High glucose induces myocardial cell injury through increasing reactive oxygen species production
2018-05-11YuJunWangXiaoYuLyuLiYu
Yu-Jun Wang, Xiao-Yu Lyu, Li Yu
1Department of Critical Care Medicine, the Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology , Wuhan City,Hubei Province 430014, China
2Department of Endocrinology, the Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology , Wuhan City, Hubei Province, 430014, China
1. Introduction
Diabetic cardiomyopathy (DCM) is one of the important complications of diabetes, mainly characterized by myocardial cell damage and intracellular mitochondrial dysfunction and clinically manifested as ventricular hypertrophy, myocardial interstitial fibrosis and cardiac vasomotor hypofunction. It can gradually progress into heart failure, and is currently the clinical common cause of disability and death for diabetic patients at present[1,2].Research on diabetic complications in recent years has shown that the increased reactive oxygen species (ROS) generation and the oxidative stress reaction activation under high glucose environment are the common bases of a variety of diabetic complications. The excessively generated ROS can cause cellular structural destruction and functional damage and result in corresponding complications[3].In the pathological process of DCM, myocardial cell injury is the most outstanding performance, and the damaging effect of high glucose on myocardial cells has also received more and more attention[4]; however, it is still not clear whether high glucose cause myocardial cell damage through increasing the ROS production and activating the oxidative stress reaction or not. In the following study, we specifically analyzed the damaging effect and molecular mechanism of high glucose on myocardial cells.
2. Materials and methods
2.1. Experimental materials
Myocardial cell lines H9c2 was purchased from ATCC Cell Company, the reagents for cell culture from Sigma Company and the consumables for cell culture from Corning Company; the ROS scavenger N-acetylcysteine (NAC) was purchased from Sigma Company; the ROS detection kit was purchased from Thermo Company, the Bax, CytC, Casaspe-3 and Casaspe-9 antibody for Western blot from Santa Cruz Company, and enzyme-linked immunosorbent assay kits and BCA quantitative kits from Shanghai Westang Company.
2.2. Experimental methods
2.2.1. Cell culture medium treatment
Myocardial cells H9c2 were regularly cultured in DMEM containing 10% fetal bovine serum, digested with trypsin and then inoculated within the culture plate for treatment, and the methods were as follows: (1) control group were treated with ordinary DMEM containing 5.5 mmol/L glucose; (2) high glucose group were treated with DMEM containing 35 mmol/L glucose; (3) NAC group were pre-treated with DMEM containing 1 000 μmol/L NAC for 1 h, and then continuously treated with DMEM containing 1 000 μmol/L NAC and 35 mmol/L glucose.
2.2.2. ROS generation detection
The cells for ROS detection were inoculated within the 24-well culture plate, and were treated to get rid of culture medium. Then the GENMED staining fluid and the staining working fluid of GENMED diluent were added and incubated for 20 min to drain the fluid. After that the 37 ℃ preheating GENMED preserving fluid was added, the fluorescence value of excitation wavelength 540 nm and emission wavelength 590 nm were detected on fluorescence microplate reader, and the ROS fluorescence value of control group after 8 h of treatment was set as 1 (100%) to calculate the relative fluorescence unit (RFU) of ROS of other groups.
2.2.3. Detection of protein expression in cells
The cells for protein expression detection were inoculated in 6-well culture plate and treated with different conditions to abandon the culture medium. In each well, 200 μL protein lysis buffer was added.The protein in cells was isolated and then mixed with the sample buffer. After 100 ℃ high-temperature degeneration the samples were added in preconfigured polyacrylamide-SDS gel for vertical electrophoresis and then the protein on the gel was transferred to NC membrane by electrophoretic transfer. The NC membrane was closed for 2 h by using 5% skim milk and then taken out to incubate the Bcl-2, Bax, CytC, Caspase-3 and Caspase-9 antibodies for the night.The next day, the HRP-labeled second antibody incubation was done, and the protein band was finally developed on the visualizer.The protein expression of control group after 8 h of treatment was set as 1 (100%) to calculate the protein expression of Bcl-2, Bax, CytC,Caspase-3 and Caspase-9 protein expression in the other groups of cells.
2.2.4. Detection of protein levels in cell culture medium
The cells for ROS detection were inoculated in the 12-well culture plate and treated to keep the culture medium, and Col-I , Col-Ⅲ ,PI NP and PⅢNP contents were determined by enzyme-linked immunosorbent assay kit; the cells were kept and added in protein lysis buffer to extract protein, and the total protein content was determined by the BCA kit. The protein levels of Col-I , Col-Ⅲ, PI NP and PⅢNP corresponding to the unit mass of total protein were calculated.
2.3. Statistical methods
SPSS20.0 software was used to analyze data, the measurement data were in terms of mean±SD and the measurement data analysis between two groups was conducted byttest, while the count data among three groups was analyzed by variance analysis and pair-wise comparison was by LSD-ttest.P<0.05 meant statistical significance in differences in test results.
3. Results
3.1. High glucose increased ROS generation in myocardial cells
After 8 h, 16 h and 24 h of treatment, ROS RFU in high glucose group of cells were (1.31±0.16), (1.88±0.24) and (2.64±0.33)respectively; ROS RFU in control group of cells were (1.00±0.16),(1.04±0.13) and (1.06±0.18) respectively. Afterttest, ROS RFU in high glucose group of cells were significantly higher than those in control group after 8 h, 16 h and 24 h of treatment (P<0.05).
3.2. High glucose promoted mitochondrial pathway apoptosis in myocardial cells
After 8 h, 16 h and 24 h of treatment, Bax, CytC, Caspase-3 and Caspase-9 protein expression in high glucose group were significantly higher than those of control group whereas Bcl-2 protein expression were significantly lower than those of control group (P<0.05) (Table 1).
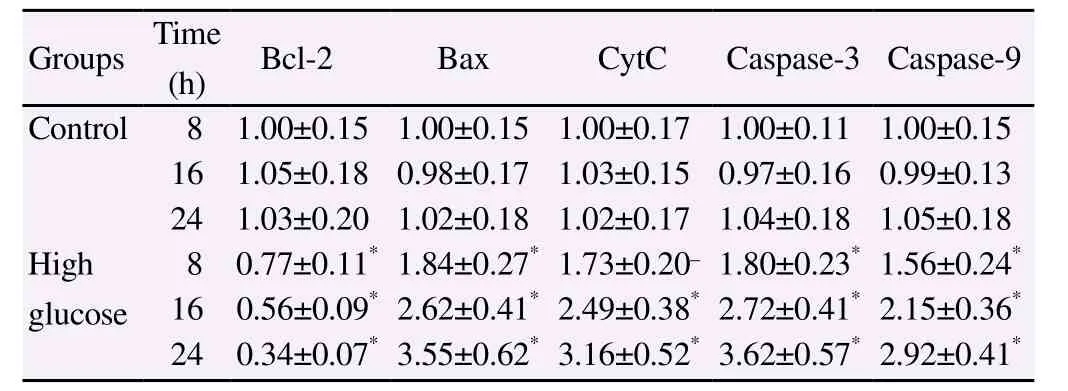
Table 1Effect of high glucose on mitochondrial pathway apoptosis in myocardial cells (mean±SD).
3.3. High glucose promotes collagen synthesis in myocardial cells
After 8 h, 16 h and 24 h of treatment, Col-I (ng/mg total protein),Col-Ⅲ (ng/mg total protein), PINP (pg/mg total protein) and PⅢNP (pg/mg total protein) protein levels in culture medium of high glucose group were significantly higher than those of control group(P<0.05), while CTX-I(pg/mg total protein) protein levels were not significantly different from those of control group (P>0.05)(Table 2).

Table 2Effect of high glucose on collagen and collagen metabolism molecule contents (mean±SD).
3.4. NAC reduced promoting effect of high glucose on mitochondrial pathway apoptosis in myocardial cells
After 24 h of treatment, Bax, CytC, Caspase-3 and Caspase-9 protein expression in high glucose group were significantly higher than those of control group whereas Bcl-2 protein expression was significantly lower than that of control group (P<0.05); Bax, CytC,Caspase-3 and Caspase-9 protein expression in NAC group were lower than those in high glucose group whereas Bcl-2 protein expression was significantly higher than that in high glucose group(P<0.05) (Table 3).

Table 3Effect of NAC combined with high glucose on mitochondrial pathway apoptosis expression in myocardial cells after 24 h of treatment (mean±SD).
3.5. NAC reduced promoting effect of high glucose on collagen synthesis in myocardial cells
After 24 h of treatment, Col-Ⅰ(ng/mg total protein), Col-Ⅲ (ng/mg total protein), PⅠNP (pg/mg total protein) and PⅢNP (pg/mg total protein) protein levels in culture medium of high glucose group were significantly higher than those of control group (P<0.05);Col- Ⅰ, Col-Ⅲ, PⅠNP and PⅢNP protein levels in culture medium of NAC group were significantly lower than those of high glucose group (P<0.05) (Table 4).

Table 4Effect of NAC combined with high glucose on collagen and collagen metabolism molecule contents after 24 h of treatment (mean±SD).
4. Discussion
DCM is one of the common complications of diabetes mellitus,which is independent of diabetic vascular complications, and mainly manifested as the damage of myocardial cell structure and function. The decreased number, dysfunction and structural damage of mitochondria in myocardial cells are the prominent pathological changes in myocardial tissue of diabetic animal models, and also accompanied by myocardial interstitial fibrosis[5,6]. The activated oxidative stress reaction and increased ROS formation caused by high glucose is one of the common pathogenesis of various diabetic complications, and the continuously generated ROS can cause cell damage within the target organ damage and result in the corresponding complications[7,8]. The damaging effect of high glucose on myocardial cells, endothelial cells and so on has been confirmed by more and more studies[9,10], but in the pathological process of DCM, it is still not clear whether high glucose causes myocardial cell injury through the activation of oxidative stress.In order to determine that, the ROS generation in the myocardial cells was analyzed in the study after high glucose treatment, and the results showed that ROS RFU in high glucose group of cells were higher than those in control group. This indicates that the high glucose condition can increase the generation of ROS in myocardial cells, which in turn can cause myocardial cell damage by oxidative stress response.
Mitochondrial pathway apoptosis is an important link in the oxidative stress injury of myocardial cells, and ROS can cause mitochondrial function disorder in cells, and thus activate mitochondrial pathway of apoptosis[11-13]. Bax and Bcl-2 are the key molecules on mitochondrial membrane that regulate CytC permeability; the homodimer formed by Bax can be the channel for CytC to leave mitochondria and enter into the cytoplasm, and the Bcl-2 can form heterodimer with Bax and antagonize its biological function[14,15]. The CytC from the mitochondria to the cytoplasm can start apoptotic cascade activation reaction; it induces Apaf allosterism and makes the caspase-9 precursor split into active mature form, and then the activated caspase-9 can further activate caspase-3 and induce apoptosis[16]. The analysis of the changes in above mitochondrial pathway apoptosis molecule expression in myocardial cells after high glucose processing in the study showed that Bax, CytC, Caspase-3 and Caspase-9 protein expression in high glucose group were higher than those of control group whereas Bcl-2 protein expression were lower than those of control group. This indicates that high glucose can affect the Bcl-2/Bax balance, increase CytC release and activate the Caspase cascade activation response to activate the mitochondrial pathway apoptosis of myocardial cells.
In the pathological process of DCM, high glucose will not only directly cause myocardial cell apoptosis, but will also cause myocardial interstitial fibrosis and affect the cardiac diastolic and systolic function so as to accelerate the occurrence of heart failure[17,18]. The excessive proliferation of fibroblasts and the abnormal accumulation of collagen in extracellular matrix are important characteristics of myocardial interstitial fibrosis. Collagen type Ⅰ and type Ⅲ are the main collagen components in the myocardial extracellular matrix, and the increase of type Ⅰ collagen and type Ⅲ collagen that are synthesized and secreted by myocardial cells can cause the occurrence of myocardial fibrosis[19,20]. In the transformation from procollagen to mature collagen, the split of procollagen C-terminal polypeptide will form the by-products P ⅠCP and PⅢCP, and the PⅠCP and PⅢCP contents can reflect the collagen anabolism process; in the process of mature collagen degradation, the C-terminal split will generate CTX-Ⅰ, and the CTX-Ⅰ can reflect the collagen catabolism process. The analysis of the changes in above collagen metabolism molecule contents in myocardial cell after high glucose processing showed that Col- Ⅰ ,Col-Ⅲ, PⅠNP and PⅢNP protein levels in high glucose group were higher than those of control group, and CTX-Ⅰ protein levels were not significantly different from those of control group. This indicates that high glucose can promote the collagen anabolism in myocardial cells without affecting the collagen catabolism so as to promote collagen deposition and myocardial fibrosis.
The mitochondrial pathway apoptosis and collagen deposition in myocardial cells are the important pathological changes in the process of myocardial injury induced by high glucose. In recent years, a growing number of studies have confirmed that the activated oxidative stress reaction and the increased oxygen free radical generation are closely related to the myocardial cell apoptosis and myocardial interstitial fibrosis[21-23], but it is still not clear about the roles that the oxidative stress play in high glucose-induced myocardial cell apoptosis and collagen deposition. As mentioned above, we have shown that high glucose can increase the production of ROS within the myocardial cells, which can activate oxidative stress response. In order to further clarify the roles of oxidative stress reaction activation in high glucose-induced myocardial cell apoptosis and collagen deposition, free radical scavenger NAC was used in the study on the basis of high glucose processing, and comparison of the changes in apoptosis molecule expression and collagen metabolism molecule contents showed that Bax, CytC, Caspase-3 and Caspase-9 protein expression as well as Col-Ⅰ, Col-Ⅲ, PⅠNP and PⅢNP protein levels in NAC group were lower than those in high glucose group whereas Bcl-2 protein expression was higher than that in high glucose group. It means that free radical scavenger can reverse the high glucose effects on promoting the mitochondria pathway apoptosis in myocardial cells and increasing the collagen deposition in myocardial cells, which also confirms high glucose can increase the generation of ROS to cause myocardial cell apoptosis, and collagen deposition.
High glucose can increase the generation of ROS in myocardial cells, cause the mitochondrial pathway apoptosis in cells and increase collagen synthesis; increasing the production of ROS is the molecular mechanism of high glucose to cause myocardial cell apoptosis and increase collagen synthesis.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Shang Y, Zhang X, Leng W, Chen L, Lei X, Zhang T, et al. Assessment of diabetic cardiomyopathy by cardiovascular magnetic resonance T1 mapping: correlation with left-ventricular diastolic dysfunction and diabetic duration.J Diabetes Res2017; 2017: 9584278.
[2] Gilca GE, Stefanescu G, Badulescu O, Tanase DM, Bararu I, Ciocoiu M.Diabetic cardiomyopathy: Current approach and potential diagnostic and therapeutic targets.J Diabetes Res2017; 2017: 1310265.
[3] Faria A, Persaud SJ. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential.Pharmacol Ther2017; 172: 50-62.
[4] Jarosz J, Ghosh S, Delbridge LM, Petzer A, Hickey AJ, Crampin EJ, et al. Changes in mitochondrial morphology and organization can enhance energy supply from mitochondrial oxidative phosphorylation in diabetic cardiomyopathy.Am J Physiol Cell Physiol2017; 312(2): 190-197.
[5] de Paula DRM, Capuano V, Filho DM, Carneiro ACDM, de Oliveira Crema V, de Oliveira LF, et al. Biological properties of cardiac mesenchymal stem cells in rats with diabetic cardiomyopathy.Life Sci2017; 188: 45-52.
[6] Liu P, Su J, Song X, Wang S. Activation of nuclear β-catenin/c-Myc axis promotes oxidative stress injury in streptozotocin-induced diabetic cardiomyopathy.Biochem Biophys Res Commun2017; 493(4): 1573-1580.
[7] Sha J, Sui B, Su X, Meng Q, Zhang C. Alteration of oxidative stress and inflammatory cytokines induces apoptosis in diabetic nephropathy.Mol Med Rep2017; 16(5): 7715-7723.
[8] Chen W, Yang J, Chen S, Xiang H, Liu H, Lin D, et al. Importance of mitochondrial calcium uniporter in high glucose-induced endothelial cell dysfunction.Diab Vasc Dis Res2017; 14(6): 494-501.
[9] Martín-Fernández B, Gredilla R. Mitochondria and oxidative stress in heart aging.Age (Dordr)2016; 38(4): 225-238.
[10] Kurian GA, Rajagopal R, Vedantham S, Rajesh M. The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling:Revisited.Oxid Med Cell Longev2016; 2016: 1656450.
[11] Yang C, Liu X, Yang F, Zhang W, Chen Z, Yan D, et al. Mitochondrial phosphatase PGAM5 regulates Keap1-mediated Bcl-xL degradation and controls cardiomyocyte apoptosis driven by myocardial ischemia/reperfusion injury.In Vitro Cell Dev Biol Anim2017; 53(3): 248-257.
[12] Wang Z, Liu D, Varin A, Nicolas V, Courilleau D, Mateo P, et al. A cardiac mitochondrial cAMP signaling pathway regulates calcium accumulation, permeability transition and cell death.Cell Death Dis2016;7: e2198.
[13] Goldenthal MJ. Mitochondrial involvement in myocyte death and heart failure.Heart Fail Rev2016; 21(2): 137-155.
[14] Blagonravov ML, Korshunova AY, Azova MM, Bryk AA, Frolov VA.Expression of Bax protein and morphological changes in the myocardium in experimental acute pressure overload of the left ventricle.Bull Exp Biol Med2016; 161(2): 312-315.
[15] Chen C, He H, Luo Y, Zhou M, Yin D, He M. Involvement of Bcl-2 signal pathway in the protective effects of apigenin on anoxia/reoxygenationinduced myocardium injury.J Cardiovasc Pharmacol2016; 67(2): 152-163.
[16] Wang Y, Cao Y, Zhu Q, Gu X, Zhu YZ. The discovery of a novel inhibitor of apoptotic protease activating factor-1 (Apaf-1) for ischemic heart:synthesis, activity and target identification.Sci Rep2016; 6: 29820.
[17] Yue Y, Meng K, Pu Y, Zhang X. Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy.Diabetes Res Clin Pract2017; 133: 124-130.
[18] Chistiakov DA, Orekhov AN, Bobryshev YV. The role of cardiac fibroblasts in post-myocardial heart tissue repair.Exp Mol Pathol2016;101(2): 231-240.
[19] Azevedo PS, Polegato BF, Minicucci MF, Paiva SA, Zornoff LA. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment.Arq Bras Cardiol2016; 106(1): 62-69.
[20] van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts.J Mol Cell Cardiol2016; 93: 133-142.
[21] Ludke A, Akolkar G, Ayyappan P, Sharma AK, Singal PK. Time course of changes in oxidative stress and stress-induced proteins in cardiomyocytes exposed to doxorubicin and prevention by vitamin C.PLoS One2017; 12(7): e0179452.
[22] Li J, Zhou Y, Zhang W, Bao C, Xie Z. Relief of oxidative stress and cardiomyocyte apoptosis by using curcumin nanoparticles.Colloids Surf B Biointerfaces2017; 153: 174-182.
[23] Torrealba N, Aranguiz P, Alonso C, Rothermel BA, Lavandero S.Mitochondria in structural and functional cardiac remodeling.Adv Exp Med Biol2017; 982: 277-306.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- An updated systematic review of Zika virus-linked complications
- Biological, chemical and pharmacological aspects of Madhuca longifolia
- Scenario of dengue infection & its control in Pakistan: An up-date and way forward
- Cytotoxic, kinetics of inhibition of carbohydrate-hydrolysing enzymes and oxidative stress mitigation by flavonoids roots extract of Dicoma anomala (Sond.)
- Evaluation of antiparasitic, anticancer, antimicrobial and hypoglycemic properties of organic extracts from Panamanian mangrove plants
- Neuroprotection by misoprostol against rotenone-induced neurotoxicity in rat brain
