苗期干旱胁迫条件下外源ABA对甘薯膜透性和抗氧化酶系统的影响
2018-05-09张海燕段文学董顺旭解备涛汪宝卿张立明
张海燕,段文学,董顺旭,解备涛,汪宝卿,张立明
(1.山东省农业科学院 作物研究所,农业部黄淮海薯类科学观测实验站,山东 济南 250100;2.山东省农业科学院,山东 济南 250100)
Drought stress is a major factor in crop yield reduction[1]. As a hardy crop,sweetpotato is widely planted in hilly regions with poor fertility,drought,and eroded soil. Drought also limits the yield of sweetpotato[2]. The seedling stage is the first point where sensitivity is observed to drought stress[3-4]. In Northern China,sweetpotato seedlings are often subjected to drought stress which results in death and loss of production. It is beneficial to study the physiological and biochemical mechanisms involved in drought tolerance which can provide a theoretical basis for increasing the yield of sweetpotato.
Drought stress is a major abiotic stress,that adversely affects crop growth and productivity[5]. Much of the damage to crops caused by abiotic stresses is associated with oxidative damage at the cellular level[6]. Oxidative damage is a vital factor in relation to water-stress[7]. Malondialdehyde (MDA),which is thought to be important inducible factor of cell apoptosis if excessively accumulated in cells,is often regarded as a widely used marker of oxidative lipid injury whose concentration varies in response to biotic and abiotic stress[8]. Abiotic stresses lead to the overproduction of reactive oxygen species (ROS) which ultimately results in oxidative stress[9]. To avoid stress induced ROS accumulation,plants have developed antioxidant defense systems including antioxidant enzymes (superoxide dismutase (SOD),peroxidase (POD),catalase (CAT),ascorbate peroxidase (APX),glutathione reductase (GR),and glutathione peroxidase (GPX)) and non-enzyme antioxidants defense systems which work to protect plants against damage from abiotic stress[9]. As a result,tolerance to stress may be improved by the enhanced capacity of antioxidant generating systems[10].
In previous studies (2013-2015),we concluded that JS21 was drought tolerant,and JZ1 was drought sensitive[20]. The objectives of this research were to (i) study the differences in the response of membrane permeability and antioxidant enzymes to drought stress in two sweetpotato cultivars with different drought-tolerant abilities,and (ii) investigate the effects of exogenous ABA on the resistance to drought stress and antioxidant defense systems in sweetpotato.
1 Materials and Methods
1.1 Plant materials
Two sweetpotato(Ipomoeabatatas(L.) Lam)cultivars,Jishu 21 (JS21) and Jizishu 1 (JZ1),were used in this study. JS21 is tolerant to drought stress,whereas JZ1 is sensitive to drought stress[20].
1.2 Drought stress conditions
Sweetpotato seedlings (20 cm in height) cutting from seedling nursery were planted in culture boxes (40 cm×60 cm) filled with 10 L 1/2 Hoagland′s solution in a special hydroponic system(Tab.1). The seedlings were established with foam collars on a lid that completely covered the boxes. Oxygen pumps were used in the hydroponic system to ensure the growth of seedlings. The culture boxes were placed in an artificial climate chamber under conditions of 75% relative humidity,a photoperiod of 16 h/8 h light/dark,and constant illumination of 420 μmol/(m2·s).
After 10 days,the seedlings were subjected to three different treatments:(A) Control (JS21-C and JZ1-C);(B) PEG+Water (JS21-PEGW and JZ1-PEGW);(C) PEG+ABA (JS21-PEGA and JZ1-PEGA) (Tab.2). In each treatment,the seedlings were cultured in five boxes (20 seedlings/box). For the groups treated with (A),the sweetpotato seedlings were cultured in Hoagland all the time. For the groups treated with (B),3 days after spraying with water,the sweetpotato seedlings were grown in Hoagland solution with PEG6000 for another 12 days. The concentration of PEG6000 was increased to 5%,10%,15% and 20% at an interval of 3 days. For the groups treated with (C),foliar spray with 150 μmol/L ABA was applied 3 days before the drought stress treatment (Tab.2).
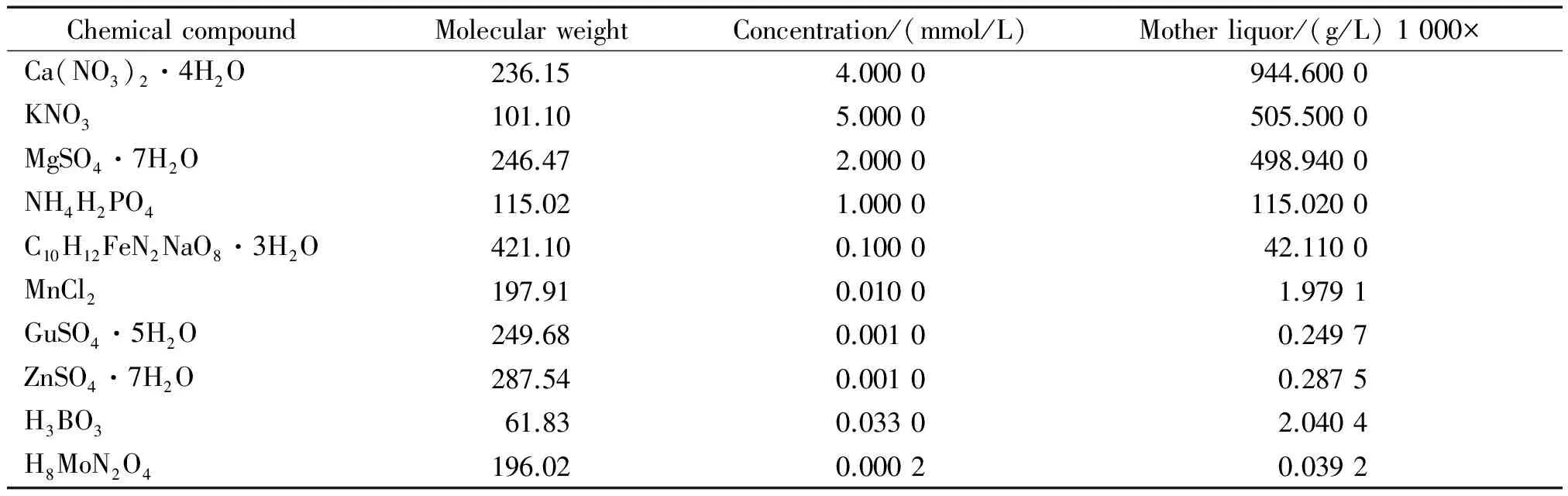
Tab.1 The formula of Hoagland′s solution

Tab.2 Timetable of solution culture,spraying,and drought stress
Note:JS21-C and JZ1-C.The control of JS21 and JZ1,always cultured in Hoagland;JS21-PEGW and JZ1-PEGW.Spraying with water before drought stress with PEG6000;JS21-PEGA and JZ1-PEGA.Spraying with 150 μmol/L ABA before drought stress with PEG6000.
1.3 Sampling methods
Five seedlings from each treated group were selected for sampling. Leaves (the 4th unfolded leaf on the main stem) and roots (the tip of roots) were sampled during three periods:10 days after growing in 1/2Hoagland solution (samples were marked as BS);3 days after foliar spray (samples were marked as BW);12 days after drought stress treatment (samples were marked as AW). The samples were frozen in liquid nitrogen until analysis.
1.4 Measurement of relative electrical conductivity
The relative electrical conductivity was measured according to Lutts et al[21]with modifications. Fresh leaves (0.1 g) were washed 2-3 times with deionized water. After a 24 h infusion in a 25 mL scale test tube with deionized water,the relative conductivity (L1) was measured. Samples were then heated at 100 ℃ for 30 min,cooled,and then supplemented with deionized water to the original scale. Then,the relative conductivity (L2) was measured for each sample. The relative conductivity=L1/L2×100%.
1.5 Measurement of malondialdehyde content
Lipid peroxidation was evaluated by measuring the malondialdehyde (MDA) content[13]. The MDA content was measured by the thiobarbituric acid (TBA) reaction method[12]with modifications. Leaf and root samples (0.5 g) were homogenized in 5 mL of 50 mmol/L cold phosphate Buffer (pH value 7.8) and cold-centrifuged at 10 000 r/min for 20 min. The supernatant (1 mL) was mixed with 2 mL 10% TCA containing 0.6% (m/V) TBA. The mixture was heated at 100 ℃ for 15 min and then cooled quickly on ice. After centrifugation at 4 000 r/min for 10 min,the absorbance of the supernatant was recorded at 600,532,450 nm. The MDA content(fresh weight) was calculated by the following formula:MDA(μmol/g) =(6.45×(D532-D600)-0.56×D450)×enzyme volume (mL)/fresh weight.
1.6 Measurement of root activity
Root activity was measured according to Zhang et al[22]with modifications. Root tips (0.5 g) were soaked in 10 mL of 0.4% triphenyltetrazolium chloride (TTC) and 0.2 mol/L cold phosphate Buffer (pH value 7),next,samples were placed in the dark for 2 h at 37 ℃,and then the reaction of root activity was terminated by 1 mol/L sulfuric acid. Following termination,samples were homogenized using 10 mL ethyl acetate,and then absorbance of the reaction solution was read at 485 nm.
1.7 Enzyme assays
Leaf and root samples (0.5 g) were homogenized using 5 mL of 50 mmol/L cold phosphate Buffer (pH value 7.8) and cold-centrifuged at 10 000 r/min for 20 min. The supernatant was subsequently used for assays of SOD,POD,CAT,and APX activities at 4 ℃.
Superoxide dismutase (SOD) activity was assayed by recording the enzyme mediated decrease in absorbance of the formazan made by superoxide radicals and nitroblue tetrazolium (NBT) dye[23]. The reaction mixture contained 50 mmol/L phosphate Buffer (pH value 7.8),130 mmol/L methionine,0.75 mmol/L NBT,0.1 mmol/L EDTA,0.02 mmol/L riboflavin and distilled water according to the proportion 15∶3∶3∶3∶3∶2.5. The 3 mL reaction mixture contained 0.02 mL of enzyme extract. The reaction was started by the addition of riboflavin,and the glass test tubes were shaken and placed under fluorescent lamps (0.06 mmol/(m2·s)). The reaction was stopped by switching off the light after 30 min. One unit of activity was defined as the amount of enzyme that would inhibit 50% of the NBT photoreduction at 560 nm.
Peroxidase (POD) activity was assayed according to Li et al[23]with modifications. The POD reaction mixture contained guaiacol (28 μL),0.1 mol/L phosphate Buffer (pH value 6.0,50 mL) and 30% H2O2(19 μL). The 3 mL reaction mixture contained 0.02 mL of enzyme extract. The reaction was initiated by adding the enzyme extract. Changes in the absorbance of the reaction solution at 470 nm were read every 30 s. The POD content(fresh weight) was calculated by the following formula:POD activity(A470/(min·g))=ΔA470× total enzyme volume (mL)×2/enzyme volume for determination/fresh weight.
Catalase (CAT) activity was assayed according to Agarwal et al[24]with modifications. The CAT reaction mixture contained 0.1 mol/L H2O2and 0.1 mol/L phosphate Buffer (pH value 7.0). The 3 mL reaction mixture contained 0.1 mL of enzyme extract. The reaction was initiated by adding the enzyme extract. Changes in absorbance of the reaction solution at 240 nm were read every 30 s. The CAT content(fresh weight) was calculated by the following formula:CAT activity(A240/(min·g))=ΔA240× total enzyme volume(mL)×2/enzyme volume for determination/fresh weight.
Ascorbate peroxidase (APX) was assayed according to Wang et al[12]with modifications. The 3 mL reaction mixture contained 50 mmol/L phosphate Buffer (pH value 7.0),5 mmol/L of ascorbic acid,0.1 mmol/L EDTA,20 mmol/L H2O2,and 0.1 mL of enzyme extract. The reaction was started by the addition of H2O2. Decrease in absorbance for a period of 30 s was measured at 290 nm. One unit of activity was defined as the amount of enzyme required to decrease 1 absorbance unit in the optical density at 290 nm per fresh weight per minute.
Glutathione reductase (GR) was assayed by using the method of Agarwal et al[24]with modifications. Leaf and root samples (0.2 g) were homogenized using 5 mL of 50 mmol/L cold phosphate Buffer (pH value 7.8) and cold-centrifuged at 12 000 r/min for 20 min. The supernatant was subsequently used for the GR activity assay. The reaction mixture contained 50 mmol/L cold phosphate Buffer (pH value 7.8),10 mmol/L oxidized glutathione (GSSG),2.4 mmol/L NADPH and 0.1 mL enzyme extract. The reaction was started by the addition of NADPH. Changes in absorbance of the reaction solution at 340 nm were read every 30 s. One unit of activity was defined as the amount of enzyme required to decrease 1 absorbance unit in the optical density at 340 nm per fresh weight per minute.
1.8 Statistical analyses
The experiment was set up in a randomized design with three replications. The data were statistically with Microsoft Excel 2016.All data presented were means over three replicates. Differences among means were compared by LSD multiple range tests at the 5% level of significance. The statistical significance of the difference between means was determined using the Duncan′s new multiple range test. Means within treatments and cultivars followed by a different letter were significantly different at the 0.05 probability level.
2 Results and Analysis
2.1 Effects of ABA on membrane permeability and antioxidant system in leaves of sweetpotato seedlings under drought stress
2.1.1 Relative electrical conductivity in leaves Before drought stress (BS and BW),the relative electrical conductivity of water treatment (JS21-PEGW and JZ1-PEGW) and ABA treatment (JS21-PEGA and JZ1-PEGA) in leaves were similar to those of the control (JS21-C and JZ1-C). After drought stress(AW),the relative electrical conductivity in leaves of JS21-PEGW significantly increased compared with JS21-C,the same as JZ1-PEGW. However the relative electrical conductivity in leaves of JS21-PEGA significantly decreased compared with JS21-PEGW,same tendency to JZ1-PEGA. Moreover,the relative electrical conductivity in leaves of JZ1-PEGW was significantly higher than that of JS21-PEGA. This indicated that drought stress resulted in the increase of the relative electrical conductivity in leaves of JS21 and JZ1,while spraying with exogenous ABA could inhibit the increase (Fig.1).
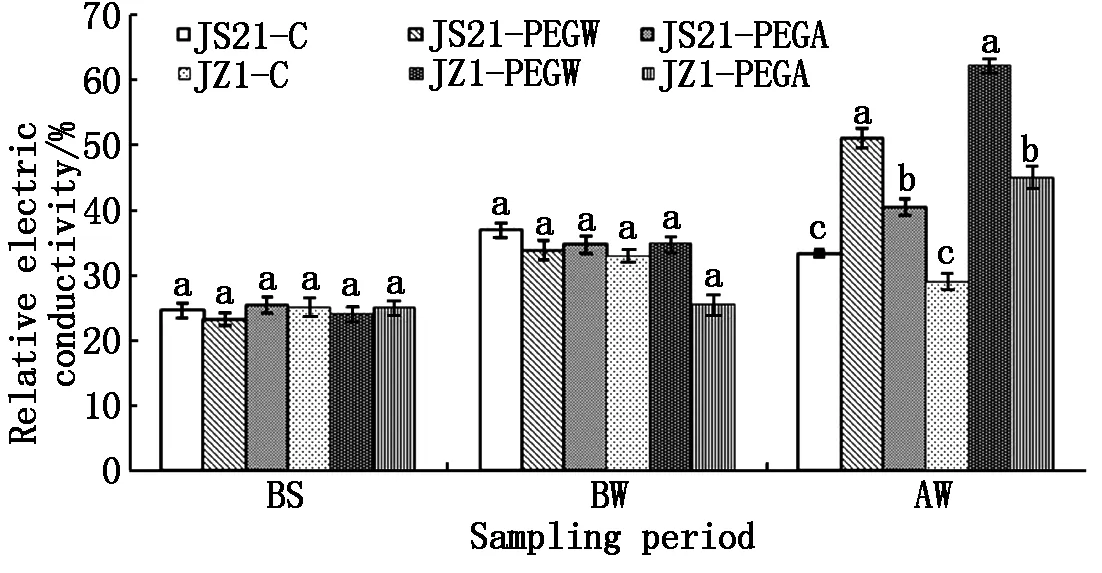
Data presented are mean of three replicates. Error bars represent standard deviations. In the same sampling period,values within treatments and cultivars followed by a different letter are significantly different at the 0.05 probability level.
Fig.1EffectsofexogenousABArelativeelectricalconductivityinleavesofsweetpotatoseedlingsunderdroughtstress
2.1.2 MDA content in leaves Before drought stress (BS and BW),the MDA content (fresh weight)of water treatment (JS21-PEGW and JZ1-PEGW) and ABA treatment (JS21-PEGA and JZ1-PEGA) in leaves were similar to those of the control (JS21-C and JZ1-C). After drought stress (AW),the MDA content in leaves of JS21-PEGW and JZ1-PEGW was significantly higher than those of JS21-C and JZ1-C,respectively. However the MDA content of JS21-PEGW,JS21-PEGA and JZ1-PEGA were all significantly lower than those of JZ1-PEGW.Moreover,the MDA content in leaves of JZ1-PEGW was significantly higher than those of JS21-PEGW and JS21-PEGA. This indicated that drought stress resulted in the increase of the MDA content in leaves of JS21 and JZ1,the drought-sensitive genotypes JZ1 was significantly higher than the drought-tolerant genotypes JS21. Spraying with exogenous ABA could significantly inhibit the increase of MDA content in leaves of the drought-sensitive genotypes JZ1(Fig.2).
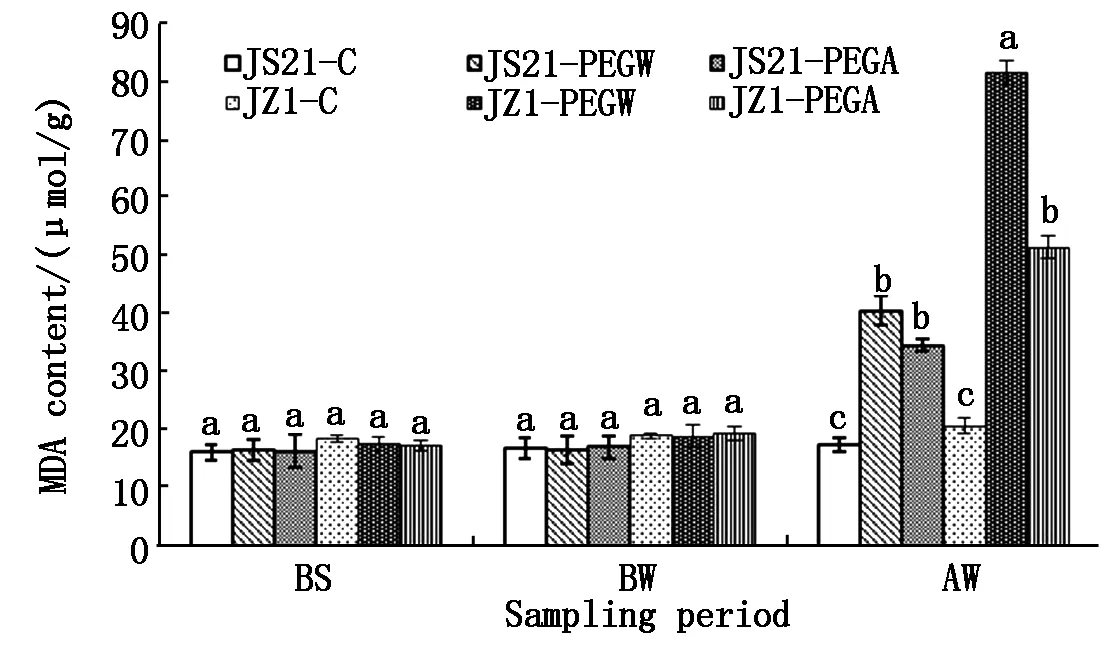
Fig.2 Effects of exogenous ABA on MDA content in leaves of sweetpotato seedlings under drought stress
2.1.3 Antioxidant enzyme activities in leaves Activities of several representative antioxidant enzymes,including SOD,POD,CAT,APX and GR in the two genotypes were determined to assess the function of ABA in regulation of these antioxidant enzymes in leaves under drought stress. After drought stress (AW),the SOD,POD,CAT,APX and GR activities in leaves were significantly higher in drought-tolerant genotypes JS21-PEGW than drought-sensitive genotypes JZ1-PEGW under drought stress. The SOD,POD and APX
activities in leaves of JS21-PEGW were significantly higher than those of JS21-C,however the GR activities in leaves of JZ1-PEGW was significantly lower than that of JZ1-C(Fig.3).
The reduced activities of antioxidant enzymes in leaves caused by drought stress in JZ1 were restored to higher level compared with their controls by application of exogenous ABA,however the POD,CAT,APX and GR activities were still significantly lower in drought-sensitive genotypes JZ1-PEGA than drought-tolerant genotypes JS21-PEGA. Exogenous ABA caused a considerable increase in the enzyme activities in both JS21 and JZ1 compared with water treatment,the POD,CAT,APX and GR activities in leaves of ABA treatment (JS21-PEGA and JZ1-PEGA) were significantly higher than those of water treatment (JS21-PEGW and JZ1-PEGW),the SOD activities in leaves of JZ1-PEGA were significantly higher than that of JZ1-PEGW. Moreover,the five enzymes activities in ABA-treated JS21-PEGA were the highest and water-treated JZ1-PEGW were the lowest(Fig.3).
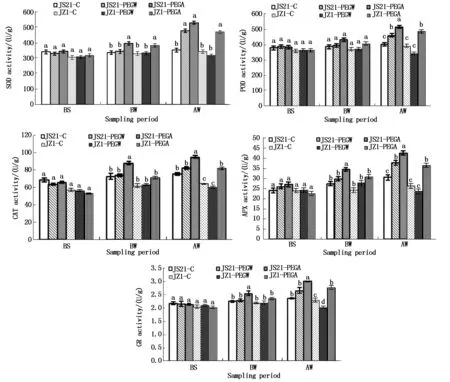
Fig.3 Effects of exogenous ABA on activities of SOD,POD,CAT,APX and GR in leaves of sweetpotato seedlings under drought stress
The results showed that antioxidant enzyme activities in leaves increased in drought-tolerant genotypes JS21,but decreased in drought-sensitive genotypes JZ1 under drought stress. Exogenous ABA could increase antioxidant enzyme activities in leaves of drought-tolerant genotypes JS21 and drought-sensitive genotypes JZ1. After drought stress (AW),antioxidant enzyme activities in leaves of drought-tolerant genotypes JS21-PEGW were always significantly higher than those of drought-sensitive genotypes JZ1-PEGW,and those of JS21-PEGA were significantly higher than those of JZ1-PEGA,except for SOD activity.
2.2 Effects of ABA on membrane permeability and antioxidant system in roots of sweetpotato seedlings under drought stress
2.2.1 Root activity Before drought stress (BS and BW),the root activity of water treatment (JS21-PEGW and JZ1-PEGW) and ABA treatment (JS21-PEGA and JZ1-PEGA) were similar to those of the control (JS21-C and JZ1-C). After drought stress (AW),the root activity for JZ1-PEGW was significantly lower than JS21-PEGW,all of which was decreased compared to their controls (JS21-C and JZ1-C). The root activity of JS21-PEGW and JZ1-PEGW for water treatment under drought stress was 0.60 and 0.32 fold compared to JS21-C and JZ1-C,respectively (Fig.4).
The reduced root activity caused by drought stress in JS21 and JZ1 were restored to higher level compared with their controls by application of exogenous ABA. The root activity of ABA treatment (JS21-PEGA and JZ1-PEGA) were significantly higher than those of water treatment (JS21-PEGW and JZ1-PEGW),respectively. However the root activity of JZ1-PEGA was still significantly lower than that of JZ1-C and JS21-PEGA.Moreover,the root activity of JZ1-PEGW was the lowest(Fig.4).
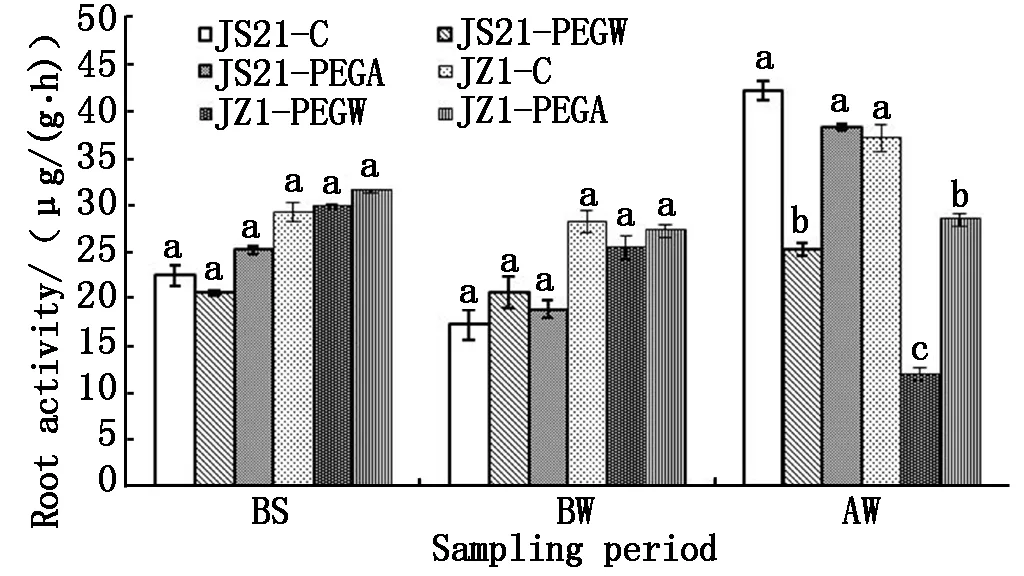
Fig.4 Effects of exogenous ABA on root activity of sweetpotato seedlings under drought stress
2.2.2 MDA content in roots Before drought stress (BS and BW),the MDA content in roots of water treatment (JS21-PEGW and JZ1-PEGW) and ABA treatment (JS21-PEGA and JZ1-PEGA) were similar to those of the control (JS21-C and JZ1-C). After drought stress (AW),the MDA content in roots of JS21-PEGW and JZ1-PEGW was significantly higher than those of JS21-C and JZ1-C,and they were increased 1.90 and 3.20 fold compared to JS21-C and JZ1-C,respectively. However the MDA content in roots of JS21-PEGW was significantly lower than that of JZ1-PEGW(Fig.5).
The increased MDA content caused by drought stress in JS21 and JZ1 were restored to lower level compared with their controls by application of exogenous ABA,the MDA content in roots of JZ1-PEGA was significantly lower than that of JZ1-PEGW,but the MDA content in roots of JS21-PEGA and JZ1-PEGA were still significantly higher than their controls (JS21-C and JZ1-C),respectively. Moreover,the MDA content in roots of JZ1-PEGW was significantly higher than those of JS21-PEGW and JS21-PEGA. Exogenous ABA inhibits the increase in MDA content in the roots of the two cultivars. The inhibition was significantly higher for the drought-sensitive cultivars than for the drought-tolerant cultivars (Fig.5).
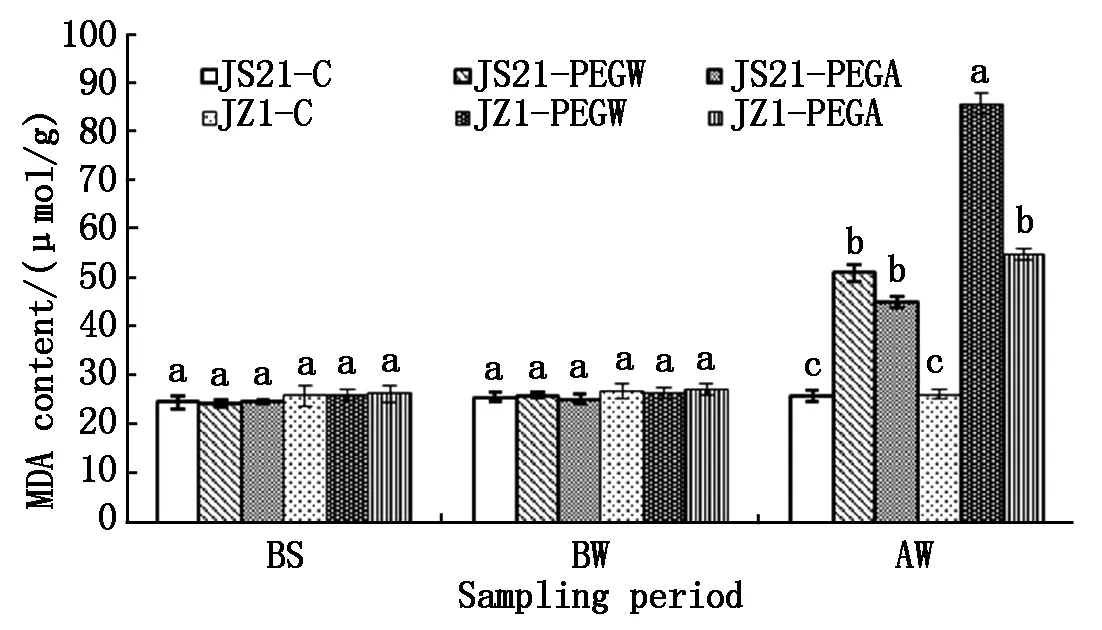
Fig.5 Effects of exogenous ABA on MDA content in roots of sweetpotato seedlings under drought stress
2.2.3 Antioxidant enzyme activities in roots Activities of SOD,POD,CAT,APX and GR in the two genotypes were determined to assess the function of ABA in regulation of these antioxidant enzymes in roots under drought stress. After drought stress (AW),the SOD,POD,CAT,APX and GR activities in roots were significantly higher in drought-tolerant genotypes JS21-PEGW than drought-sensitive genotypes JZ1-PEGW under drought stress. The SOD,POD and APX activities in roots of JS21-PEGW were significantly higher than those of JS21-C,however the APX activities in roots of JZ1-PEGW was significantly lower than that of JZ1-C (Fig.6).
The reduced activities of antioxidant enzymes in roots caused by drought stress were restored to higher level compared with their controls by application of exogenous ABA. The SOD,POD,CAT,APX and GR activities in roots of JZ1-PEGA was significantly higher than that of JZ1-PEGW and JZ1-C. However the POD,CAT,APX and GR activities in roots were still significantly lower in drought-sensitive genotypes JZ1-PEGA than drought-tolerant genotypes JS21-PEGA. Moreover,the five enzymes activities in roots of water-treated JZ1-PEGW were the lowest (Fig.6).
Exogenous ABA also caused a considerable increase in the enzyme activities in drought-tolerant genotypes JS21 compared with water treatment,the five activities in roots of JS21-PEGA was significantly higher than that of JS21-C,and the POD,CAT and GR was significantly higher than that of JS21-PEGW.Moreover,the five enzymes activities in ABA-treated JS21-PEGA were the highest (Fig.6).
The results showed that antioxidant enzyme activities in roots increased in drought-tolerant genotypes JS21,but decreased in drought-sensitive genotypes JZ1 under drought stress. Exogenous ABA could increase antioxidant enzyme activities in roots of drought-tolerant genotypes JS21 and drought-sensitive genotypes JZ1. After drought stress (AW),antioxidant enzyme activities in roots of drought-tolerant genotypes JS21-PEGW were always significantly higher than those of drought-sensitive genotypes JZ1-PEGW,and those of JS21-PEGA were significantly higher than those of JZ1-PEGA,except for SOD activities.
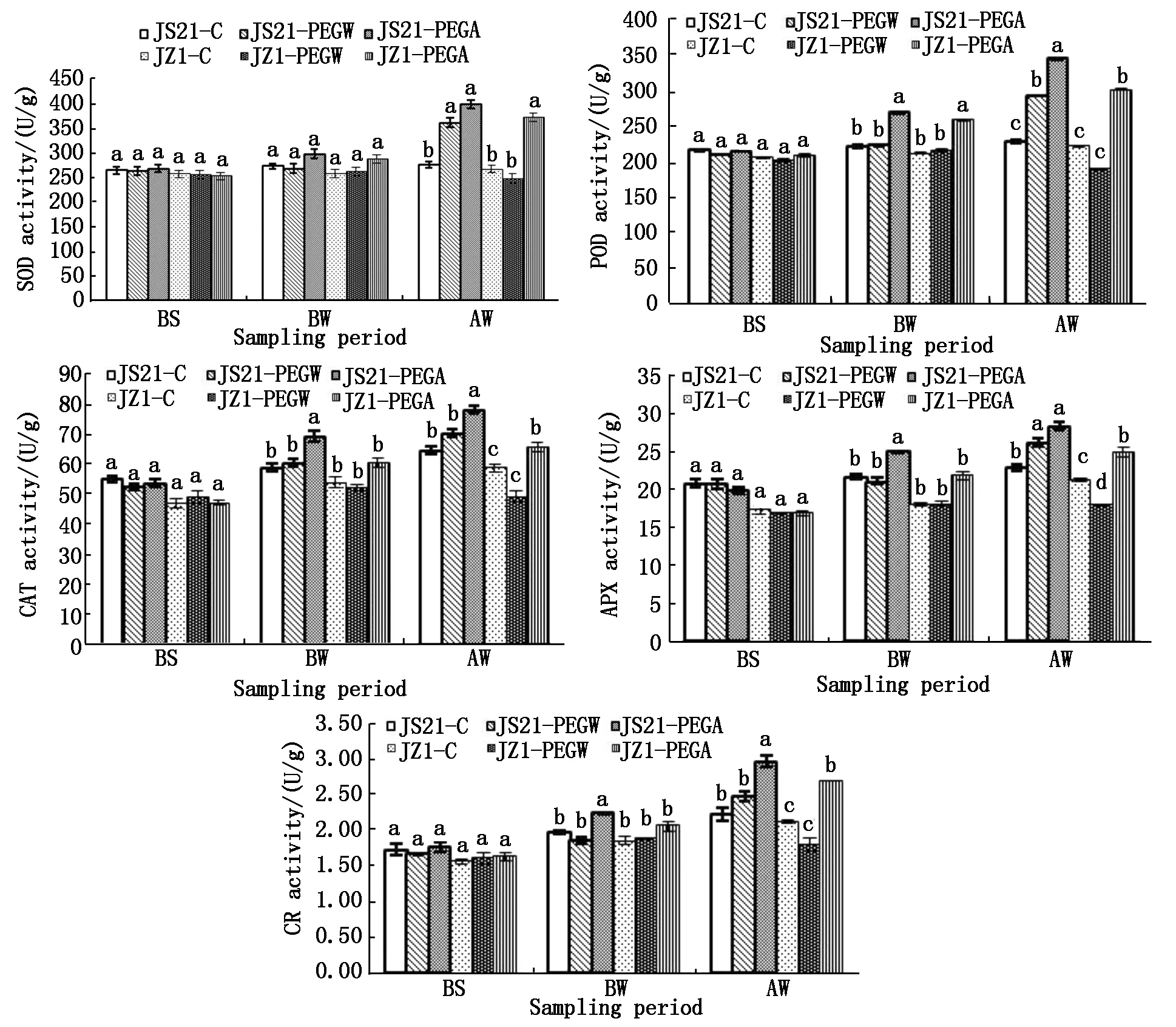
Fig.6 Effects of exogenous ABA on activities of SOD,POD,CAT,APX and GR in roots of sweetpotato seedlings under drought stress
3 Discussion and Conclusion
Drought stress is a constraint to sweetpotato production in many parts of China. Previous studies have revealed that drought stress is one of the major abiotic stresses accompanied by suppression of sweetpotato storage root yield and quality[2,25]. Thus,it is necessary to study the mechanism of drought resistance in sweetpotato. Many studies on the improvement of stress resistance of crops by exogenous ABA have been reported[12,18-19]. The aims of this study were to investigate the effects of exogenous ABA on membrane permeability and the antioxidant defense systems of seedlings on protection against drought stress by the use of two sweetpotato cultivars with different drought-tolerant abilities.
Drought damage to crops is associated with various abiotic stresses. Various abiotic stresses lead to the overproduction of ROS in plants which causes damage to proteins,lipids,carbohydrates,and DNA,which results in oxidative stress[9]. The relative electrical conductivity is an important measure of membrane permeability. MDA is a membrane-lipid peroxidation product,which reflects damage to membrane integrity. In this study,the relative electrical conductivity in leaves,as well as the MDA content in leaves and roots were increased significantly compared to their control for both the drought-sensitive cultivar JZ1 and the drought-tolerant cultivar JS21. Under drought stress,the MDA content in leaves and roots of JZ1 were significantly higher than those of JS21. Therefore,the results showed that drought stress resulted in the increase of the relative electrical conductivity and the MDA content,the MDA content of the drought-sensitive genotypes JZ1-PEGW was significantly higher than that of the drought-tolerant genotypes JS21-PEGW. The results presented here were consistent with the results of previous studies that show oxidative damage to membrane permeability was significant in drought-sensitive cultivars under drought stress in many crops[26-27].
Root activity is a physiological index reflecting the ability of roots to absorb water and nutrients,to synthesize certain compounds,and to either oxidize or reduce elements in the surrounding rhizosphere[28]. Previous studies have confirmed that roots activity decreased under drought stress[29]. The results was that,root activity suppressed in comparison to control in seedling stage for JS21 and JZ1 under drought stress. And it was significantly lower for drought-sensitive cultivar JZ1 than that of the drought-tolerant cultivar JS21. The results indicated that drought stress firstly restrained the growth of roots,and then affect the growth of shoots and leaves,ultimately lead to the damage of membrane permeability and antioxidant system in leaves and roots. Exogenous ABA significantly increased root activity of both cultivars,the results showed that ABA pre-treatment could improve root traits and enhance water absorption,therefore increased the resistance of sweetpotato to drought stress.
In this study,exogenous ABA significantly inhibited the increase of the relative conductivity in leaves,the increase of the MDA content in leaves and roots,and the decrease of root activity in two cultivars under drought stress. The results were in accordance with previous reports which found that the increased tolerance with exogenous ABA was associated with a lower accumulation of MDA[12]. ABA pre-treatment could increase SOD,POD,CAT,APX and GR activities in crops under environmental stresses such as drought stress[17,30],cold stress[12]and other oxidative stresses[16,31-32]. In this study,we observed that exogenous ABA caused a significant increase in SOD,POD,CAT,APX and GR activities in leaves and roots in two cultivars under drought stress. In addition,we found that the activities of antioxidant enzymes were significantly higher in the drought-tolerant cultivars (JS21-PEGA) for ABA treatment than those in the drought-sensitive cultivars (JZ1-PEGA). This showed that the effect of exogenous ABA-improved drought tolerance was more significant in drought-tolerant cultivars than in drought-sensitive cultivars.The results were in accordance with previous reports which have shown a higher accumulation of ABA in drought tolerant wheat cultivars than susceptible ones in response to drought stress[33]. Additional reports have shown that exogenous ABA is vital in increasing drought tolerance and plays an important role in enhancing drought stress induced antioxidant defense against oxidative stress[34]. Therefore,the study indicated that exogenous ABA could increase the drought tolerance of sweetpotato by improving the capacity of antioxidant defense systems under drought stress.
The studies showed that the drought tolerance of sweetpotato was closely correlated to the activities of antioxidant enzyme and the responses of membrane permeability to drought stress. Drought-tolerant cultivars have better resistance to drought stress compared with drought-sensitive cultivars by maintaining lower relative electrical conductivity and MDA content,higher root activities and antioxidant enzyme activities,all of which enhanced the tolerance of sweetpotato to drought stress. Additionally,the results suggest that ABA has play an important role in conferring drought tolerance in sweetpotato. Exogenous ABA increased antioxidant defense capacity and root activity,decreased MDA and ROS level and relative conductivity,which alleviated the damage of drought stress on membrane integrity and subsequently increased the tolerance to drought stress in sweetpotato.
参考文献:
[1] Hall A J,Richards R A. Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops[J]. Field Crops Research,2013,143(SI):18-33.
[2] Lewthwaite S L,Triggs C M. Sweetpotato cultivar response to prolonged drought[J]. Agronomy New Zeal ,2012,42:1-10.
[3] Zhu L D,Shao X H,Zhang Y C,et al. Effects of potassium fertilizer application on photosynthesis and seedling growth of sweet potato under drought stress[J]. Journal of Food Agriculture & Environment,2012,10(3/4,1):487-491.
[4] Solis J,Villordon A,Baisakh N,et al. Effect of drought on storage root development and gene expression profile of sweetpotato under greenhouse and field conditions[J]. Journal of the American Society for Horticultural Science,2014,139(3):317-324.
[5] Kim Y H,Jeong J C,Lee H S,et al. Comparative characterization of sweetpotato antioxidant genes from expressed sequence tags of dehydration-treated fibrous roots under different abiotic stress conditions[J]. Mol Biol Rep,2013,40(4):2887-2896.
[6] Fang Y J,Xiong L Z. General mechanisms of drought response and their application in drought resistance improvement in plants[J]. Cellular and Molecular Life Sciences,2015,72(4):673-689.
[7] Srivalli B,Sharma G,Khanna-Chopra R. Antioxidative defense system in an upland rice cultivar subjected to increasing intensity of water stress followed by recovery[J]. Physiologia Plantarum,2003,119(4):503-512.
[8] Liu H Z,Zhang G S,Wang J S,et al. The relation ship between male sterility and membrane lipid peroxidation and antioxidant enzymes in wheat (TriticumaestivumL.)[J]. Turk J Field Crops,2015,20(2):179-187.
[9] Gill S S,Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants[J]. Plant Physiology and Biochemistry,2010,48(12):909-930.
[10] Li Z,Zhou H,Peng Y,et al. Exogenously applied spermidine improves drought tolerance in creeping bentgrass associated with changes in antioxidant defense,endogenous polyamines and phytohormones[J]. Plant Growth Regulation,2015,76(1):71-82.
[11] Elansary H O,Yessoufou K. Growth regulators and mowing heights enhance the morphological and physiological performance of seaspray turfgrass during drought conditions[J]. Acta Physiologiae Plantarum,2015,37(11):1-11.
[12] Wang G J,Miao W,Wang J Y,et al. Effects of exogenous abscisic acid on antioxidant system in weedy and cultivated rice with different chilling sensitivity under chilling stress[J]. Journal of Agronomy and Crop Science,2013,199(3):200-208.
[13] Ding W,Song L,Wang X,et al. Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes ofPhragmitescommunis[J]. Biologia Plantarum,2010,54(4):607-613.
[14] Zhang M S,Xie B,Tan F. Relationship between changes of endogenous hormone in sweet potato under water stress and variety drought-resistance[J]. Agr Sci China,2002,1(6):626-630.
[15] Li J,Wang X Q,Watson M B,et al. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase[J]. Science,2000,287(5451):300-303.
[16] Pal M,Janda T,Szalai G. Abscisic acid May alter the salicylic acid-related abiotic stress response in maize[J]. Journal of Agronomy and Crop Science,2011,197(5):368-377.
[17] Du Y L,Wang Z Y,Fan J W,et al. Exogenous abscisic acid reduces water loss and improves antioxidant defence,desiccation tolerance and transpiration efficiency in two spring wheat cultivars subjected to a soil water deficit[J]. Functional Plant Biology,2013,40(5):494-506.
[18] Marcinska I,Czyczylo-Mysza I,Skrzypek E A,et al. Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings[J]. International Journal of Molecular Sciences,2013,14(7):13171-13193.
[19] Mahouachi J,Argamasilla R,Gomez-Cadenas A. Influence of exogenous glycine betaine and abscisic acid on papaya in responses to water-deficit stress[J]. Journal of Plant Growth Regulation,2012,31(1):1-10.
[20] Yuan Z,Wang B Q,Jiang Y,et al. Seedling screening of drought resistance varieties of sweetpotato and drought resistance index research[J]. Shandong Agricultural Science,2015,47:22-26,36.
[21] Lutts S,Kinet J M,Bouharmont J. NaCl-induced senescence in leaves of rice (OryzasativaL.) cultivars differing in salinity resistance[J]. Ann of Bot,1996,78(3):389-398.
[22] Zhang D,Liu G S,Zhang J X,et al. Effect of different topping time on activity of root system and accumulation of nicotine in tobacco plants[J]. Chin Tob Sci,2006(1):38-41.
[23] Li H,Sun Z Q,Sun Z. Principles and techniques of plant physiological biochemical experiment[M]. Beijing:Higher Education Press,2000.
[24] Agarwal S,Sairam R K,Srivastava G C,et al. Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes[J]. Biologia Plantarum,2005,49(4):541-550.
[25] Mwije A,Mukasa S B,Gibson P,et al. Heritability analysis of putative drought adaptation traits in sweet potato[J]. African Crop Sci J,2014,22:79-87.
[26] Nikolaeva M K,Maevskaya S N,Voronin P Y. Activities of antioxidant and osmoprotective systems and photosynthetic gas exchange in maize seedlings under drought conditions[J]. Russian Journal of Plant Physiology,2015,62(3):314-321.
[27] Khoyerdi F F,Shamshiri M H,Estaji A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress[J]. Scientia Horticulturae,2016,198:44-51.
[28] Luo H H,Zhang Y L,Zhang W F. Effects of water stress and rewatering on photosynthesis,root activity,and yield of cotton with drip irrigation under mulch[J]. Photosynthetica,2016,54(1):65-73.
[29] Liu H L,Zheng G Z,Guan J F,et al. Changes of root activity and membrane permeability under drought stress in maize[J]. Acta Agriculturae Boreali-Sinica,2002,17(2):20-22.
[30] Wu M Y,Li X,He Y F,et al. Drought response in overexpression of maize phosphoenolpyruvate carboxylase rice seedlings treated by inhibitors of ABA and HXK pathway[J]. Bulletin of Botanical Research, 2017,37:402-415.
[31] Xiao Q,Wang G,Yi Y J,et al. Enhancing the salt tolerance of sweet potato seedlings through exogenous abscisic acid[J]. Journal of Plant Nutrition and Fertilizer, 2016,22(1):201-208.
[32] Li J,Zhang L F,Jian J,et al. Effect of exogenous abscisic acid on growth of maize seedling under low temperature stress[J]. Journal of Northeast Agricultural University, 2015,46:1-7.
[33] Chandrasekar V,Sairam R K,Srivastava G C. Physiological and biochemical responses of hexaploid and tetraploid wheat to drought stress[J]. Journal of Agronomy & Crop Science,2000,185(4):219-227.
[34] Jiang M Y,Zhang J H. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves[J]. Journal of Experimental Botany,2002,53(379):2401-2410.
