y B, SHAO D D, CHEN C L, et al. Highly efficient enrichment of radionuclides on graphene oxide supported polyaniline [J]. Environmental Science & Technology, 2013, 47: 9904-9910.
[81] WU Q Y, LAN J H, WANG C Z, et al. Understanding the interactions of neptunium and plutonium ions with graphene oxide:Scalar-relativistic DFT investigations[J]. The Journal of Physical Chemistry A, 2014, 118 (44):10273-10280.
[82] 王祥学,李洁,于淑君,等. 放射性核素在天然黏土和人工纳米材料上的吸附机理研究[J]. 核化学与放射化学, 2015, 37 (5): 329-340.
WANG X X, LI J, YU S J, et al. Sorption mechanism of radionuclides on clay minerals and manmade nanomaterials[J]. Journal of Nuclear & Radiochemistry, 2015, 37 (5): 329-340.
[83] 杜毅,王建,王宏青,等. 人工纳米材料吸附放射性核素的机理研究[J]. 农业环境科学学报, 2016, 35 (10): 1837-1847.
DU Y, WANG J, WANG H Q, et al. Research on sorption mechanism of radionuclides by manufactured nanomaterials[J]. Journal of Agro-Environment Science, 2016, 35 (10): 1837-1847.
[84] WU Q Y, WANG C Z, LAN J H, et al. Theoretical investigation on multiple bonds in terminal actinide nitride complexes[J]. Inorganic Chemistry, 2014, 53 (18): 9607-9614.
[85] LIU S, ZENG T H, HOFMANN M, et al. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress[J]. ACS Nano, 2011, 5 (9): 6971-6980.
[86] AKHAVAN O, GHADERI E. Toxicity of graphene and graphene oxide nanowalls against bacteria[J]. ACS Nano, 2010, 4 (10): 5731-5736.
[87] DUCH M C, BUDINGER G R S, LIANG Y T, et al. Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung [J]. Nano Letters, 2011, 11 (12): 5201-5207.
[88] 吕小慧,陈白杨,朱小山. 氧化石墨烯的水环境行为及其生物毒性[J]. 中国环境科学, 2016, 36 (11): 3348-3359.
LÜ X H, CHEN B Y, ZHU X S. Fate and toxicity of graphene oxide in aquatic environment [J]. China Environmental Science, 2016, 36 (11): 3348-3359.
