High expression of TRAF4 in hepatocelullar carcinoma as an independent negative survival and recurrence predictor
2018-05-09RuCuiYuPingShengFanWeiWangWeiJia
Ru-Cui Yu, Ping-Sheng Fan, Wei Wang, Wei Jia
1School of Clinical Medicine, Shan Dong University, China
2Department of Chinese Medicine Oncology, Anhui Provincial Hospital, China
3The Cancer Hospital of Anhui Province, Provincial Hospital of Anhui Medical University, China
4Department of Medical Oncology, Anhui Provincial Hospital, Anhui Medical University, Hefei, China
1. Introduction
Hepatocellular carcinoma (HCC), the sixth most common cancer and the third most common cause of cancer mortality worldwide[1],is a major problem worldwide, had more than 748 000 new cases and responsible for more than 695 000 deaths in 2008[2].Appropriate markers for early diagnosis of this tumor are still lacking and liver transplant, interventional radiology treatment,and chemoembolization for unresectable HCC remain the main choices for HCC therapy today, even if the survival probability is limited[3]. Most patients with resection have a high tendency to have recurrent diseases, particularly those with larger tumors or tumors displaying vascular invasion[4]. High recurrence rate and low long-term survival rate are still common in patients with unresectable HCC, so more effective and safer treatments are urgently needed[5]. Many patients with HCC suffer from severe prognosis because of the lack of early specific diagnosis[6]. Alpha fetoprotein is the most commonly used to diagnose liver cancer.Similarly, descarboxyprothrombin, also known as prothrombin induced by vitamin K absenceⅡlevels promote in most patients with HCC[7]. The screening of more biomarkers for progression and recurrence of HCC in targeted therapy is urgently demanded. As a highly angiogenic cancer and with the display of marked vascular abnormalities[8], its aggressiveness and poor prognosis is conduced to tumor angiogenesis[9]. As a result, a potential therapeutic treatment can be achieved by the targeting of angiogenesis.
Tumor necrosis factor receptor-associated factor 4 (TRAF4),formerly known as CART1 (cysteinerich motif associated with RING and TRAF domains), was found by an including lymph node that contained metastatic tumor cells from breast cancer[10]. Data analysis from TCGA database shows thatTRAF4gene is amplified in many other types of cancer, such as ovarian cancer and bladder cancer, which indicates that TRAF4 is carcinogenic and its role is not limited to breast cancer[11]. Further studies have shown that TRAF4 high expression is a tumor but has a common feature.In vivoexperiment involves establishing animal metastasis models, and it has a crucial role, which is further confirmed by the sample analysis of human patients, but it is associated with poor prognosis[12]. Tumor angiogenesis contributes to the invasiveness and poor prognosis of liver cancer. The mechanism of TRAF4 in the angiogenesis of HCC is still unclear. Vascular endothelial growth factor (VEGF) is one of the earliest angiogenic peptides found and the most widely studied angiogenic factors. Immunohistochemical method was used to observe the expression of TRAF4, VEGF and CD34 labeled microvessel density (MVD) in hepatocellular carcinoma tissues and adjacent tumor tissues. In addition, survival analysis was performed by Kaplan Mayer and Cox regression. All the above mentioned purposes were to prove the relationship between the expression of TRAF4 in HCC and prognosis and clinicopathological factors.
2.Materials and methods
2.1. Tissue samples
There were 90 consecutive cases of locally advanced HCC(Karnofsky≥60%/Child-Pugh≤12) and paired adjacent normal hepatic tissues were collected from patients undergoing curative liver resection during August 2006 to November 2009 at Anhui Provincial Hospital in our single-center retrospective study. Locally advanced HCC were defined as one nodule was larger than 5 cm or more than 3 nodules were larger than 3 cm in diameter. All the patients had neither other malignant tumors nor any anticancer therapies before surgery. The tumor differentiation degree is defined based on the Edmondson classification system[13]. All cases had complete clinical data including disease-free survival time (DFS) and overall survival time (OS). The pathological diagnosis was squamous cell carcinoma and the double blind method was applied. The duration of follow-up was from surgery to death or deadline (September 20th, 2013). All patients detected by TRAF4 signed informed consent. The study was approved by the ethics committee of Provincial Hospital of Anhui Medical University.
2.2. Immunohistochemistry
The detection of the expression levels of TRAF4 protein was achieved by immunohistochemistry staining using Envision kit method. A primary rabbit polyclonal anti-TRAF4 antibody,bought from Abcam Ltd, Shanghai, China, was used at a working concentration of 1:300. In short, 4 microns thick slices were divided from all formalin fixed paraffin embedded specimens. Dewaxing,after rinsing by heating the specimen of incubated antigen repair in pH 6 citrate buffer, was completed with a microwave oven.The sections, washed three times in a buffer, were then incubated with primary antibody above room temperature for 2 h following blocking endogenous peroxidases. Then the coloring of the sections was completed after incubating with secondary antibody (mouse antirabbit IgG, Zhongshan Jinqiao Co., Beijing, China) at room temperature for 30 min. The positive tissue slices and glass slides treated with phosphate buffer saline were positively and negatively controlled.
2.3. Result evaluation
An independent and blind estimation of the stained section was fulfilled by two experienced pathologists. The staining intensity grading were negative, 0; light yellow, 1; brownish yellow, 2; brown,3. Staining positive tumor cells were classified into positive cells<5%, 0; positive cells 5%-25%, 1; positive cells were 25%-50%, 2;positive cells >50%, 3. The score=grade in stain intensity×grade in percentage of positive cell. Scores more than 4 was regarded as high expressions; On the other hand, low and even no expressive slices were contained in low expression groups.
2.4. MVD assessment
The staining of vascular endothelial cells with CD34 antibody and the calculation of CD34 positive MVD were achieved. Five distinctly prominent vascular (hot) regions were selected, and the number of ships is at a high multiple (400×). An average count was calculated by average measurements. An independent evaluation of all the biopsies was done by two pathologists who did not have the same pathology, and the differences were resolved by consensus.
2.5. Statistical analysis
SPSS software (version 13.0) was used to analyze the data.The expression of TRAF4 was correlated with clinicopathologic characteristics byχ2or Kruskal-Wallis analysis. The detection of TRAF4 expression was performed by Spearman rank correlation test and the comparison of the correlation between survival records and the expression of proteins was conducted by Kaplan-Meier curve and log-rank test. The assessment of Multivariate analysis was realized by using the Cox regression model. AllP< 0.05 was viewed as being full of statistical significances.
3. Results
3.1. TRAF4 expression in HCC and its relationship with clinical pathological factors
In this study, TRAF4 expression was mainly found in tumor cytoplasm around HCC cells (Figure 1), only a weak or no TRAF4 expression was shown in healthy hepatic tissues according to immunohistochemitry detection. The positive rate of TRAF4 expression was 57.8% (52/90) in HCC cancer tissues and 22.2%(20/90) in adjacent non-tumoral hepatic tissues showing significant difference between cancer tissues and cancer adjacent hepatic tissues (P<0.001) (Table 1). Besides, significant relationship was found between the positive expression of TRAF4 in HCC and tumor size (P=0.001) and tumor stage (P<0.05). However, no significant relationship was found between TRAF4 expression and gender, age,differentiation of grade, venous invasion (P>0.05, Table 2).
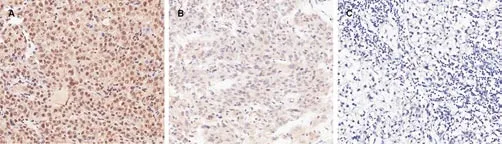
Figure 1. Expression of TRAF4 in HCC tissues by immunohistochemistry.TRAF4 expression showed diffuse cytoplasmic staining in tumor cells.A. ++TRAF4 staining(200×); B. +TRAF4 staining (200×); C. Negative expression of TRAF4 (200×).

Table 1Expression of TRAF4 in HCC tissues and corresponding paracarcinomatous tissues.

Table 2Relationship between TRAF4 and clinicopathological characteristics in 90 locally advanced HCC patients.
3.2. Correlation between TRAF4 and VEGF protein expression in HCC tissues
Immunohistochemical method was applied to detect expression rate of VEGF and CD34 in patients with HCC in order to confirm whether TRAF4 is indeed associated with aniogenesis in HCC tissues or not. The results showed stained VEGF was mainly localized in cytoplasm. A total of 56 out of the 90 HCC samples showed VEGF positivity (62.2%; Figure 2B); and 42.2% (38/90)of the samples were positive for both TRAF4 and VEGF. A total of 22.2% (20/90) samples were negative for both TRAF4 and VEGF expressins. A total of 64.4% (58/90) showed consistency and 35.6% (32/90) showed the discrepancy between TRAF4 and VEGF expression. There was a positive correlation between TRAF4 and VEGF over-expression (r=0.262;P=0.013, Table 3).

Figure 2. Expression of TRAF4 ,VEGF and CD34 in HCC tissues by immunohistochemistry.

Table 3The expression correlation bewteen TRAF4 and VEGF cases.
3.3. Relationship between TRAF4 expression and MVD in HCC tissues
A measuring of MVD by CD34 immunohistochemical staining of the endothelial cells is conducted in order to explore the relationship between TRAF4 expression and angiogenesis (Figure 3). The MVD in HCC tissues ranged from 0 to 138/200 per field (median, 64/200 per field). Immunohistochemical staining detection of CD34 for MVD in HCC tissues showed tumor tissues with positive TRAF4 expression had a significantly higher MVD compared to tumors with negative TRAF4 expression (41.6±6.3vs.24.5±6.7,P<0.001, Figure 3).

Figure 3. Immunohistochemical staining of CD34 for MVD in HCC tissues.Tumors with positive TRAF4 expression had a significantly higher MVD compared to tumors with negative TRAF4 expression (P<0.001). Data are expressed as the number of CD34-positive microvessels per field.
3.4. Survival analysis of TRAF4
Kaplan Meier analysis showed that DFS time in locally advanced HCC patients with positive expression of TRAF4 was shorter than those with negative expression rate of TRAF4 (median: 23.0 monthsvs.49.6 months,P=0.001; Figure 4A). Similarly, OS time of patients with positive TRAF4 expression was shorter than that with negative TRAF4 expression (29.0vs.53.3 months,P<0.001; Figure 4B).
What’s more, a significant relation between the prognosis and TRAF4 expression (P=0.001), tumor size (P=0.001) and tumor stage (P<0.05) (Table 4) according to univariate analysis. It was shown that TRAF4 was an independent prognostic factor for HCC according to multivariate Cox regression analysis (Table 5).

Figure 4. Kaplan-Meier analysis of DFS and OS curves of patients with HCC based on TRAF4 expression as positive or negative. A. DFS curve of patients with HCC based on TRAF4 expression. B. OS curve of patients with HCC based on TRAF4 expression.

Table 4Univariate analysis of clinicopathologic features associated with survival time.

Table 5Multivariate analysis of characteristics associated with OS and DFS.
4. Discussion
TRAF4, commonly over-expressed in cancer and initially identified as a gene amplified and over-expressed in breast carcinoma,located at 17q11.2 which was a region of amplification devoid of known oncogenes[14], might be involved in the development and progression of primary breast cancer and metastasis. TRAF4 protein high expression was limited to cancer cells and the subcellular localization was consistently cytoplasmic in a large majority of cases. It is considered that TRAF4 is the only traffic regulator and regulated by tumor suppressorp53, which is analyzed inp53regulated gene microarrays[15]. TRAF4 may play a role in thep53mediated apoptotic signaling of cellular stress responses. In response to TGF-βmanner, TRAF4 was observed to be recruited to the TGF-βreceptor complex[11]. TRAF4 increases NF-κB activation through the glucocorticoid-induced TNF-R (GITR), a receptor expressed on T cells, B cells and macrophages[16]. Immunohistochemical experiments showed a strong cytoplasmic TRAF4 staining, but mainly localized in the basal epithelial cells[17]. The analyses have suggested that high TRAF4 expression is an indicator of poor outcomes in cancer patients. No extensive studies have yet been carried out to establish the significance of TRAF4 expression in diagnosis or prognosis.
HCC, a most common human malignancy, makes up for 70%-85%of the primary liver tumors and contributes to cancer-related death as a third leading cause worldwide[18,19]. Because HCC are highly vascular tissues and angiogenesis matters a lot in its development,progression, and metastasis, angiogenesis is a key process to tumor progression[8]. Accordingly, the prevention or delay of the development of HCC can be accomplished by the interference with angiogenesis. Tumor angiogenesis is a complex process of multi-factor regulation[20]. Kinds of important roles are played by angiogenesis in many cancers, including HCC by VEGF, which,highly expressed in HCC, was the most studied angiogenic factor in vascular endothelial cell carcinoma. Besides, VEGF imposes key effects in stimulating angiogenesis and in enhancing vascular permeability[21]. MVD is a commonly used parameter to evaluate tumor neovascularization formation level number. MVD is usually quantized by immunohistochemical staining of endothelial cell marker. CD34 is a commonly used marker of endothelial cells[22],and it might stand for MVD in HCC more accurately due to the fact that MVD by CD34 staining is an independent prognostic factor of patient survival after resection of HCC[23].
To examine the involvement of TRAF4 in tumor angiogenesis in HCC, the association between the expression of TRAF4, VEGF,CD34 and the expression of MVD in HCC tissues was studied. The expression of VEGF showed a significantly positive correlation. In addition, HCC cases with high TRAF4 expression had a significantly greater MVD than those with low TRAF4 expression. These results indicate the expression of TRAF4 in the angiogenesis in HCC may be associated with VEGF .
TRAF4 as an independent prognostic factor for advanced HCC is the most important finding of my study. Survival analysis also showed significantly shorter DFS and OS on patients with TRAF4-high expression than those with TRAF4-low expression. That TRAF4 was an independent poor predictor of DFS and OS was confirmed in univariate and multivariate analyses in following surgical resection of HCC patients.
Our present findings provide evidence that the detection of TRAF4 as a positive prognostic marker in the expression of liver cancer is very important. We believe that TRAF4 protein, by immunohistochemical examination, the diagnosis of liver cancer markers can improve the early detection of HCC and the detection rate and prognosis of patients with HCC.
Overall, the occurrence and development of advanced HCC is displayed in our research, which shows that TRAF4 is closely related to the abnormal tumor. Therefore, the prognostic value of combined detection of TRAF4 may provide a potential malignant tumor strategy.
Conflict of interest statement
All authors declare that they have no potential conflicts of interests.
[1] Lai Q, Lerut JP. Hepatocellular cancer: how to expand safely inclusion criteria for liver transplantation.Curr Opin Organ Transplant2014; 19:229-234.
[2] Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics.CA Cancer J Clin2011; 61(2): 69-90.
[3] Pascual S, Herrera I, Irurzun J. New advances in hepatocellular carcinoma.World J Hepatol2016; 8(9): 421-438.
[4] Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma.Ann Surg2009; 249(5):799-805.
[5] Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma.Lancet2012;379(9822): 1245-1255.
[6] Intaraprasong P, Siramolpiwat S, Vilaichone RK. Advances in management of hepatocellular carcinoma.Asian Pac J Cancer Prev2016;17(8): 3697-3703.
[7] Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients.Hepatology2003; 37(5): 1114-1121.
[8] Yang ZF, Poon RT. Vascular changes in hepatocellular carcinoma.Anat Rec (Hoboken)2008; 291(6): 721-734.
[9] Muto J, Shirabe K, Sugimachi K, Maehara Y. Review of angiogenesis in hepatocellular carcinoma.Hepatol Res2014; 45(1): 1-9.
[10] Regnier CH, Tomasetto C, Moog-Lutz C, Chenard MP, Wendling C,Basset P, et al. Presence of a new conserved domain in CART1, a novel member of the tumor necrosis factor receptor-associated protein family,which is expressed in breast carcinoma.J Biol Chem1995; 270(43):25715-25721.
[11] Zhang L, Zhou F, Garcia de Vinuesa A, de Kruijf EM, Mesker WE, Hui L, et al. TRAF4 promotes TGF-βreceptor signaling and drives breast cancer metastasis.Mol Cell2013; 51(5): 559-572.
[12] Zhou FF, Li F, Xie F, Zhang Z, Huang H, Zhang L. TRAF4 mediates activation of TGF-βsignaling and is a biomarker for oncogenesis in breast cancer.Sci China Life Sci2014; 57(12): 1172-1176.
[13] Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48 900 necropsies.Cancer1954; 7(3): 462-503.
[14] Camilleri-Broet S, Cremer I, Marmey B, Comperat E, Viguié F, Audouin J, et al. TRAF4 overexpression is a common characteristic of human carcinomas.Oncogene2007; 26(1): 142-147.
[15] Sax JK, El-Deiry WS. Identification and characterization of the cytoplasmic protein TRAF4 as ap53-regulated proapoptotic gene.J Biol Chem2003; 278(38): 36435-36444.
[16] Kedinger V, Rio MC. TRAF4, the unique family member.Adv Exp Med Biol2007; 597(4): 60-71.
[17] Krajewska M, Krajewski S, Zapata JM, Van Arsdale T, Gascoyne RD, Berern K, et al. TRAF-4 expression in epithelial progenitor cells.Analysis in normal adult, fetal, and tumor tissues.Am J Pathol1998;152(6): 1549-1561.
[18] El-Serag HB. Hepatocellular carcinoma.N Engl J Med2011; 365(12):1118-1127.
[19] Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality.Hepatology2014; 61(1): 191-199.
[20] Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, et al.Hepatocellular carcinoma: Consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting.J Clin Oncol2010;28(25): 3994-4005.
[21] Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma.Cancer Res2008; 68(16): 6779-6788.
[22] Lau CP, Poon RT, Cheung ST, Yu WC, Fan ST. SPARC and Hevin expression correlate with tumour angiogenesis in hepatocellular carcinoma.J Pathol2006; 210(4): 459-468.
[23] Tanigawa N, Lu C, Mitsui T, Miura S. Quantitation of sinusoidlike vessels in hepatocellular carcinoma: its clinical and prognostic significance.Hepatology1997; 26(5): 1216-1223.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- An updated systematic review of Zika virus-linked complications
- Biological, chemical and pharmacological aspects of Madhuca longifolia
- Scenario of dengue infection & its control in Pakistan: An up-date and way forward
- Cytotoxic, kinetics of inhibition of carbohydrate-hydrolysing enzymes and oxidative stress mitigation by flavonoids roots extract of Dicoma anomala (Sond.)
- Evaluation of antiparasitic, anticancer, antimicrobial and hypoglycemic properties of organic extracts from Panamanian mangrove plants
- Neuroprotection by misoprostol against rotenone-induced neurotoxicity in rat brain
