An updated systematic review of Zika virus-linked complications
2018-05-09BrairaWahidAmjadAliMuhammadWaqarMuhammadIdrees
Braira Wahid, Amjad Ali, Muhammad Waqar, Muhammad Idrees,3
1Centre for Applied Molecular Biology, 87-West Canal Bank Road Thokar Niaz Baig, University of the Punjab, Lahore, Pakistan
2Genome Centre for Molecular Based Diagnostics and Research Centre, Cl-25 Block B Al-Sudais Plaza Abdalian Cooperative Society, Lahore,Pakistan
3Hazara University, Mansehra, Pakistan
1. Introduction
Zika virus (ZIKV) is enveloped, positive sense ssRNA virus that belongs to family Flaviviridae. Since last one year it has spread to almost 84 countries and territories with an increased incidence of central nervous system (CNS) malformations in 33 countries. The first case of ZIKV in human was reported in 1954 in Nigeria[1].Only 14 cases were confirmed from all across the globe until 2007.However, in 2007, large outbreak was reported in Micronesia, Yap Island where about 5 000 people were infected. Other outbreaks occurred in French Polynesia, Cook Island, Easter Island, Solomon Island, and New Caledonia between 2013 and 2015. ZIKV spread to 20 countries with highest prevalence in Brazil during 2015. The global bioburden has been raised to 3-4 million cases worldwide with 1.5 million cases confirmed in Brazil[2,3]. Several experimental evidences suggest that this virus is behind the growing incidence of congenital malformation, i.e., microcephlay in neonates and other neurological Guillain-Barre syndrome (GBS), meningoencephalitis,hydrancephaly/hydrops fetalis, myasthenia gravis, and myelitis in adults. The link between ZIKV and catastrophic neurological complications has led to global health emergency. In response to this emerging epidemic, researches have been stirred into action to explore ZIKV vaccine, therapeutics, and consequences. Many cohort studies and case control studies involving pregnant women with ZIKV symptoms have been evaluated to determine the correlation between ZIKV and neurological dysfunctions[4].
In Brazil, a significant increase in newborns with microcephaly during 2015 has strengthened the connection of ZIKV with microcephaly[2]. From January 2007 to June 2016, 1 551 cases of microcephaly were reported in Brazil, 32 in Colombia, 8 in French Polynesia, 6 in Cabo Verde, 5 in Panama, 4 in Martinique,2 in America, 1 in Slovenia, 1 in Spain, 1 in Puerto Rico, and 1 in Marshall Islands[5,6]. Ministry of Health, Brazil reported about 4 700 suspected cases of microcephaly during mid-2015 to January 2016,and this incidence was 20 times higher than that between 2010 and 2014.
As of 10 March 2017, 23 countries have reported an increased incidence of GBS. According to World Health Organization, on average 242 ZIKV-linked GBS cases are reported annually in Colombia[1]. As of 8 February 2017, the confirmed number of ZIKV cases in Western Hemisphere countries was 202 008 whereas, 2 588 cases of ZIKV-linked microcephaly in this region has also been reported[7].
Likewise, 252 cases of GBS with ZIKV symptoms were documented during January 2016 in state of Venezuela out of which 66 were reported in state of Zulia[8]. About 169 cases of GBS are reported annually in El Salvador; however, 46 cases including two deaths were reported in this region during ZIKV outbreak (from 1 December 2015 to 6 January 2016)[9]. PAN American Health Organization (PAHO) reported 100 cases of GBS associated with ZIKV from 1stweek until 17thweek of 2016 in Dominican Republic.Dominican Republic Ministry of Public Health documented a significant increase in GBS cases from 14thweek of 2016 and onwards[10]. In Pernambuco state, Brazil seven cases of GBS-linked to ZIKV infection were reported on 25 November, 2015.Immediately after start of ZIKV outbreak during April and June 2015, increased incidence of GBS was confirmed in several states of Brazil. About 130 cases were reported in Pernambuco,55 in Bahia, 24 in Rio Grande do Norte, 14 in Maranhao, and 6 cases in Paraiba[6]. Likewise, other neural abnormalities such as hydrancephaly/hydrops fetalis, myasthenia gravis,meningoencephalitis and myelitis have also been reported in patients who were positive for ZIKV.
This systematic review presents the key evidences of reported neurological complications in ZIKV-infected people and also speculates increasing risk of teratogenic outcomes during recent outbreak of ZIKV. This study demonstrated the breadth of neurological manifestations of ZIKV.
2. Material and methods
Different databases and PROSPERO-International prospective register for systematic reviews were searched thoroughly to determine if there was any systematic review already published on topics related to ZIKV-linked neurological effects in adults and teratogenic outcomes. None was found. Then, literature search was conducted from June 2016 to March 2017 using different electronic databases such as ScienceDirect, Pubmed, Medline,Scopus, and Global Health Library according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)guidelines. We searched databases for publications in English language from early 2015 up to March 2017 to identify potentially eligible studies. Our search terms included ZIKV, neurological abnormalities, hydrancephaly/hydrops fetalis, myasthenia gravis,epidemiology, microcephaly, ovular abnormalities, GBS, myelitis,meningoencephalitis, case-studies, case-cohort studies, crosssectional studies, organizational survey reports and case-control studies. We reviewed all titles, abstracts, and conclusions of publications. We also studied letters, editorials, and review articles and interacted with experts in the field.
Out of total 72 records, 50 met the inclusion criteria based on reported brain anomalies associated with ZIKV. All articles were reviewed to scan for exclusion criteria. All authors independently screened abstracts, article titles and year of publications to select records for full-text read. Then, we assessed full-texts of articles and consulted other reviewers in case of disagreements so that final decision can be made with mutual consensus. After removing duplicates, review articles, organizational survey reports, letters, and editorials only 39 studies were finally selected for review. Eligible publications included original studies related to ZIKV-linked neurological disorders in fetuses and adults. We finally selected 35 studies for systematic analysis and used spreadsheet to record information such as authors, study area, study period, and finding. A flow chart of our research strategy can be seen in Figure 1.

Figure 1. Flowchart defining search strategy.
3. Results
As of March 12, 2017, we retrieved 64 articles that reported neurological fetal outcome in ZIKV-infected pregnant women and other neuro-related disorders in adults. About 35 articles were screened for eligibility and all 35 articles included simple case-studies, case-cohort studies, case-control studies, and crosssectional studies. The year of publication, location, and frequency of ZIKV-related neuro-disorders in adults as well as neonates were recorded. Out of 35 studies, 15 were related to microcephaly, 16 were related to GBS, 2 studies showed the association of ZIKV with meningoencephalitis, and 2 studies reported acute myelitis in ZIKV-positive patients. Two patients with confirmed diagnosis of ZIKV experienced hydrops fetalis and myasthenia gravis. According to laboratory confirmed cases and clinical profile studies, microcephaly,GBS, acute myelitis, meningoencephalitis, hydrancephaly/Hydrops fetalis and myasthenia gravis are presented in Tables 1-3 respectively.
Literature survey shows that microcephaly, GBS, hydrops fetalis,myasthenia gravis, myelitis, and meningoencephalitis are the possible adverse outcomes of ZIKV syndrome. An unprecedented increase in cases of aforementioned brain abnormalities specifically microcephaly during the emergence of ZIKV outbreak has been observed worldwide.
Significant increase in microcephalic newborns was recorded in Brazilian states during 2013 and 2016 compared to previous years.The baseline prevalence of microcephaly was found to be two cases(95%CI: 0-8) per 10 000 neonates in French Polynesia[11,12]. From August to October 2015 about 74% cases of microcephaly related to ZIKV were reported in Brazil[13]. Two studies (simple case report and case-cohort study) demonstrated the association of ZIKV with congenital ocular findings concomitant with microcephaly[14,15]. Two other cases of microcephaly were reported in Rio Grande do Norte and mothers of both fetuses were found to be ZIKV-positive[16]. In another case-control study, ocular findings were confirmed in 34.5%of ZIKV-linked microcephalic infants[17]. Likewise, mothers of two other microcephalic infants were tested positive for ZIKV[18].Another case-cohort study reported 28% brain anomalies including microcephaly in newborns whose mothers had infected with ZIKV[19]. Risk of ZIKV-associated microcephaly has increased to 30% (usually varies between 0% to 5%)[20]. In 2015, alarming rise in cases of microcephaly during epidemiologic weeks 45-47 had been observed in three different states of Brazil[20]. Marked increase(> 14 times of previous findings) in confirmed cases of microcephaly was observed in 2016[22]. One study has shown dramatic rise in microcephaly in Paraiba, Bahia, and Pernanmbuco[51]. Three other prospective studies reported CNS malformations such as brain or cerebral lesions and microcephaly in ZIKV-positive dead and live fetuses[21,23-25].
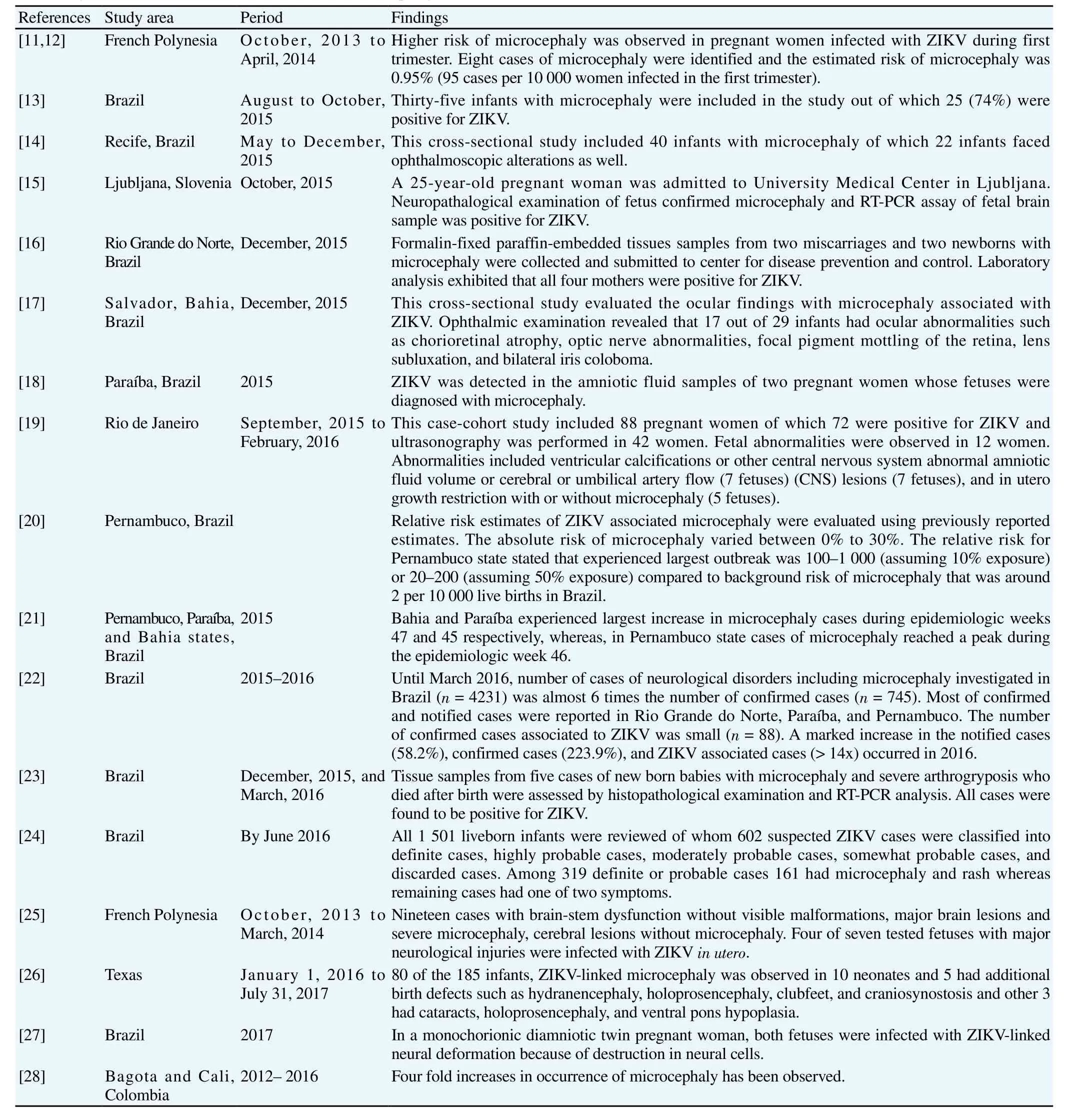
Table 1Laboratory confirmed cases and clinical studies of microcephaly.

Table 2Confirmed cases and clinical profile studies of Guillain-Barre syndrome (GBS).

Table 3Laboratory confirmed cases and clinical studies of acute myelitis, meningoencephalitis, hydrancephaly/hydrops fetalis, and myasthenia gravis.
About 42 cases of GBS were reported in French Polynesia during ZIKV outbreak 2013-2014[52], of which 26 were reported within short period of 8 weeks and this number actually exceeded the GBS baseline incidence that is 3-8 cases a year[53]. During early 2016,two more GBS cases have also been confirmed in Martinique,French West Indies Island[32]. During the current ZIKV outbreak 2016, ZIKV has been confirmed in the urine and serum samples of GBS patients found in Netherlands, Colombia, Haiti, Rio de Janeiro,Spain and Martinique.
Araujo and Ferreira had provided additional evidences of GBS in their review article[54]. A neurologist Mario Emilio Dourado reported seven ZIKV-linked GBS cases in the State of Rio Grande do Norte, Natal City, Northeast Brazil. Four other cases of GBS and two cases of acute disseminated encephalomyelitis associated with ZIKV infection were documented in State of Pernambuco, Northeast Brazil during July 2015. Researchers demonstrated that Brazil experienced 5 times increase in ZIKV-linked GBS since 2015[54].According to center for disease prevention and control, seven cases of GBS linked to ZIKV infection were reported on 25 November,2015 in Pernambuco state, Brazil. Immediately after start of ZIKV outbreak during April and June 2015, increased incidence of GBS was confirmed in several states of Brazil. About 130 cases were reported in Pernambuco, 55 in Bahia, 24 in Rio Grande do Norte, 14 in Maranhao, and 6 cases in Paraiba[6]. One case of GBS has also been confirmed in patient co-infected with ZIKV and chikungunya virus[41]. Another male patient in 40s suffered from GBS after travelling to ZIKV endemic region Guyana[40]. A case-control study exhibited 42 cases of GBS in ZIKV-infected patients and showed 20-fold increase in incidence of GBS[31,55]. Nine other cases-reports have also confirmed ZIKV-linked GBS in different regions of world during 2014 to 2017[33-39,42-44,56].
Evidence of ZIKV-linked acute myelitis[45] and meningoencephalitis[47] has been published in The Lancet and New England Journal of Medicine respectively. Evidences of hydrops fetalis and myasthenia gravis have been confirmed in Brazil and New Caledonia, respectively[49,50].
The evidence of ZIKV causing these adverse outcomes in adults and pregnant women has been the hot topic of debate since ZIKV outbreak in Brazil. Although accumulating evidence have now clarified the lingering questions regarding the confirmed link of ZIKV with potential teratogenic outcomes, the wide spectrum of congenital brain defects discussed in our systematic review also supports the causal relationship of ZIKV with brain anomalies because of increased incidence of neurological malformations reported during recent 2015-2016 outbreak of ZIKV.
4. Discussion
We systematically reviewed many studies reporting the fetal teratogenic effects of ZIKV. Apart from laboratory confirmed cases,animal model studies have also supported the possible association of neurological diseases with ZIKV. It is notable that in this current systematic review, GBS is the most common neurological malformation followed by microcephaly, acute myelitis and meningencephalitis, hydrops fetalis and myasthenia gravis.
According to Tanget al., ZIKV infects human cortical neural progenitor cells (hCNPCs) and the infected cells lead to production of infectious ZIKV particles which cause cell death of hCNPCs.ZIKV infection impedes cell-cycle progression, increases cell death and attenuates growth of hCNPCs[57]. Likewise, ZIKV induced inflammasome activation in the glial cell line U87-MG[58].Another study reported birth defects caused by Brazilian ZIKV strain in experimental models. In mice, this strain was found to cause microcephaly and intrauterine growth restriction in fetuses.Results of the study exhibit that Brazilian ZIKV cross the placenta,target cortical progenitor cells, induce cell death and impair neurodevelopment[59].
Nowakowskiet al. elucidated the underlying cellular and molecular mechanisms that associated ZIKV infection to neurological defects[60]. Single-cell RNA expression profiles for cell populations in the human fetal brain were generated and then candidate receptors for ZIKV were surveyed from that dataset. High level of mRNA expression of candidate viral entry receptor AXL was observed in human radial glial cells, endothelial cells, astrocytes, and microglia in developing human cortex; therefore, AXL has been proved as a candidate ZIKV entry receptor in neural stem cells[60]. Likewise,Danget al. developed human embryonic stem cell derived organoid to recapitulate first trimester fetal brain growth. ZIKV was found to infect neural progenitor cells in neurosphere models. ZIKV activated Toll like receptor-3 in cerebral organoids and led to attenuation of neurogenesis[61]. In addition to this, ZIKV activate the death of infected neural cells because of interaction of its capsid protein with mouse double-minute-2 homolog that plays an important role in P53-mediated apoptosis pathway[62]. One study has also shown oncolytic activity of ZIKV against glioblastoma stem cells[63]. ZIKV encoded NS2A degrades adherens junction proteins resulting in disruption of mammalian cortical neurogenesis[64,65].
Garcezet al. showed the effects of ZIKV in human neural stem cells growing as brain organoids and neurospheres. Immunocytochemistry and electron chemistry showed that ZIKV reduced the viability and growth of human brain cells[66]. Another ZIKV strain SZ01 targets different neuronal lineages and replicates in the embryonic mouse brain. ZIKV impair cell-cycle, cause apoptosis, and inhibit differentiation of neural precursor cells, leading to microcephaly and cortical thinning. This study provided direct link between ZIKV and brain anomalies, because gene expression analysis of infected brain reveals upregulation of candidate flavirus entry receptors and dysregulation of genes associated with apoptosis,immune response, and microcephaly[67]. Another study involving two mouse models exhibited affinity of ZIKV to murine neuronal cells thereby supporting the link between ZIKV infection and microcephaly[68]. A recent study retrospectively evaluates the fetal abnormalities due to ZIKV outbreak and found a significant rise in neurological complications in neonates[69]. The main neuroimaging findings observed in different studies include cortical development(e.g., lissencephaly, heterotopia, etc.), brainstem and cerebral,hypoplasia, agenesis of the cavum septum pellucidum, parenchymal calcifications, dysgenesis of the corpus callosum, unilateral or bilateral ventriculomegaly, agenesis of the cavum septum pellucidum, and enlarged extra-axial cerebrospinal fluid spaces[70].A recent study presented neurological disorders in 12 of 16 patients co-infected with ZIKV, chikungunya virus, and dengue virus in Guayaquil, Ecuador. One patients experienced CNS vasculitis, three had GBS whereas, and six patients were diagnosed with meningitis or encephalitis[71]. Our study reinforces the growing body of evidences associating upsurge in congenital brain malformations with ZIKV by pointing to the worrisome increase in the clinical severity and bioburden of life-threatening brain anomalies[72].
Geographical expansion of ZIKV and unusual rise in magnitude of cerebral malformations also provide basis of ZIKV link with neurological disorders. The causative link of ZIKV with congenital microcephaly and neurological complications worsened the explosive nature of recent epidemics of ZIKV. GBS and microcephaly are the most common brain anomalies whereas, hydrancephaly/hydrops fetalis, myasthenia gravis, meningoencephalitis and myelitis have also been observed in ZIKV infected patients. The findings of our study provide the spectrum of ZIKV-associated brain anomalies.Moreover, this study will act as baseline data for further research.Given the substantial public health implications and rapid geographic spread of ZIKV in recent years, a coordinated global effort and a lot of research work are needed to effectively curb the expansion of current outbreak.
Conflict of interest statement
The authors declare that they have no conflict of interest.
[1] World Health Organization. Zika virus, microcephaly and Guillain-Barré syndrome. [Online]. Available from: http://www.who.int/emergencies/zika-virus/situation-report/10-march-2017/en/. Accessed on March 10,2017.
[2] Korzeniewski K, Juszczak D, Zwolińska E. Zika-another threat on the epidemiological map of the world.Int Marit Health2016; 67(1): 31-37.
[3] Blázquez AB, Saiz JC. Neurological manifestations of Zika virus infection.World J Virol2016; 5(4): 135.
[4] Carod-Artal FJ. Epidemiology and neurological complications of infection by the Zika virus: A new emerging neurotropic virus.Rev Neurol2016; 62(7): 317-328.
[5] World Health Organization. Zika virus, microcephaly and Guillain-Barré syndrome. [Online]. Available from: http://www.who.int/emergencies/zika-virus/situation-report/9-june-2016/en/. Accessed on June 9, 2016.
[6] European Centre for Disease Prevention and Control. Epidemiological update: Complications potentially linked to the Zika virus outbreak,Brazil and French Polynesia. [Online]. Available from: https://ecdc.europa.eu/en/news-events/epidemiological-update-outbreaks-zika-virusand-complications-potentially-linked-24. Accessed on: 11, 2015.
[7] Department of Defense, Armed Forces Health Surveillance Branch Global Zika Virus Surveillance Summary. [Online]. Available from:https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=6&cad=rja&uact=8&ved=0ahUKEwjCytn64o7XAhVEuhoKHUMYBQQFghFMAU&url=http%3A%2F%2Fwww.med.navy.mil%2Fsites%2 Fnmcphc%2FDocuments%2Fprogram-and-policy-support%2FAFHSBDetecting-and-Reporting-DoD-Cases-of-Zika-17%2520MAY-2016.pdf&usg=AOvVaw2J7xMDPfF1a9KUj8BqcpqW. Accessed on January 25,2017.
[8] World Health Organization. Guillain-Barré syndrome - Colombia and Venezuela. [Online]. Available from: http://www.who.int/csr/don/12-february-2016-gbs-colombia-venezuela/en/. Accessed on 12 February,2016.
[9] World Health Organization, Guillain-Barré syndrome - El Salvador.[Online]. Available from: http://www.who.int/csr/don/21-january-2016-gbs-el-salvador/en/. Accessed on: 21 January 2016.
[10] PAN Americna Health Organization. Zika - Epidemiological Update. [Online]. Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=40222&l ang=en. Accessed on May 26, 2016.
[11] Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly.N Engl J Med2016; 375(1): 1-4.
[12] Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P,Eyrolle-Guignot, et al. Association between Zika virus and microcephaly in French Polynesia, 2013-15: A retrospective study.Lancet2016;387(10033): 2125-2132.
[13] Schuler-Faccini L. Possible association between Zika virus infection and microcephaly-Brazil, 2015.MMWR Morb Mortal Wkly Rep2016; 65(3):59-62.
[14] Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R. Zika virus in Brazil and macular atrophy in a child with microcephaly.Lancet2016;387(10015): 228.
[15] Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, MrazJ, et al.Zika virus associated with microcephaly.N Engl J Med2016; 10(374):951-958.
[16] Martines RB. Notes from the field: Evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses-Brazil, 2015.MMWR Morb Mortal Wkly Rep2016;65(6): 159-160.
[17] de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA,Ko AI, Maia M, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador,Brazil.JAMA Ophthalmol2016; 134(5): 529-535.
[18] Calvet GA, Filippis AM, Mendonça MC, Sequeira PC, Siqueira AM,Veloso VG, et al. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro, Brazil.J Clin Virol2016;31(74): 1-3.
[19] Brasil P, Pereira Jr JP, Moreira ME, RibeiroNogueira RM, Damasceno L, Wakimoto, et al. Zika virus infection in pregnant women in Rio de Janeiro.N Engl J Med2016; 375: 2321-2334.
[20] Jaenisch T, Rosenberger K, Brito C, Brady O, Brasil P, Marques E.Estimating the risk for microcephaly after Zika virus infection in Brazil.Bull World Health Organ2017 95(3): 191-198.
[21] de Oliveira WK. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy-Brazil, 2015.MMWR Morb Mortal Wkly Rep2016; 65: 242-247
[22] Cunha AJ, Magalhães-Barbosa MC, Lima-Setta F, Prata-Barbosa A.Evolution of cases of microcephaly and neurological abnormalities suggestive of congenital infection in Brazil: 2015-2016.Bull World Health Organ2016; 94: DOI: http://www.who.int/bulletin/online_first/zika_open/en/.
[23] Martines RB, Bhatnagar J, de Oliveira Ramos AM, Davi HP, Iglezias SD,Kanamura CT et al. Pathology of congenital Zika syndrome in Brazil: A case series.Lancet2016; 388(10047): 898-904.
[24] França GV, Schuler-Faccini L, Oliveira WK, Henriques CM, Carmo EH,Pedi VD, et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation.Lancet2016;388(10047): 891-897.
[25] Besnard M, Eyrolle-Guignot D, Guillemette-Artur P, Lastère S, Bost-Bezeaud F, Marcelis L, et al. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia.Euro Surveill2016; 21(13): doi:10.2807/1560-7917.ES.2016.21.13.30181.
[26] Hall NB. Notes from the Field: Zika Virus-Associated Neonatal Birth Defects Surveillance-Texas, January 2016-July 2017.MMWR Morb Mortal Wkly Rep2017; 66(31): 835-836.
[27] Santos VS, Oliveira SJ, Gurgel RQ, Lima DR, dos Santos CA, Martins-Filho PR. Case Report: Microcephaly in Twins due to the Zika Virus.Am J Trop Med Hyg2017; 97(1): 151-154.
[28] Hurtado-Villa P, Puerto AK, Victoria S, Gracia G, Guasmayán L, Arce P, et al. Raised frequency of microcephaly related to Zika virus infection in two birth defects surveillance systems in Bogotá and Cali, Colombia.Pediatr Infect Dis2017; 36(10): 1017-1019.
[29] Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, et al. Zika virus infection complicated by Guillain-Barre syndrome-case report, French Polynesia, December 2013.Eurosurveill2014; 19(9):20720.
[30] Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study.Lancet2016;387(10027): 1531-1539.
[31] Watrin L, Ghawché F, Larre P, Neau JP, Mathis S, Fournier E. Guillain-Barré syndrome (42 Cases) occurring during a Zika virus outbreak in French Polynesia.Medicine (Baltimore)2016; 95: 3257-3261.
[32] Rozé B, Najioullah F, Fergé JL, Apetse K, Brouste Y, Cesaire R, et al.Zika virus detection in urine from patients with Guillain-Barré syndrome on Martinique, January 2016.Eurosurveill2016; 21(9): 30154-30156.
[33] van den Berg B, van den Beukel JC, Alsma J, van der Eijk AA, Ruts L,van Doorn PA, et al. Guillain-Barre syndrome following infection with the Zika virus.Ned Tijdschr Geneeskd2016; 160: 155-161.
[34] Duijster JW, Goorhuis A, van Genderen PJ, Visser LG, Koopmans MP,Reimerink JH, et al. Zika virus infection in 18 travellers returning from Surinam and the Dominican Republic, The Netherlands, November 2015-March 2016.Infection2016; 44(6): 797-802.
[35] Kassavetis P, Joseph JM, Francois R, Perloff MD, Berkowitz AL. Zika virus-associated Guillain-Barré syndrome variant in Haiti.Neurology2016; 87(3): 336-337.
[36] Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, et al. Guillain-Barré syndrome associated with Zika virus infection.Lancet2016; 387(10026): 1482-1486.
[37] Reyna-Villasmil E, Lopez-Sanchez G, Santos-Bolívar J. Guillain-Barre syndrome due to Zika virus during pregnancy.Med Clin2016; 146(7):331-337.
[38] Fontes CA, dos Santos AA, Marchiori E. Magnetic resonance imaging findings in Guillain-Barré syndrome caused by Zika virus infection.Neuroradiology2016; 58(8): 837-838.
[39] do Rosário MS, de Jesus PA, Vasilakis N, Farias DS, Novaes MA,Rodrigues SG, et al. Guillain-Barré Syndrome after Zika virus infection in Brazil.Am J Trop Med Hyg2016; 95(5): 1157-1160.
[40] Fabrizius RG, Anderson K, Hendel-Paterson B, Kaiser RM, Maalim S, Walker PF. Guillain-Barré Syndrome associated with Zika virus infection in a traveler returning from Guyana.Am J Trop Med Hyg2016;95(5): 1161-1165.
[41] Zambrano H, Waggoner JJ, Almeida C, Rivera L, Benjamin JQ, Pinsky BA. Zika virus and chikungunya virus coinfections: A series of three cases from a single center in Ecuador.Am J Trop Med Hyg2016; 95(4):894-896.
[42] Arias A, Torres-Tobar L, Hernández G, Paipilla D, Palacios E, Torres Y, et al. Guillain-Barré syndrome in patients with a recent history of Zika in Cucuta, Colombia: A descriptive case series of 19 patients from December 2015 to March 2016.J Crit Care2017; 37: 19-23.
[43] Parra B, Lizarazo J, Jiménez-Arango JA, Zea-Vera AF, González-Manrique G, Vargas J, et al. Guillain-Barré syndrome associated with Zika virus infection in Colombia.N Engl J Med2016; 375(16): 1513-1523.
[44] Langerak T, Yang H, Baptista M, Doornekamp L, Kerkman T,Codrington J, et al. Zika virus infection and Guillain-Barré syndrome in three patients from Suriname.Front Neurol2016; 7: 233-237.
[45] Mécharles S, Herrmann C, Poullain P, Tran TH, Deschamps N, Mathon G, et al. Acute myelitis due to Zika virus infection.Lancet2016;387(10026): 1481-1486.
[46] Schwartzmann PV, Ramalho LN, Neder L, Vilar FC, Ayub-Ferreira SM, Romeiro MF, et al. Zika virus meningoencephalitis in an immunocompromised patient.Mayo Clin Proc2017; 92(3): 460-466.
[47] Carteaux G, Maquart M, Bedet A, Contou D, Brugières P, Fourati S, et al. Zika virus associated with meningoencephalitis.N Engl J Med2016;374(16): 1595-1596.
[48] Soares CN, Brasil P, Carrera RM, Sequeira P, De Filippis AB, Borges VA, et al. Fatal encephalitis associated with Zika virus infection in an adult.J Clin Virol2016; 83: 63-65.
[49] Sarno M, Sacramento GA, Khouri R, do Rosário MS, Costa F, Archanjo G. Zika virus infection and stillbirths: A case of hydropsfetalis,hydranencephaly and fetal demise.PLOS Negl Trop Dis2016; 10(2):517-521.
[50] Molko N, Simon O, Guyon D, Biron A, Dupont-Rouzeyrol M, Gourinat AC. Zika virus infection and myasthenia gravis: Report of 2 cases.Neurology2017; 88(11): 1097-1098.
[51] Oliveira CS, Vasconcelos PF. Microcephaly and Zika virus.J Pediatr2016; 92(2): 103-105.
[52] Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP,et al. Zika virus, French polynesia, South pacific, 2013.Emerg Infect Dis2014; 20(6): 1085-1090.
[53] Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. Zika: The origin and spread of a mosquito-borne virus.Bull World Health Organ2016;94(9): 675-686.
[54] Ramos da Silva S, Gao SJ. Zika virus: An update on epidemiology,pathology, molecular biology, and animal model.J Med Virol2016;88(8): 1291-1296.
[55] Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP,et al. Zika virus, French polynesia, South pacific, 2013.Emerg Infect Dis2014; 20(6): 1085-1090.
[56] Oehler E, Fournier E, Leparc-Goffart I, Larre P, Cubizolle S, Sookhareea C, et al. Increase in cases of Guillain-Barré syndrome during a Chikungunya outbreak, French Polynesia, 2014 to 2015.Euro Surveill2015; 20(48): 30079.
[57] Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al.Zika virus infects human cortical neural progenitors and attenuates their growth.Cell stem cell2016; 18(5): 587-590.
[58] Tricarico PM, Caracciolo I, Crovella S, D'Agaro P. Zika virus induces inflammasome activation in the glial cell line U87-MG.Biochem Biophys Res Commun2017; 492(4): 597-602.
[59] Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimarães KP,et al. The Brazilian Zika virus strain causes birth defects in experimental models.Nature2016; 534(7606): 267-271.
[60] Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells.Cell stem cell2016; 18(5):591-596.
[61] Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, et al.Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3.Cell stem cell2016; 19(2): 258-265.
[62] Teng Y, Liu S, Guo X, Liu S, Jin Y, He T, et al. An integrative analysis reveals a central role of P53 activation via MDM2 in Zika virus infection induced cell death.Front Cell Infect Microbiol2017; 7: 327-329.
[63] Zhu Z, Gorman MJ, McKenzie LD, Chai JN, Hubert CG, Prager BC, et al. Zika virus has oncolytic activity against glioblastoma stem cells.J Exp Med2017; 214(10): 2843-2857.
[64] Yoon KJ, Song G, Qian X, Pan J, Xu D, Rho HS, et al. Zika-virusencoded NS2A disrupts mammalian cortical neurogenesis by degrading adherens junction proteins.Cell Stem Cell2017; 21(3): 349-358.
[65] Oh Y, Zhang F, Wang Y, Lee EM, Choi IY, Lim H, et al. Zika virus directly infects peripheral neurons and induces cell death.Nat Neurosci2017; 20(9):1209-1212.
[66] Garcez PP, Loiola EC, da Costa RM, Higa LM, Trindade P, Delvecchio R, et al. Zika virus impairs growth in human neurospheres and brain organoids.Science2016; 352(6287): 816-818.
[67] Garcez PP, Loiola EC, da Costa RM, Higa LM, Trindade P, Delvecchio R, et al. Zika virus impairs growth in human neurospheres and brain organoids.Science2016; 352(6287): 816-818.
[68] Tetro JA. Zika and microcephaly: Causation, correlation, or coincidence.Microbes Infect2016; 18(3): 167-168.
[69] Silva AC, Moreira JM, Romanelli RM, Teixeira AL. Zika virus challenges for neuropsychiatry.Neuropsychiatr Dis Treat2016; 12: 1747-1751.
[70] Mehrjardi MZ, Keshavarz E, Poretti A, Hazin AN. Neuroimaging findings of Zika virus infection: A review article.Jpn J Radiol2016;34(12): 765-770.
[71] Acevedo N, Waggoner J, Rodriguez M, Rivera L, Landivar J, Pinsky B,et al. Zika virus, Chikungunya virus, and dengue virus in cerebrospinal fluid from adults with neurological manifestations, Guayaquil, Ecuador.Front Microbiol2017; 8: 42-45.
[72] Cumberworth SL, Barrie JA, Cunningham ME, de Figueiredo DP,Schultz V, Wilder-Smith AJ, et al. Zika virus tropism and interactions in myelinating neural cell cultures: CNS cells and myelin are preferentially affected.Acta Neuropathol Commun2017; 5(1): 50-51.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Biological, chemical and pharmacological aspects of Madhuca longifolia
- Scenario of dengue infection & its control in Pakistan: An up-date and way forward
- Cytotoxic, kinetics of inhibition of carbohydrate-hydrolysing enzymes and oxidative stress mitigation by flavonoids roots extract of Dicoma anomala (Sond.)
- Evaluation of antiparasitic, anticancer, antimicrobial and hypoglycemic properties of organic extracts from Panamanian mangrove plants
- Neuroprotection by misoprostol against rotenone-induced neurotoxicity in rat brain
- Effects of aqueous extract of Notobasis syriaca on lipopolysaccharideinduced inflammation in rats
