Kidney disease models: tools to identify mechanism s and potential therapeutic targets
2018-05-07YinWuBaoYuanYuanJiangHuaChenWeiQiangLin
Yin-Wu Bao, Yuan Yuan, Jiang-Hua Chen, Wei-Qiang Lin,*
1 Kidney Disease Center, First Affiliated Hospita l, School of Medicine, Zhejiang University, Hangzhou Zhejiang 310058, China
2 Institute of Translational Medicine, Schoo l of Medicine, Zhejiang University, Hangzhou Zhejiang 310058, China
INTRODUCTION
Acute kidney injury (AKI) and chronic kidney disease (CKD) are linked to high morbidity and mortality. AKI is regarded as a rapid and reversible decline in renal function and is associated with accelerated CKD (Siew & Davenport, 2015). The ability to diagnose AKI has progressed significantly. Recent consensus diagnostic criteria include an increase in serum creatinine ≥0.3 mg/dL (≥26.5 µmol/L) w ithin 48 h; an increase in serum creatinine to ≥1.5 times baseline; or urine volume <0.5 m L/kg/h for 6 h (Khwaja, 2012). Many risk factors such as drugs/toxins,sepsis, and ischem ia-reperfusion (IR) commonly result in AKI and lead to reduced glomerular filtration rate (GFR) as well as acute tubular cell death (Sanz et al., 2013). CKD is a significant medical problem globally, with a rapid increase in incidence due to the rise in hypertension and diabetes (Tom ino, 2014). CKD is usually diagnosed by the presence of album inuria or estimated GFR from serum creatinine <60 m L/m in/1.73 m2(Andrassy, 2013).
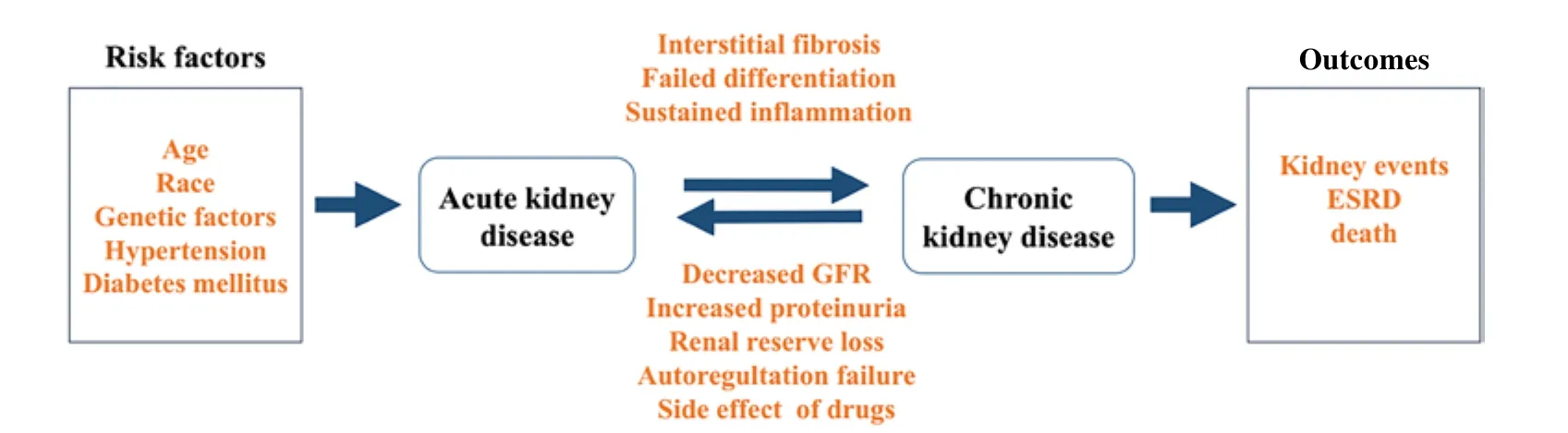
There is increasing recognition that AKI and CKD are closely linked and are therefore regarded as an integrated clinical syndrome (Chaw la & Kimmel, 2012) (Figure 1). Key biological processes such as cell death, cell proliferation, inflammation,and fibrosis, as well as common biomarkers, are detected in both kinds of nephropathy (Andreucci et al., 2017; Endre et al.,2011). Generally, tubular cell death, which includes necrosis,apoptosis, or necroptosis, is the main histological feature in early stage AKI, whereas fibrosis tends to occur under CKD. An increasing number of studies have shown that AKI is a major risk factor that can accelerate CKD progression (Pannu, 2013).Clinical observations have also found a strong relationship between AKI and CKD. Compared to patients with no history of AKI or CKD, AKI patients are more likely to develop new CKD or end-stage renal disease (ESRD) (Chaw la et al., 2014;Chaw la & Kimmel, 2012). Conversely, CKD also plays an im portant role in AKI. Patients with CKD may suffer higher risk of transient decreases in renal function consistent with AKI(Chaw la et al., 2014). The underlying mechanism that results in acute renal dysfunction may involve decreased GFR, increased proteinuria, renal auto-regulation failure, and drug side effects(Hsu & Hsu, 2016).1
Mechanisms of disease generation and progression in AKI and CKD remain incom pletely understood (Singh et al., 2013;Tam pe et al., 2017). Although several clinical studies have investigated early stage predictive biomarkers of kidney disease,few has been applied in clinical practice (Endre et al., 2013;Francoz et al., 2016; Peng et al., 2008; Soto et al., 2010). Our group identified urinary fractal kine as a marker of acute kidney transp lant rejection (Peng et al., 2008). However, considerable challenges still lay ahead for the design and implementation of clinical kidney disease trials. Large sample size and long foll owup duration a re essential in a multicenter clinical trial toguarantee the quality, efficiency, and safety of intervention and treatments as there are many different types and causes of kidney disease and treatment can be protracted (Luyckx et al.,2013). Moreover, serious comp lications greatly contribute to total mortality in kidney disease (Di Lullo et al., 2015; Pálsson &Patel, 2014; Ross & Banerjee, 2013), making it difficult to determine the major cause and best treatment. Thus, mature animal models are an indispensable part of scientifically
designed kidney disease studies, and play an important role in resolving the bottleneck issue in treatment.
Figu re 1 Relationship betw een acu te kidney in ju ry (AKI) and ch ronic kidney d isease (CKD)

Figure 2 Summ ary o f m ajor acute kidney in jury (AKI) and chronic kidney disease (CKD) m odels
Animal models have been extensively used to clarify the pathogenesis and underlying mechanism s of renal disease.Among these models, mice and rats are the most commonly used to study nephropathy events and potential therapeutic targets and to identify specific biomarkers of disease. Mice and rats are easily bred and are relatively inexpensive to house and maintain (Wei & Dong, 2012). Classic acute kidney disease can be induced in a variety of murine models by surgery or administration of drugs or toxins (Ortiz et al., 2015; Singh et al.,2012). Furthermore, genetically engineered mice and inbred strains provide a new platform for investigating complex human nephropathy (such as IgAnephropathy and diabetic nephropathy)(Marchant et al., 2015; Suzuki et al., 2014). In this review, we focused on murine models of AKI and CKD (Figure 2).
ACUTE KIDNEY INJURY MODELS
Recently, several reviews of available models, including their advantages and disadvantages, have been discussed (Ortiz et al., 2015; Ramesh & Ranganathan, 2014); however, the types of models are incom plete and many details, such as model techniques and modeling time, are not mentioned. Current models of AKI can be induced by IR (pre-renal acute kidney failure), injection of drugs, toxins, or endogenous toxins (sepsisassociated AKI), and ureteral obstruction (post-renal acute kidney failure) (Sanz et al., 2013; Singh et al., 2012) (Table 1).This section will discuss experimental AKI models, surgical operations, model tim e courses, and drug/toxin dose ranges.

Tab le 1 Com parison o f conven tiona l acu te kidney in ju ry (AKI) m ice m odels
Sepsis-associated AKI
Sepsis-associated AKI (SA-AKI) is characterized by severe inflammatory complications and high morbidity and mortality(Swam inathan et al., 2015). Frequently used experimental models of SA-AKI can be divided into two types: (1) injection of bacteria or endogenous toxins (e.g., LPS) into the peritoneum or blood; and (2) release of intestinal excreta by cecal ligation and puncture (CLP) or colon ascendens stent peritonitis (CASP)(Xu et al., 2014; Liu et al., 2015a).
LPS modelsLPS-induced AKI has mainly been studied in rats and mice. Com pared with other species, rodents are significantly more resistant to the toxic or lethal effects of LPS.The dose of LPS commonly used in research is 10–15 mg/kg(Fink, 2014; Liu et al., 2015b; Venkatachalam & Weinberg,2012). After LPS interacts with specific receptors such as Tolllike receptor 4 (TLR-4) (Solov'eva et al., 2013) on host immune cells, inflammatory cytokines like IL-1, TNF-α, and IL-6 are secreted, leading to hem odynamic alteration, widespread inflammation, and sepsis (Solov'eva et al., 2013). This is an acute model that usually term inates at 72–96 h.
CLP modelThe CLP model is the most frequently used model due to its simplicity. Firstly, ligation of the cecum from the distal to the ileocecal valve is made. After that, two needle punctures are made to extrude stool into the abdominal cavity(Liu et al., 2016b; Poli-De-Figueiredo et al., 2008). CLP in mice can develop the typical symptoms of bacterial peritonitis observed in humans and yield good results (Fink, 2014).However, it is difficult to control the severity of sepsis and the differences in age and strain in CLP models (Zarjou & Agarwal,2011). Moreover, reproducible AKI cannot be developed in a CLP model (Dejager et al., 2011).
Although experimental models have extended our understanding of sepsis and sepsis-associated AKI, there is still no effective clinical therapy (Alobaidi et al., 2015). Several clinical trials targeting specific signaling pathways based on convincing results in murine models have failed to im prove survival in septic patients.
Is chemia-reperfusion (IR) model
Currently, IR is the most widely used model for clinical AKI and renal transp lant studies (Hesketh et al., 2014). Among the variety of existing models, the mouse clamping model is often applied due to its low costs and choice of transgenic models(Sanz et al., 2013). According to previous studies, commonly used models contain bilateral renal IR (Huang et al., 2012; Hu et al., 2010; Kim et al., 2012) and unilateral renal IR (Braun et al., 2012; Chen et al., 2011; Gall et al., 2011).
First, 50–60 mg/kg of pentobarbital (5 mg/m L) is used to anesthetize mice by intraperitoneal (i.p.) injection, with body temperatures then maintained at 36.5–37 °C during surgery.Second, the renal artery and vein are clamped by m icroaneurysm clips for a variable length of time to induce different severities of kidney injury. In general, clam ping the renal pedicle for 30 m in is used to induce IR injury (Huang et al.,2015; Liu et al., 2016a). Successful is chemia can be confirmed by gradual darkening of the kidney (from red to dark purple). The clam p is then removed at the desired time to achieve reperfusion,with the kidney color immediately reverting to red (Hesketh et al.,2014; Wei & Dong, 2012). Ischemia-reperfusion will trigger tubular cell necrosis and apoptosis, inflammation, and oxidative stress (Rovcanin et al., 2016; Sanz et al., 2013; Zhou et al.,2015), which can result in a decline of renal function, as evaluated by blood urea nitrogen (BUN) and serum creatinine.Despite the view that the IR model is less stable, experimental factors such as anesthesia dose, mouse strain, age, gender, and feeding conditions can be well-controlled (Wei & Dong, 2012).
Obstructive AKI
Unilateral ureteric obstruction (UUO) is the most common rodent model used to study AKI and CKD (Ucero et al., 2014).This model can result in hydronephrosis and blood flow changes. Ischem ia, hypoxia, and oxidative stress (Dendooven et al., 2011) contribute to the tubular cell death, followed by interstitial inflammation. Additionally, transformed fibroblasts can interact with extracellular matrix deposition to cause renal fibrosis (Xiao et al., 2016; Zhou et al., 2014).
Recent studies using the UUO model have shown that adenosine levels (Tang et al., 2015), nuclear factor-erythroid-2-related factor 2 (Nrf2) (Chung et al., 2014), interleukin-10 (Jin et al., 2013), and the JAK/STAT signaling pathway (Koike et al.,2014) are related to renal fibrosis, thus offering a potential therapeutic target for renal injury. The UUO model is relatively straightforward. Male animals, which are recommended in this model, undergo a m id line abdominal incision under anesthesia,with the left ureter then ligated with 4–0 silk. After 24 h, the ureter obstruction is removed (Bander et al., 1985). Different from the complete UUO model, a partial UUO is created by inserting the ureter into a surgically created tunnel in the psoas muscle (Sugandhi et al., 2014). Reversible partial UUO is generally performed in neonatal mice to investigate kidney recovery after obstruction (Ucero et al., 2014). However, the complete UUO model is more popular because it is less technical and more easily reproduced.
Toxin-induced AKI
Exogenous drugs or poisons and endogenous toxins are used to stimulate AKI by their side or poisoning effects. Among these models, 6–20 mg/kg cisplatin can result in acute tubular injury within 72 h, whereas administration of 40–200 mg/kg gentamicin in rats for 4–10 d can induce acute renal failure.Aristolochic acid and high dose folic acid (FA) are frequently used to study AKI-CKD transition, with AKI models developed by warfarin and glycerol also used.
Cisp latin-induced AKI
Cisplatin is a chemotherapy agent that is widely used in the treatment of solid tumors (Karasawa & Steyger, 2015).However, high doses of cisplatin can induce prom inent nephrotoxicity in humans (Humanes et al., 2012; Malik et al.,2015). Among cisplatin’s adverse effects, direct proximal tubular toxicity is significant. Tubular cell necrosis and apoptosis are mediated by inflammation, oxidative stress, and calcium overload. These modes of cell death both lead to increased vascular resistance and decreased GFR (Ozkok & Edelstein,2014). The pathology and recovery phase of cisplatin-induced AKI models are com parable with those of humans. Many studies have reported that single i.p. injection of 6–20 mg/kg cisplatin can induce AKI within 72 h in rodent models (Ko et al.,2014; Lee et al., 2009; Morsy & Heeba, 2016; Xu et al., 2015).Furthermore, other groups have developed AKI models by injecting higher doses of cisplatin, including 30 mg/kg, i.p. (Lu et al., 2008; Mitazaki et al., 2009) and 40 mg/kg, i.p. (Zhang et al.,2016). Based on results from this experimental model, several therapeutic targets have been established.
Aristo loch ic acid neph ropathy
It has been reported that i.p. injection of aristolochic acid (AA)(5 mg/kg/d for 5 d) can induce AKI (Matsui et al., 2011; Wu et al., 2014). The pathology of acute aristolochic acid nephropathy(AAN) involves proximal tubular cell injury and necrosis with oxidative stress and progressive interstitial renal fibrosis(Baudoux et al., 2012; Nortier et al., 2015; Yang et al., 2010).Rabbit and rat models were first used to recapitulate human CKD and confirmed that aristolochic acid is related to Chinese herb nephropathy and Balkan endem ic nephropathy (De Broe,2012; Sanz et al., 2013). Recently, studies on AA in AKI-CKD transition have increased. Signaling pathways such as nuclear factor erythroid 2-related factor 2 (Nrf2) (Wu et al., 2014) and Jun N-term inal kinases (JNK) signaling (Rui et al., 2012; Yang et al., 2010) have been shown to play important roles in AA-induced acute kidney lesions, thus providing several new therapeutic targets.
Folic acid-induced AKI
A high dose of FA can also induce AKI in mice (Wen et al.,2012). Intraperitoneal injection of 250 mg/kg of FA (dissolved in 0.3 mmol/L NaHCO3) can cause acute renal toxicity and injury in rodents (Soofi et al., 2013; Wen et al., 2012). The mechanism of FA nephropathy might be due to FA crystal deposition in the tubular lumen, which results in obstruction and extensive necrosis (Kumar et al., 2015; Szczypka et al., 2005).A more recent study showed that inhibition of ferroptosis can protect kidneys from FA-induced AKI, implicating its important role in FA nephropathy (Martin-Sanchez et al., 2017).Additionally, mitochondrial dysfunction and early renal fibrosis,which are related to CKD pathology, can be found in the FA-induced AKI model, thus providing a new way in which to investigate AKI-CKD transition (Stallons et al., 2014).
Warfarin-induced AKI
A new model of warfarin-induced hematuric AKI based on 5/6 renal nephrectomized rats (Ware et al., 2011) was established to study the pathology of warfarin-related nephropathy (WRN)in patients with excessive anticoagulant (Rizk & Warnock,2011). The 5/6 nephrectomy was performed in Sprague Dawley rats, with animals allowed three weeks recovery from the surgery before warfarin treatment. Warfarin was given orally via drinking water, and warfarin dosage was based on rat weight(Brodsky, 2014). Extensive glomerular hemorrhage and tubular obstruction can occur in rats after seven days adm inistration of warfarin (0.4 mg/kg/d), as well as increased serum creatinine(Brodsky, 2014; Ozcan et al., 2012). Besides, WRN can also induce AKI, accelerate CKD, and increase the mortality rate in warfarin-treated patients (Brodsky et al., 2011). However, the mechanism and therapeutic strategies to ameliorate WRN-induced AKI rem ain to be demonstrated.
Glycerol-induced AKI
Rhabdom yolysis is a syndrome in which the breakdown of skeletal muscle leads to the release of intracellular proteins and toxic com pounds into circulation (Hamel et al., 2015). AKI is a common complication of rhabdomyolysis and accounts for the high mortality (Elterman et al., 2015; Zhang et al., 2012).Presently, oxidative damage and inflammation are the two major causes of rhabdom yolysis-induced AKI (Tom ino, 2014).To reproduce the typical symptoms observed in humans, rats or mice are deprived of water for 24 h, after which a 8–10 m L/kg dose of 50% glycerol is adm inistrated in the hindlimb muscle(Geng et al., 2014; Kim et al., 2014b). Although studies have reported that vitam in C (Ustundag et al., 2008), L-carnitine(Aydogdu et al., 2006; Ustundag et al., 2009), and resveratrol(Aydogdu et al., 2006) can ameliorate rhabdom yolysis-induced AKI, there is currently no effective therapy for this disease except aggressive rehydration (Gu et al., 2014).
Gentamicin nephropathy
Gentamicin is an aminoglycoside antibiotic commonly used to prevent gram-negative bacterial infection. Nevertheless,nephrotoxicity lim its its use in clinical practice (He et al., 2015).Doses of gentam icin ranging from 40–200 mg/kg administered for 4–10 d (Bledsoe et al., 2008; Boroushaki et al., 2014;Heidarian et al., 2017; Jabbari et al., 2011) can induce acute renal failure in rats. Administration of 100 mg/kg i.p. for 5 d is recommended to mimic gentamicin-induced nephrotoxicity (Hur et al., 2013; Stojiljkovic et al., 2008; Stojiljkovic et al., 2012).This acute model is characterized by increased levels of serum urea and creatinine, decreased GFR, tubular lesions, and fibrosis (Romero et al., 2009; Al-Shabanah et al., 2010;Balakumar et al., 2010).
CHRONIC KIDNEY DISEASE MODELS
CKD models mainly include diabetic/hypertensive nephropathy,glomerular injury, polycystic kidney disease (PKD), and chronic tubulointerstitial nephritis (Table 2). In this section, key information on various rodent models of CKD is discussed.
Renal m ass reduction
The remnant kidney model has been one of most commonly used experimental models of CKD. The 5/6 subtotal nephrectom y approach is widely used to mimic human CKD in rats. The right kidney is removed and the upper and lower poles(2/3 of the left kidney) are resected after ligation of the left renal artery (He et al., 2012). After surgery, activation of the reninangiotensin system (RAS) can cause glomerular hypertension/hyperfiltration (Ergür et al., 2015; Tapia et al.,2012). Together with oxidative stress and inflammation, the glomerular hypertension/hyperfiltration finally results in glomerulosclerosis, tubulointerstitial injury, renal atrophy,proteinuria, and possible ESRD (Gong et al., 2016; Kim et al.,2009). The remnant kidney model is highly influenced by the animal strain used. C57BL/6 mice are resistant to fibrosis or progressive CKD, whereas other animal strains such as rats and CD-1, 129/Sv, and Swiss-Webster mice are susceptible(Leelahavanichkul et al., 2010; Orlando et al., 2011). In addition, high mortality and little renal tissue after 5/6 nephrectomy are also challenges to this model.

Tab le 2 Advan tages and d isadvan tageso f experim en tal CKD m ice m odels
Diabetic nephropathy
Diabetic nephropathy (DN) is the leading cause of ESRD.There are many kinds of rodent models relevant to diabetic nephropathy, but none of them perfectly m im ics the human disease (Deb et al., 2010). The Animal Models of Diabetic Com plications Consortium (AMDCC) defines the ideal rodent model of human diabetic nephropathy and comp lications (Kong et al., 2013; Kitada et al., 2016). The latest validated criteria are: (1) more than 50% decrease in GFR; (2) greater than 10-fold increase in album inuria compared with controls; and (3)pathological changes in kidneys including advanced mesangial matrix expansion±nodular sclerosis and mesangiolysis,glomerular basement membrane (GBM) thickening by >50%over baseline, arteriolar hyalinosis, and tubulointerstitial fibrosis.Classical type 1 diabetes can be modeled by the adm inistration of streptozotocin (a toxin to β-cells that results in insulin deficiency), with spontaneous autoimmunity (e.g., NOD mice or BB-DP rat) or with gene mutation (Akita and OVE26 mice)(G raham & Schuurman, 2015; Kitada et al., 2016). A high fat diet is commonly used to induce obesity and insulin resistance and develop glomerular lesions in mice (Soler et al., 2012).Typical type 2 diabetes nephropathy (DN) model can be establised by leptin deficiency (e.g., ob/ob mice) or inactivation of the leptin receptor (e.g., db/db mice, Zuker rat) (Soler et al.,2012). To exhibit more pathological features of human DN,recent studies have focused on (1) targeted gene knockout in mice (e.g., eNOS-deficient mice (Takahashi & Harris, 2014)), (2)selection of more susceptible rodent species and strains (e.g.,FVB (Chua et al., 2010) and DBA/2J mice (Østergaard et al.,2017), and (3) monogenic manipulations or superim posing additional key factors to accelerate nephropathy (e.g., STZ-eNOS-/-, db/db eNOS-/-) (Betz & Conway, 2014; Nakayama et al., 2009).
Hypertension-induced renal in jury
Spontaneously hypertensive rats are usually used to investigate hypertension-induced nephropathy. Additionally, unilateral nephrectomy is required to promote significant renal injury with increased glomerular pressure and flow (Zhong et al., 2016).Chronic injection of angiotensin II for weeks also results in persistent hypertension and renal injury (Dikalov et al., 2014).Vascular endothelial grow th factor (Lankhorst et al., 2015),Smad signaling (Liu & Davidson, 2012a), and inflammatory cytokines (Guo et al., 2015) are also involved in this process.
Primary glomerular nephropathy Focal segmental glomerulosclerosis (FSGS)
FSGS is a common primary glomerular disorder characterized by podocyte injury and loss and marked proteinuria (Fogo,2015). Although there is currently no primary FSGS model available, several secondary FSGS models have been established. Adriamycin (ADR) and puromycin are widely used to study FSGS. Single injection of these specific toxins can result in podocyte foot process effacement, deficient filtration barrier, and nephrotic syndrome (Fogo, 2003;Zhang et al.,2013). However, the dosage of adriamycin is highly dependent on species and strain. Most rat species are susceptible to low doses of ADR ranging from 1.5–7.5 mg/kg (Lee & Harris, 2011),whereas most mouse strains are resistant to ADR. To produce a successful model, higher doses of ADR are required, for example 9.8–12 mg/kg in male BALB/C (Wada et al., 2016) and 13–25 mg/kg in C57BL/c mice (Cao et al., 2010; Hakroush et al., 2014; Jeansson et al., 2009; Maimaitiyiming et al., 2016;Wang et al., 2000).
Gene modification approaches in mice, such as inactivation of Mpv-17 (Casalena et al., 2014; Viscom i et al., 2009),knockout α-actinin-4 (De Mik et al., 2013; Henderson et al.,2008) or NPHS2 (Mollet et al., 2009), or introducing the expression of Thy-1.1 antigen on podocytes, can also lead to proteinuria and FSGS (Smeets et al., 2004).
Crescentic glomerulonephritis
Antibodies fixation in the whole glomeruli (nephrotoxic nephritis)or GBM (anti-GBM nephritis) are the primary models used to mimic human crescentic glomerulonephritis (Hénique et al.,2014). Intraperitoneal injection of heterologous antibodies to heterologous whole glomeruli can induce nephrotoxic nephritis(Gigante et al., 2011). Anti-GBM nephritis can be caused by immunization with the non-collagenous domains of the alpha-3 chain of type IV collagen or passive transfer of anti-GBM antibodies (Cheungpasitporn et al., 2016; Kambham, 2012;Kvirkvelia et al., 2015). After treatment, severe proteinuria and azotem ia appear in the following weeks.
Mem b ranous neph ropathy
Membranous nephropathy (MN) is a major cause of nephrotic syndrome in the elderly and is characterized by subepithelial deposits and diffuse thickening of the GBM (Makker & Tramontano,2011). Active and passive Heymann nephritis model in rats closely resemble human MN and have been used to study MN(Sendeyo et al, 2013).
Autologous antibodies are exposed to target antigens by injection of kidney extracts or antiserum to antigen generated in another animal species (Cybulsky, 2011; Jefferson et al., 2010),resulting in immune deposits associated with heavy proteinuria(Cybulsky et al., 2005). In rat models, megalin and receptor associated protein (RAP) are the major podocyte antigens targeted by the circulating antibodies (Ronco & Debiec, 2010).However, studies have shown that megalin is neither expressed in human podocytes nor detected in patients with membranous nephropathy (Beck & Salant, 2010; Ma et al., 2013). Recently,M-type phospholipase A2 receptor (PLA2R) was identified as a target antigen for autoantibodies in human MN (Debiec &Ronco, 2011; Herrmann et al., 2012; Kao et al., 2015).Additionally, circulating thrombospondin type-1 domaincontaining 7A (THSD7A) has been detected in a subgroup of patients with idiopathic MN rather than PLA2R, suggesting a new target antigen in human MN (Tomas et al., 2014).
The cationic BSA mouse model also produces features of human MN. Mice are preimmunized with cationic bovine serum album in (cBSA) every other day for a week. Two weeks later,mice are reimm unized with cBSA in Freund’s ad juvant (Motiram Kakalij et al., 2016). Mice will develop symptoms of MN,including severe proteinuria, diffuse thickening of the GBM,subepithelial deposits, and GBM spikes.
IgA neph ropathy (IgAN)
IgAN is the most common form of glomerulonephritis, and is characterized by mesangial immune com plex depositions that contain IgA1, IgG, complement C3, and IgM (Daha & Van Kooten, 2016). Inducible IgAN models include intravenous injection of IgA containing immune comp lexes to develop m ild and transient IgAN (Rifai et al., 1979), and oral adm inistration of protein antigens that result in mesangial IgA deposits(Emancipator et al., 1983). The ddY mouse is a spontaneous IgAN model derived from a non-inbred strain that develops glomerulonephritis and m ild proteinuria without hematuria(Suzuki et al., 2014). Mouse line HIGA, an inbred strain with high levels of circulating IgA, shows significant early-onset immune deposits (Eitner et al., 2010). Other genetically modified mice, such as uteroglobin-deficient mice (Lee et al.,2006) and CD89-transgenic mice (Moura et al., 2008; Papista et al., 2015), can also be used to investigate IgA nephropathy.Although some models are available, the underlying mechanism of IgAN is still not fully understood.
Secondary neph ro tic synd rom e
In this section, murine models of system ic lupus erythematosus and am yloidosis are reviewed. Transgenic murine models are widely used to investigate these com plex diseases, especially system ic lupus erythematosus (SLE). Both MRL and CD95 gene mutant animals can serve as research models to develop SLE sym ptom s and investigate potential therapies.
Am y lo id A (AA) am y lo idosis
Am yloid A (AA) am yloidosis is a serious com p lication of chronic inflammation. AA-type amyloid deposition can cause alteration in tissue structure and function, with the kidney noted to be a major target organ (Simons et al., 2013). Injection of chemical or biological com pounds such as casein, lipopolysaccharide(Kisilevsky & Young, 1994; Skinner et al., 1977), an extract of am yloidotic tissue or purified amyloidogenic light chains (Teng et al., 2014) are widely used to create AA am yloidosis mouse models. However, unlike clinical AA-amyloid patients, these models rarely develop renal failure (Simons et al., 2013). In recent years, a striking transgenic murine model has been developed. Mice carrying the human interleukin-6 gene under the control of the metallothionein-I promoter or with doxycycline-inducible transgenic expression of SAA provide another way to investigate AA-am yloid (Simons et al., 2013).
Systemic lupus erythematosus (SLE)
Lupus nephritis is characterized by autoantibodies against nuclear autoantigens such as DNA, histones, and nucleosomes(Liu & Davidson, 2012b). Most studies on SLE are based on murine models. Genetically modified models include MRL and CD95 mutants such as MRLlprand FasLgldmice (Nickerson et al., 2013; Otani et al., 2015), BXSB mice (McGaha et al., 2005;McPhee et al., 2013), and NZB/NZW F1mice (Fagone et al.,2014), which are widely used to develop proteinuria,lym phoproliferation, and similar features relevant to human lupus nephritis (McGaha & Madaio, 2014). Recently, TWEAKFn14 signaling has been reported to play an important role in the progression of lupus nephritis and anti-TWEAK blocking antibodies can preserve renal function and increase survival rate in experimental models of CKD (Gomez et al., 2016; Sanz et al., 2014). Although multiple mouse models have been used to investigate lupus nephritis, each model has limitations that impede our understanding of the pathogenesis and clinical manifestations of this disease. Subsequently, no effective therapy for lupus nephritis currently exists.
Hereditar ynephritis Po lycystic kidney disease (PKD)
PKD includes a group of human monogenic disorders inherited in an autosomal dominant (ADPKD) or recessive (ARPKD)fashion. PKD is mainly restricted to the liver and kidney, and occurs in a range of ages from children to the elderly. In children and adults, ADPKD and ARPKD are the most common genetic nephropathies and leading causes of ESRD (Liebau &Serra, 2013). ADPKD is caused by mutation of either PKD1(85%) or PKD2 (15%) (Kim et al., 2014a), whereas ARPKD is caused by PKHD1 gene mutations (Sweeney & Avner, 2011).Although hereditary PKD is comp lex and diverse, it is normally induced by single mutations in single genes. Therefore,genetically engineered murine models are widely used to mimic human PKD. As homozygous mice of PKD1 or PKD2 result in embryonic lethality (Woudenberg-Vrenken et al., 2009),conditional knockouts, inducible strategies, or the introduction of unstable alleles are the major ways to establish experimental models (Ko & Park, 2013). There have been some successful clinical trials based on results from these models. For example,tolvaptan has been proven to be effective in ADPKD and is now marketed in Japan (Torres et al., 2012). Moreover, combination therapy of tolvaptan and pasireotide has brought significant reduction in cystic and fibrotic volume in a PKD1 mouse model(Hopp et al., 2015).
Alport syndrome
Alport syndrome (AS) is a hereditary glomerulopathy resulting from mutations in the type IV collagen genesCOL4A3,COL4A4, orCOL4A5, and is characterized by hematuria, renal failure, hearing loss, ocular lesions (Savige et al., 2011), and abnormal collagen IV com position in the GBM (Savige et al.,2013). TheCOL4A3gene knockout mouse is the major model used to study the pathogenesis of AS. Homozygous mice can develop proteinuria at 2–3 months of age and die from renal failure at 3–4 months (Kashtan & Segal, 2011). InCOL4A3-/-mice, studies have shown that TNF-α contributes to Alport glomerulosclerosis by inducing podocyte apoptosis (Ryu et al.,2012). Furthermore, spontaneousCOL4A4mutation in NONcNZO recombinant inbred mice exhibits early stage proteinuria associated with glomerulosclerosis. These genetically modified mice provide valuable models for potential therapy testing and help understand the mechanisms of AS(Korstanje et al., 2014).
CONCLUSIONS
Although AKI and CKD are significantly increasing worldwide and cause high mortality, clinical diagnosis and therapeutic interventions are lagging. AKI-CKD transition and the underlying mechanisms of complex CKD such as IgAnephropathy, diabetic nephropathy, and FSGS are still unclear and impede the search for potential therapies. Despite the valuable new insights into kidney disease gained from existing models, many do not fully reproduce human clinical diseases.Thus, improved murine models are still desperately needed to investigate potential diagnostic and therapeutic approaches. In AKI models, obtaining new mouse strains susceptible to toxins/drugs is urgent, and finding new approaches to develop stable and reproducible AKI models is necessary. As for CKD models, to develop complex and specific pathologies, mice with multiple genetically modified will be widely used to develop complex and specific pathology in the near future. Additionally,models that faithfully develop common conditions such as DN or SLE are also imperative.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Y.W.B. wrote the manuscript with W.Q.L.’s input.W.Q.L., Y.Y., and J.H.C.revised the manuscript. All authors read and approved the final manuscript.
Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM. 2015. Sepsis-associated acute kidney injury.Seminars in Nephrology,35(1): 2–11.
Al-Shabanah OA, Aleisa AM, Al-Yahya AA, Al-Rejaie SS, Bakheet SA,Fatani AG, Sayed-Ahmed MM. 2010. Increased urinary losses of carnitine and decreased intramitochondrial coenzyme a in gentamicin-induced acute renal failure in rats.Nephrology Dialysis Transplantation,25(1): 69–76.
Andrassy KM. 2013. Comments on 'KDIGO 2012 clinical practice guideline for the evaluation and managem ent of chronic kidney disease'.Kidney International,84(3): 622–623.
Andreucci M, Faga T, Pisani A, Perticone M, Michae l A. 2017. The ischem ic/nephrotoxic acute kidney injury and the use of renal biomarkers in clinical practice.European Journal of Internal Medicine,39: 1–8.
Aydogdu N, Atmaca G, Yalcin O, Taskiran R, Tastekin E, Kaymak K. 2006.Protective effects of L-carnitine on myoglobinuric acute renal failure in rats.Clinical and Experimental Pharmacology and Physiology,33(1–2): 119–124.Ba lakum ar P, Rohilla A, Thangathirupathi A. 2010. Gentam icin-induced nephrotoxicity: Do we have a prom ising therapeutic approach to blunt it?Pharmacological Research,62(3): 179–186.
Bander SJ, Buerkert JE, Martin D, Klahr S. 1985. Long-term effects of 24-hr unilateral uretera l obstruction on renal function in the rat.Kidney International,28(4): 614–620.
Baudoux TER, Pozdzik AA, Arlt VM, De Prez EG, Antoine MH, Quellard N,Goujon JM, Nortier JL. 2012. Probenecid prevents acute tubular necrosis in a mouse mode l of aristolochic acid nephropathy.Kidney International,82(10): 1105–1113.
Beck LH Jr, Salant DJ. 2010. Membranous nephropathy: recent travels and new roads ahead.Kidney International,77(9): 765–770.
Betz B, Conway BR. 2014. Recent advances in animal models of diabetic nephropathy.Nephron Experimental Nephrology,126(4): 191–195.
Bledsoe G, Shen B, Yao YY, Hagiwara M, Mizell B, Teuton M, Grass D,Chao L, Chao JL. 2008. Ro le of tissue kallikrein in prevention and recovery of gentam icin-induced renal injury.Toxicological Sciences,102(2): 433–443.Boroushaki MT, Asadpour E, Sadeghnia HR, Dolati K. 2014. Effect of pom egranate seed oil against gentam icin -induced nephrotoxicity in rat.Journal of Food Science and Technology,51(11): 3510–3514.
Borza DB, Hudson BG. 2002. O f mice and men: m urine models of anti-GBM antibody nephritis.Kidney International,61(5): 1905–1906.
Braun H, Schm idt BMW, Raiss M, Baisantry A, Mircea-Constantin D, Wang SJ, Gross ML, Serrano M, Schm itt R, Melk A. 2012. Ce llu lar senescence lim its regenerative capacity and allogra ft survival.Journal of the American Society of Nephrology,23(9): 1467–1473.
Brodsky SV. 2014. Anticoagulants and acute kidney injury: clinical and patho logy considerations.Kidney Research and Clinical Practice,33(4):174–180.
Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM,Bhatt UY, Hebert LA. 2011. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate.Kidney International,80(2): 181–189.
Cao Q, Wang YP, Zheng D, Sun Y, Wang Y, Lee VWS, Zheng GP, Tan TK,Ince J, Alexander SI, Harris DCH. 2010. IL-10/TGF-β-modified macrophages induce regulatory T cells and protect against adriam ycin nephrosis.Journal of the American Society of Nephrology,21(6): 933–942.
Casalena G, Krick S, Daehn I, Yu LP, Ju WJ, Shi SL, Tsai SY, D'Agati V,Lindenmeyer M, Cohen CD, Sch londorff D, Bottinger EP. 2014. Mpv17 in mitochondria protects podocytes against mitochondrial dysfunction and apoptosis in vivo and in vitro.American Journal of Physiology-Renal Physiology,306(11): F1372–F1380.
Chaw la LS, Kimmel PL. 2012. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome.Kidney International,82(5): 516–524.
Chaw la LS, Eggers PW, Star RA, Kimm el PL. 2014. Acute kidney injury and chronic kidney disease as interconnected syndrom es.The New England Journal of Medicine,371(1): 58–66.
Chen JL, Hartono JR, John R, Bennett M, Zhou XJ, Wang YX, Wu QQ,W interberg PD, Nagam i GT, Lu CY. 2011. Early interleukin 6 production by leukocytes during ischem ic acute kidney injury is regulated by TLR4.Kidney International,80(5): 504–515.
Cheungpasitporn W, Zacharek CC, Fervenza FC, Cornell LD, Sethi S,Herrera Hernandez LP, Nasr SH, Alexander MP. 2016. Rapid ly progressive glomeru lonephritis due to coexistent anti-glomerular basement membrane disease and fibrillary glomerulonephritis.Clinical Kidney Journal,9(1): 97–101.
Chevalier RL, Forbes MS, Thornhill BA. 2009. Ureteral obstruction as a model of rena l interstitial fibrosis and obstructive nephropathy.Kidney International,75(11): 1145–1152.
Chua S Jr, Li YF, Liu SM, Liu RJ, Chan KT, Martino J, Zheng ZY, Susztak K,D'Agati VD, Gharavi AG. 2010. A susceptibility gene for kidney disease in an obese mouse model of type II diabetes maps to chrom osome 8.Kidney International,78(5): 453–462.
Chung S, Yoon HE, Kim SJ, Kim SJ, Koh ES, Hong YA, Park CW, Chang YS, Shin SJ. 2014. Oleanolic acid attenuates renal fibrosis in mice with unilateral ureteral obstruction via facilitating nuclear translocation of Nrf2.Nutrition & Metabolism,11(1): 2.
Cybulsky AV. 2011. Membranous nephropathy.Contributions to Nephrology,169(1): 107–125.
Cybulsky AV, Quigg RJ, Salant DJ. 2005. Experimenta l m embranous nephropathy redux.American Journal of Physiology-Renal Physiology,289(4): F660–F671.
De Broe ME. 2012. Chinese herbs nephropathy and Balkan endem ic nephropathy: toward a sing le entity, aristo lochic acid nephropathy.Kidney International,81(6): 513–515.
De Mik SM, Hoogduijn MJ, De Bruin RW, Dor FJ. 2013. Pathophysiology and treatment of focal segmental glomerulosclerosis: the role of animal models.BMC Nephrology,14: 74.
Deb DK, Sun T, Wong KE, Zhang ZY, Ning G, Zhang Y, Kong J, Shi H,Chang A, Li YC. 2010. Combined vitam in D ana log and AT1 receptor antagonist synergistically block the deve lopment of kidney disease in a model of type 2 diabetes.Kidney International,77(11): 1000–1009.
Debiec H, Ronco P. 2011. PLA2R autoantibodies and PLA2R glomerular deposits in mem branous nephropathy.The New England Journal of Medicine,364(7): 689–690.
Dejager L, Pinheiro I, Dejonckheere E, Libert C. 2011. Cecal ligation and puncture: the gold standard model for po lym icrobial sepsis?Trends in Microbiology,19(4): 198–208.
Dendooven A, Ishola DA Jr, Nguyen TQ, Van Der Giezen DM, Kok RJ,Goldschmeding R, Jo les JA. 2011. Oxidative stress in obstructive nephropathy.International Journal of Experimental Pathology,92(3): 202–210.
Di Lu llo L, House A, Gorini A, Santoboni A, Russo D, Ronco C. 2015.Chronic kidney disease and cardiovascular com p lications.Heart Failure Reviews,20(3): 259–272.
Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassègue B,Griend ling KK, Harrison DG, Dikalova AE. 2014. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension.Antioxidants & Redox Signaling,20(2): 281–294.
Eitner F, Boor P, Floege J. 2010. Models of IgA nephropathy.Drug Discovery Today: Disease Models,7(1–2): 21–26.
Elterman J, Zonies D, Stewart I, Fang R, Schreiber M. 2015.Rhabdom yolysis and acute kidney injury in the injured war fighter.The Journal of Trauma and Acute Care Surgery,79(4 Supp l 2): S171–S174.
Emancipator SN, Gallo GR, Lamm ME. 1983. Experimental IgAnephropathy induced by oral immunization.The Journal of Experimental Medicine,157(2): 572–582.
Endre ZH, Kellum JA, Di Somma S, Doi K, Goldstein SL, Koyner JL,Macedo E, Mehta RL, Murray PT. 2013. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference.Contributions to Nephrology,182: 30–44.
Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, Fram pton CM, Bennett MR, Ma Q, Sabbisetti VS, Vaidya VS, Wa lcher AM, Shaw GM, Henderson SJ, Nejat M, Schollum JBW, George PM. 2011.Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and base line rena l function.Kidney International,79(10): 1119–1130.
Ergür BU, Çilaker MS, Yilmaz O, Akokay P. 2015. The effects of α-lipoic acid on aortic injury and hypertension in the rat remnant kidney (5/6 nephrectom y) model.Anatolian Journal of Cardiology,15(6): 443–449.
Fagone P, Mangano K, Mammana S, Quattrocchi C, Magro G, Coco M,Imene S, Di Marco R, Nico letti F. 2014. Acceleration of SLE-like syndrome development in NZBxNZW F1 mice by beta-glucan.Lupus,23(4): 407–411.Fink MP. 2014. Animal models of sepsis.Virulence,5(1): 143–153.
Fogo AB. 2015. Causes and pathogenesis of focal segmental glomerulosclerosis.Nature Reviews Nephrology,11(2): 76–87.
Francoz C, Nadim MK, Durand F. 2016. Kidney biomarkers in cirrhosis.Journal of Hepatology,65(4): 809–824.
Gall JM, Wong V, Pimental DR, Havasi A, Wang ZY, Pastorino JG, Bonegio RGB, Schwartz JH, Borkan SC. 2011. Hexokinase regulates Bax-mediated mitochondria l membrane injury fo llow ing ischem ic stress.Kidney International,79(11): 1207–1216.
Geng YQ, Zhang L, Fu B, Zhang JR, Hong Q, Hu J, Li DG, Luo CJ, Cui SY,Zhu F, Chen XM. 2014. Mesenchymal stem cells ameliorate rhabdom yolysis-induced acute kidney injury via the activation of M 2 m acrophages.Stem Cell Research & Therapy,5(3): 80.
Gigante M, Piemontese M, Gesualdo L, Iolascon A, Aucella F. 2011.Molecu lar and genetic basis of inherited nephrotic syndrome.International Journal of Nephrology,2011: 792195.
Gomez IG, Roach AM, Nakagawa N, Am atucci A, Johnson BG, Dunn K,Kelly MC, Karaca G, Zheng TS, Szak S, Peppiatt-Wildman CM, Burkly LC,Duffield JS. 2016. TWEAK-Fn14 Signaling Activates Myofibrob lasts to Drive Progression of Fibrotic Kidney Disease.Journal of the American Society ofNephrology,27(12): 3639–3652.
Gong W, Mao S, Yu J, Song JY, Jia ZJ, Huang SM, Zhang AH. 2016.NLRP3 deletion protects against renal fibrosis and attenuates mitochondrial abnormality in mouse with 5/6 nephrectomy.American Journal of Physiology-Renal Physiology,310(10): F1081–F1088.
Graham ML, Schuurman HJ. 2015. Validity of animal m odels of type 1 diabetes, and strategies to enhance their utility in translational research.European Journal of Pharmacology,759: 221–230.
Gu HX, Yang M, Zhao XM, Zhao B, Sun XJ, Gao X. 2014. Pretreatment with hydrogen-rich saline reduces the damage caused by g lycerol-induced rhabdom yolysis and acute kidney in jury in rats.Journal of Surgical Research,188(1): 243–249.
Guo ZT, Sun H, Zhang HM, Zhang YF. 2015. Anti-hypertensive and renoprotective effects of berberine in spontaneously hypertensive rats.Clinical and Experimental Hypertension,37(4): 332–339.
Hakroush S, Cebulla A, Schaldecker T, Behr D, Mundel P, Weins A. 2014.Extensive podocyte loss triggers a rapid parietal epithelial cell response.Journal of the American Society of Nephrology,25(5): 927–938.
Hamel Y, Mamoune A, Mauvais FX, Habarou F, Lallement L, Romero NB,Ottolenghi C, De Lonlay P. 2015. Acute rhabdomyolysis and inflammation.Journal of Inherited Metabolic Disease,38(4): 621–628.
He J, Wang Y, Sun S, Yu MN, Wang CY, Pei XH, Zhu B, Wu JQ, Zhao WH.2012. Bone marrow stem cells-derived microvesicles protect against rena l injury in the mouse remnant kidney model.Nephrology,17(5): 493–500.
He LY, Peng XF, Zhu JF, Liu GY, Chen X, Tang CY, Liu H, Liu FY, Peng YM.2015. Protective effects of curcum in on acute gentam icin-induced nephrotoxicity in rats.Canadian Journal of physiology and Pharmacology,93(4): 275–282.
Heidarian E, Jafari-Dehkordi E, Valipour P, Ghatreh-Samani K, Ashrafi-Eshkaftaki L. 2017. Nephrop rotective and anti-inflammatory effects ofPistacia atlanticaleaf hydroethanolic extract against gentam icin-induced nephrotoxicity in rats.Journal of Dietary Supplements,14(5): 489–502.
Henderson JM, Al-Waheeb S, Weins A, Dandapani SV, Pollak MR. 2008.Mice with altered α-actinin-4 expression have distinct morphologic patterns of glomerular disease.Kidney International,73(6): 741–750.
Herrmann SMS, Sethi S, Fervenza FC. 2012. Membranous nephropathy:the start of a paradigm shift.Current Opinion in Nephrology and Hypertension,21(2): 203–210.
Hesketh EE, Czopek A, Clay M, Borthw ick G, Ferenbach D, Kluth D,Hughes J. 2014. Renal ischaem ia reperfusion injury: a mouse model of injury and regeneration.Journal of Visualized Experiments,(88). doi:10.3791/51816.
Hénique C, Papista C, Guyonnet L, Lenoir O, Tharaux PL. 2014. Update on crescentic glomerulonephritis.Seminars in Immunopathology,36(4): 479–490.
Hopp K, Hommerding CJ, Wang XF, Ye H, Harris PC, Torres VE. 2015.Tolvaptan p lus pasireotide shows enhanced efficacy in a PKD1 model.Journal of the American Society of Nephrology,26(1): 39–47.
Hsu RK, Hsu CY. 2016. The role of acute kidney injury in chronic kidney disease.Seminars in Nephrology,36(4): 283–292.
Hu MC, Shi MJ, Zhang JN, Quiñones H, Kuro-O M, Moe OW. 2010. Klotho deficiency is an early biomarker of renal is chem ia-reperfusion injury and its rep lacem ent is p rotective.Kidney International,78(12): 1240–1251.
Huang LP, Be lousova T, Chen MY, Dimattia G, Liu DJ, Sheikh-Hamad D.2012. Overexpression of stannioca lcin-1 inhibits reactive oxygen species and renal ischem ia/reperfusion injury in mice.Kidney International,82(8):867–877.
Huang QS, Niu ZG, Tan J, Yang J, Liu Y, Ma HJ, Lee VWS, Sun SM, Song XF, Guo MH, Wang YP, Cao Q. 2015. IL-25 elicits innate lym phoid cells and multipotent progenitor type 2 cells that reduce renal ischem ic/reperfusion injury.Journal of the American Society of Nephrology,26(9): 2199–2211.
Humanes B, Lazaro A, Camano S, Moreno-Gordaliza E, Lazaro JA,Blanco-Codesido M, Lara JM, Ortiz A, Gom ez-Gomez MM, Martin-Vasallo P,Te jedor A. 2012. Cilastatin protects against cisp latin-induced nephrotoxicity w ithout com prom ising its anticancer efficiency in rats.Kidney International,82(6): 652–663.
Hur E, Garip A, Cam yar A, llgun S, Ozisik M, Tuna S, O lukman M, Narli Ozdem ir Z, Yildirim Sozmen E, Sen S, Akcicek F, Duman S. 2013. The effects of vitam in d on gentam icin-induced acute kidney injury in experimental rat m odel.International Journal of Endocrinology,2013:313528.
Jabbari M, Rostam i Z, Jenabi A, Bahram i A, Mooraki A. 2011. Sim vastatin ame liorates gentam icin-induced renal injury in rats.Saudi Journal of Kidney Diseases and Transplantation: An Official Publication of the Saudi Center for Organ Transplantation, Saudi Arabia,22(6): 1181–1186.
Jeansson M, Björck K, Tenstad O, Haraldsson B. 2009. Adriamycin alters glomeru lar endothe lium to induce proteinuria.Journal of the American Society of Nephrology,20(1): 114–122.
Jefferson JA, Pippin JW, Shankland SJ. 2010. Experimental m ode ls of membranous nephropathy.Drug Discovery Today: Disease Models,7(1–2):27–33.
Jin Y, Liu RJ, Xie JY, Xiong HB, He JC, Chen N. 2013. Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model.Laboratory Investigation,93(7): 801–811.Kambham N. 2012. Crescentic glomerulonephritis: an update on Pauciimmune and Anti-GBM diseases.Advances in Anatomic Pathology,19(2):111–124.
Kao L, Lam V, Wa ldm an M, Glassock RJ, Zhu QS. 2015. Identification of the immunodom inant epitope region in phospholipase A2receptormediating autoantibody binding in idiopathic membranous nephropathy.Journal of the American Society of Nephrology,26(2): 291–301.
Karasawa T, Steyger PS. 2015. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity.Toxicology Letters,237(3): 219–227.
Kashtan CE, Segal Y. 2011. G lomerular basement membrane disorders in experimental mode ls for renal diseases: impact on understanding pathogenesis and improving diagnosis.Contributions to Nephrology,169(1):175–182.
Khwa ja A. 2012. KDIGO clinical practice guide lines for acute kidney in jury.Nephron. Clinical Practice,120(4): c179–C184.
Kim H, Kang AY, Ko AR, Park HC, So I, Park JH, Cheong HI, Hwang YH,Ahn C. 2014a. Calpain-mediated proteolysis of polycystin-1 C-terminus induces JAK2 and ERK signal alterations.Experimental Cell Research,320(1): 62–68.
Kim HJ, Moradi H, Yuan J, Norris K, Vaziri ND. 2009. Rena l mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney.American Journal of Physiology-Renal Physiology,296(6): F1297–F1306.
Kim HJ, Lee JS, Kim JD, Cha HJ, Kim A, Lee SK, Lee SC, Kwon BS, Mittler RS, Cho HR, Kwon B. 2012. Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation.Proceedings of the National Academy of Sciences of the United States of America,109(1): E13–E22.
Kim JH, Lee DW, Jung MH, Cho HS, Jeon DH, Chang SH, Park DJ. 2014b.Macrophage dep letion ameliorates glycerol-induced acute kidney injury in mice.Nephron. Experimental Nephrology,128(1–2): 21–29.
Kisilevsky R, Young ID. 1994. Pathogenesis of am yloidosis.Baillière's Clinical Rheumatology,8(3): 613–626.
Kitada M, Ogura Y, Koya D. 2016. Rodent models of diabetic nephropathy:their utility and limitations.International Journal of Nephrology and Renovascular Disease,9: 279–290.
Ko JW, Lee IC, Park SH, Moon C, Kang SS, Kim SH, Kim JC. 2014.Protective effects of pine bark extract against cisplatin-induced hepatotoxicity and oxidative stress in rats.Laboratory Animal Research,30(4): 174–180.
Ko JY, Park JH. 2013. Mouse models of polycystic kidney disease induced by defects of ciliary proteins.BMB Reports,46(2): 73–79.
Koike K, Ueda S, Yamagishi S, Yasukawa H, Kaida Y, Yokoro M, Fukam i K,Yoshim ura A, Okuda S. 2014. Protective ro le of JAK/STAT signaling against rena l fibrosis in mice with unilateral ureteral obstruction.Clinical Immunology,150(1): 78–87.
Kong LL, Wu H, Cui WP, Zhou WH, Luo P, Sun J, Yuan H, M iao LN. 2013.Advances in m urine models of diabetic nephropathy.Journal of Diabetes Research,2013: 797548.
Korstanje R, Caputo CR, Doty RA, Cook SA, Bronson RT, Davisson MT,Miner JH. 2014. A mouseCOL4A4mutation causing Alport glomeru losclerosis with abnorm al collagen α3α4α5(IV) trimers.Kidney International,85(6): 1461–1468.
Kumar D, Singla SK, Puri V, Puri S. 2015. The restrained expression of NF-kB in renal tissue ameliorates folic acid induced acute kidney injury in mice.PLoS One,10(1): e115947.
Kvirkvelia N, McMenam in M, Gutierrez VI, Lasareishvili B, Madaio MP.2015. Human anti-α3(IV)NC1 antibody drug conjugates target glomeru li to resolve nephritis.American Journal of Physiology-Renal Physiology,309(8):F680–F684.
Lankhorst S, Saleh L, Danser AJ, Van Den Meiracker AH. 2015. Etiology of angiogenesis inhibition-re lated hypertension.CurrentOpinionin Pharmacology,21: 7–13.
Lee DW, Kwak IS, Lee SB, Song SH, Seong EY, Yang BY, Lee MY, So l MY.2009. Post-treatment effects of erythropoietin and nordihydroguaiaretic acid on recovery from cisplatin-induced acute renal failure in the rat.Journal of Korean Medical Science,24(Supp l 1): S170–S175.
Lee VW, Harris DC. 2011. Adriamycin nephropathy: a m odel of focal segmental glomerulosclerosis.Nephrology,16(1): 30–38.
Lee YC, Zhang ZJ, Mukherjee AB. 2006. Mice lacking uteroglobin are highly susceptible to developing pulmonary fibrosis.FEBS Letters,580(18):4515–4520.
Lee lahavanichkul A, Yan Q, Hu XZ, Eisner C, Huang YN, Chen R, Mizel D,Zhou H, W right EC, Kopp JB, Schnerm ann J, Yuen PST, Star RA. 2010.Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse mode l.Kidney International,78(11): 1136–1153.
Liebau MC, Serra AL. 2013. Looking at the (w)ho le: magnet resonance imaging in polycystic kidney disease.Pediatric Nephrology,28(9): 1771–1783.
Liu DJ, Shang HP, Liu Y. 2016a. Stanniocalcin-1 protects a m ouse m odel from renal ischem ia-reperfusion injury by affecting ROS-mediated multip le signaling pathways.International Journal of Molecular Sciences,17(7):1051.
Liu J, Abdel-Razek O, Liu ZY, Hu FQ, Zhou QS, Cooney RN, Wang GR.2015a. Role of surfactant proteins A and D in sepsis-induced acute kidney injury.Shock,43(1): 31–38.
Liu L, Song YJ, Zhao M, Yi ZW, Zeng QY. 2015b. Protective effects of edaravone, a free radical scavenger, on lipopolysaccharide-induced acute kidney injury in a rat model of sepsis.International Urology and Nephrology,47(10): 1745–1752.
Liu X, Wang N, Wei G, Fan SJ, Lu YL, Zhu YF, Chen Q, Huang M, Zhou H,Zheng J. 2016b. Consistency and pathophysiological characterization of a rat polym icrobial sepsis m ode l via the im p roved cecal ligation and puncture surgery.International Immunopharmacology,32: 66–75.
Liu Z, Davidson A. 2012a. Tam ing lupus-a new understanding of pathogenesis is leading to clinica l advances.Nature Medicine,18(6): 871–882.
Liu Z, Huang XR, Lan HY. 2012b. Smad3 mediates ANG II-induced hypertensive kidney disease in mice.American Journal of Physiology-Renal Physiology,302(8): F986–F997.
Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, Edelstein CL. 2008.Increased macrophage infiltration and fractalkine expression in cisp latininduced acute renal failure in mice.The Journal of Pharmacology and Experimental Therapeutics,324(1): 111–117.
Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, Vikse BE. 2013. Effect of fetal and child health on kidney development and longterm risk of hypertension and kidney disease.The Lancet,382(9888): 273–283.
Ma H, Sandor DG, Beck LH Jr. 2013. The role of com plement in membranous nephropathy.Seminars in Nephrology,33(6): 531–542.
Maimaitiyim ing H, Zhou Q, Wang SX. 2016. Thrombospondin 1 deficiency am eliorates the developm ent of adriam ycin-induced proteinuric kidney disease.PLoS One,11(5): e0156144.
Makker SP, Tram ontano A. 2011. Idiopathic mem branous nephropathy: an autoimmune disease.Seminars in Nephrology,31(4): 333–340.
Ma lik S, Sucha l K, Gamad N, Dinda AK, Arya DS, Bhatia J. 2015.Telm isartan ameliorates cisp latin-induced nephrotoxicity by inhibiting MAPK mediated inflammation and apoptosis.European Journal of Pharmacology,748: 54–60.
Marchant V, Droguett A, Valderrama G, Burgos ME, Carpio D, Kerr B, Ruiz-Ortega M, Egido J, Mezzano S. 2015. Tubular overexpression of Grem lin in transgenic mice aggravates renal damage in diabetic nephropathy.American Journal of Physiology-Renal Physiology,309(6): F559–F568.
Martin-Sanchez D, Ruiz-Andres O, Poveda J, Carrasco S, Cannata-Ortiz P,Sanchez-Niño MD, Ortega MR, Egido J, Linkermann A, Ortiz A, Sanz AB.2017. Ferroptosis, but not necroptosis, is im portant in nephrotoxic folic acid-induced AKI.Journal of the American Society of Nephrology,28(1):218–229.
Matsui K, Kam ijo-Ikemorif A, Sugaya T, Yasuda T, Kimura K. 2011. Renal liver-type fatty acid binding protein (L-FABP) attenuates acute kidney injury in aristo lochic acid nephrotoxicity.The American Journal of Pathology,178(3): 1021–1032.
McGaha TL, Madaio MP. 2014. Lupus nephritis: animal modeling of a com p lex disease syndrome pathology.Drug Discovery Today: Disease Models,11: 13–18.
McGaha TL, Sorrentino B, Ravetch JV. 2005. Restoration of tolerance in lupus by targeted inhibitory receptor expression.Science,307(5709): 590–593.
McPhee CG, Bubier JA, Sproule TJ, Park G, Steinbuck MP, Schott WH,Christianson GJ, Morse III HC, Roopenian DC. 2013. IL-21 is a doubleedged sword in the system ic lupus erythematosus-like disease of BXSB.Yaamice.The Journal of Immunology,191(9): 4581–4588.
Mitazaki S, Kato N, Suto M, Hiraiwa K, Abe S. 2009. Interleukin-6 deficiency acce lerates cisp latin-induced acute renal failure but not system ic injury.Toxicology,265(3): 115–121.
Mollet G, Ratelade J, Boyer O, Muda AO, Morisset L, Lavin TA, Kitzis D,Dallman MJ, Bugeon L, Hubner N, Gubler MC, Antignac C, Esquivel EL.2009. Podocin inactivation in mature kidneys causes focal segmental g lom erulosclerosis and nephrotic syndrome.Journal of the American Society of Nephrology,20(10): 2181–2189.
Morsy MA, Heeba GH. 2016. Nebivolol ameliorates cisplatin-induced nephrotoxicity in rats.Basic & Clinical Pharmacology & Toxicology,118(6):449–455.
Motiram Kaka lij R, Tejaswini G, Patil MA, Dinesh Kum ar B, Diwan PV. 2016.Vanillic acid ameliorates cationic bovine serum album in induced immune com p lex glomeru lonephritis in BALB/c mice.Drug Development Research,77(4): 171–179.
Moura IC, Benhamou M, Launay P, Vrtovsnik F, Blank U, Monteiro RC.2008. The glomerular response to IgA deposition in IgA nephropathy.Seminars in Nephrology,28(1): 88–95.
Nakayama T, Sato W, Kosugi T, Zhang L, Cam pbell-Thom pson M,Yoshimura A, Croker BP, Johnson RJ, Nakagawa T. 2009. Endothelial injury due to eNOS deficiency accelerates the progression of chronic rena l disease in the mouse. Am ericanJournal of Physiology-Renal Physiology,296(2): F317–F327.
Nickerson KM, Cullen JL, Kashgarian M, Shlom chik MJ. 2013. Exacerbated autoimmunity in the absence of TLR9 in MRL.Faslprmice depends onIfnar1.The Journal of Immunology,190(8): 3889–3894.
Nortier J, Pozdzik A, Roum eguere T, Vanherweghem JL. 2015. [Aristo lochic acid nephropathy ("Chinese herb nephropathy")].Nephrologie &Therapeutique,11(7): 574–588.
Orlando LA, Belasco EJ, Pate l UD, Matchar DB. 2011. The chronic kidney disease model: a general purpose model of disease progression and treatment.BMC Medical Informatics and Decision Making,11: 41.
Ortiz A, Sanchez-Niño MD, Izquierdo MC, Martin-Cleary C, Garcia-Bermejo L, Moreno JA, Ruiz-Ortega M, Draibe J, Cruzado JM, Garcia-Gonzalez MA,Lopez-Novoa JM, Soler MJ, Sanz AB. 2015. Translational value of anima l models of kidney failure.European Journal of Pharmacology,759: 205–220.Otani Y, Ichii O, Otsuka-Kanazawa S, Chihara M, Nakamura T, Kon Y. 2015.MRL/Mp J-Faslprmice show abnormalities in ovarian function and morphology with the progression of autoimm une disease.Autoimmunity,48(6): 402–411.
Ozcan A, Ware K, Calomeni E, Nadasdy T, Forbes R, Satoskar AA,Nadasdy G, Rovin BH, Hebert LA, Brodsky SV. 2012. 5/6 nephrectomy as a validated rat model mimicking human warfarin-related nephropathy.American Journal of Nephrology,35(4): 356–364.
Ozkok A, Edelstein CL. 2014. Pathophysiology of cisplatin-induced acute kidney injury.BioMed Research International,2014: 967826.
Østergaard MV, Pinto V, Stevenson K, Worm J, Fink LN, Coward RJM.2017. DBA2Jdb/dbmice are susceptible to early album inuria and glomerulosclerosis that correlate with systemic insulin resistance.American Journal of Physiology-Renal Physiology,312(2): F312–F321.
Pálsson R, Patel UD. 2014. Cardiovascular com p lications of diabetic kidney disease.Advances in Chronic Kidney Disease,21(3): 273–280.
Pannu N. 2013. Bidirectional relationships between acute kidney in jury and chronic kidney disease.Current Opinion in Nephrology and Hypertension,22(3): 351–356.
Papista C, Lechner S, Ben Mkaddem S, Lestang MB, Abbad L, Bex-Coud rat J, Pillebout E, Chemouny JM, Jab lonski M, Flamant M, Daugas E,Vrtovsnik F, Yiangou M, Berthelot L, Monteiro RC. 2015. G luten exacerbates IgAnephropathy in hum anized mice through gliadin-CD89 interaction.Kidney International,88(2): 276–285.
Peng WH, Chen JH, Jiang YG, Wu JY, Shou ZF, He Q, Wang YM, Chen Y,Wang HP. 2008. Urinary fractalkine is a marker of acute rejection.Kidney International,74(11): 1454–1460.
Poli-De-Figueiredo LF, Garrido AG, Nakagawa N, Sannomiya P. 2008.Experimental models of sepsis and their clinical relevance.Shock,30(Supp l 1): 53–59.
Ramesh G, Ranganathan P. 2014. Mouse models and methods for studying hum an disease, acute kidney in jury (AKI).In: Singh S, Coppola V. Mouse Genetics. New York, NY: Humana Press,1194: 421–436.
Rifai A, Sm all PA Jr, Teague PO, Ayoub EM. 1979. Experimental IgAnephropathy.The Journal of Experimental Medicine,150(5): 1161–1173.
Rizk DV, Warnock DG. 2011. Warfarin-related nephropathy: another newly recognized complication of an old drug.Kidney International,80(2): 131–133.
Romero F, Pérez M, Chávez M, Parra G, Durante P. 2009. Effect of uric acid on gentamicin-induced nephrotoxicity in rats - role of matrix meta lloproteinases 2 and 9.Basic & Clinical Pharmacology & Toxicology,105(6): 416–424.
Ronco P, Debiec H. 2010. Antigen identification in membranous nephropathy moves toward targeted monitoring and new therapy.Journal of the American Society of Nephrology,21(4): 564–569.
Ross L, Banerjee D. 2013. Cardiovascular com p lications of chronic kidney disease.International Journal of Clinical Practice,67(1): 4–5.
Rovcanin B, Medic B, Kocic G, Cebovic T, Ristic M, Prostran M. 2016.Mo lecular dissection of renal is chemia-reperfusion: oxidative stress and cellular events.Current Medicinal Chemistry,23(19): 1965–1980.
Rui HL, Wang YY, Cheng H, Chen YP. 2012. JNK-dependent AP-1 activation is required for aristolochic acid-induced TGF-β1 synthesis in human renal p roxim al epithelial cells.American Journal of Physiology-Renal Physiology,302(12): F1569–F1575.
Ryu M, Mulay SR, Miosge N, Gross O, Anders HJ. 2012. Tumour necrosis factor-α drives Alport glomerulosclerosis in mice by promoting podocyte apoptosis.The Journal of Pathology,226(1): 120–131.
Sanz AB, Sanchez-Niño MD, Martin-Cleary C, Ortiz A, Ramos AM. 2013.Progress in the development of animal models of acute kidney injury and its impact on drug discovery.Expert Opinion on Drug Discovery,8(7): 879–895.
Sanz AB, Izquierdo MC, Sanchez-Nino MD, Ucero AC, Egido J, Ruiz-Ortega M, Ramos AM, Putterman C, Ortiz A. 2014. TWEAK and the progression of renal disease: clinical translation.Nephrology Dialysis Transplantation,29(Suppl 1): i54–i62.
Savige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F. 2013. Expert guide lines for the management of Alport syndrome and thin basem ent membrane nephropathy.Journal of the American Society of Nephrology,24(3): 364–375.
Savige J, Ratnaike S, Colville D. 2011. Retinal abnormalities characteristic of inherited renal disease.Journal of the American Society of Nephrology,22(8): 1403–1415.
Siew ED, Davenport A. 2015. The grow th of acute kidney injury: a rising tide or just closer attention to detail?Kidney International,87(1): 46–61.
Simons JP, Al-Shaw i R, Ellmerich S, Speck I, Aslam S, Hutchinson WL,Mangione PP, Disterer P, Gilbertson JA, Hunt T, Millar DJ, Minogue S,Bodin K, Pepys MB, Hawkins PN. 2013. Pathogenetic mechanism s of amyloid a amyloidosis.Proceedings of the National Academy of Sciences of the United States of America,110(40): 16115–16120.
Singh AP, Junemann A, Muthuraman A, Jaggi AS, Singh N, Grover K,Dhawan R. 2012. Animal models of acute renal failure.Pharmacological Reports,64(1): 31–44.
Singh P, Ricksten SE, Bragadottir G, Red fors B, Nordquist L. 2013. Renal oxygenation and haemodynam ics in acute kidney injury and chronic kidney disease.Clinical and Experimental Pharmacology and Physiology,40(2):138–147.
Skinner M, Shirahama T, Benson MD, Cohen AS. 1977. Murine amyloid protein AA in casein-induced experimental amyloidosis.Laboratory Investigation; A Journal of Technical Methods and Pathology,36(4): 420–427.
Sm eets B, Te Loeke NAJM, Dijkman HBPM, Steenbergen MLM, Lensen JFM, Begieneman MPV, Van Kuppevelt TH, Wetzels JFM, Steenbergen EJ.2004. The parietal epithelial cell: a key p layer in the pathogenesis of focal segmental glomeruloscleros is in Thy-1.1 transgenic mice.Journal of the American Society of Nephrology,15(4): 928–939.
Soler MJ, Riera M, Batlle D. 2012. New experimental models of diabetic nephropathy in mice models of type 2 diabetes: efforts to replicate human nephropathy.Experimental Diabetes Research,2012: 616313.
So lov'eva T, Davydova V, Krasikova I, Yerm ak I. 2013. Marine com pounds with therapeutic potential in gram-negative sepsis.Marine Drugs,11(6):2216–2229.
Soofi A, Zhang P, Dressler GR. 2013. Kielin/chordin-like protein attenuates both acute and chronic renal in jury.Journal of the American Society of Nephrology,24(6): 897–905.
Soto K, Coelho S, Rodrigues B, Martins H, Frade F, Lopes S, Cunha L,Papoila AL, Devarajan P. 2010. Cystatin C as a marker of acute kidney injury in the emergency department.Clinical Journal of the American Society of Nephrology,5(10): 1745–1754.
Stallons LJ, Whitaker RM, Schnellmann RG. 2014. Suppressed m itochondrial biogenesis in folic acid-induced acute kidney injury and early fibrosis.Toxicology Letters,224(3): 326–332.
Stojiljkovic N, Mihailovic D, Veljkovic S, Stoiljkovic M, Jovanovic I. 2008.G lom erular basement membrane alterations induced by gentam icin adm inistration in rats.Experimental and Toxicologic Pathology,60(1): 69–75.
Stojiljkovic N, Stoiljkovic M, Rand jelovic P, Veljkovic S, Mihailovic D. 2012.
Cytoprotective e ffect of vitam in C against gentam icin-induced acute kidney injury in rats.Experimental and Toxicologic Pathology,64(1–2): 69–74.
Sugandhi N, Srinivas M, Agarwa la S, Gupta DK, Sharma S, Sinha A, Dinda A, Mohanty S. 2014. Effect of stem ce lls on renal recovery in rat model of partial unilateral upper ureteric obstruction.Pediatric Surgery International,30(2): 233–238.
Suzuki H, Suzuki Y, Novak J, Tom ino Y. 2014. Development of animal models of hum an IgA nephropathy.Drug Discovery Today: Disease Models,11: 5–11.
Swam inathan S, Rosner MH, Okusa MD. 2015. Emerging therapeutic targets of sepsis-associated acute kidney in jury.Seminars in Nephrology,35(1): 38–54.
Sweeney WE Jr, Avner ED. 2011. Diagnosis and m anagement of childhood polycystic kidney disease.Pediatric Nephrology,26(5): 675–692.
Szczypka MS, Westover AJ, Clouthier SG, Ferrara JLM, Hum es HD. 2005.Rare incorporation of bone marrow-derived cells into kidney after fo lic acidinduced injury.Stem Cells,23(1): 44–54.
Takahashi T, Harris RC. 2014. Role of endothelia l nitric oxide synthase in diabetic nephropathy: lessons from diabetic eNOS knockout mice.Journal of Diabetes Research,2014: 590541.
Tampe B, Steinle U, Tampe D, Carstens JL, Korsten P, Zeisberg EM, Müller GA, Kalluri R, Zeisberg M. 2017. Low-dose hydralazine prevents fibrosis in a murine mode l of acute kidney injury-to-chronic kidney disease progression.Kidney International,91(1): 157–176.
Tang J, Jiang XZ, Zhou YH, Xia B, Dai YB. 2015. Increased adenosine levels contribute to ischem ic kidney fibrosis in the unilateral ureteral obstruction model.Experimental and Therapeutic Medicine,9(3): 737–743.Tapia E, Soto V, Ortiz-Vega KM, Zarco-Márquez G, Mo lina-Jijón E,Cristóbal-García M, Santamaria J, García-Niño WR, Correa F, Zazueta C,Pedraza-Chaverri J. 2012. Curcum in induces Nrf2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectom ized rats.Oxidative Medicine and Cellular Longevity,2012: 269039.
Teng JM, Turbat-Herrera EA, Herrera GA. 2014. An anima l model of glomerular light-chain-associated amyloidogenesis depicts the crucial role of lysosomes.Kidney International,86(4): 738–746.
Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Po lski B, Ma H,Zahner G, Dolla G, Hoxha E, Helm chen U, Dabert-Gay AS, Debayle D,Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G. 2014.
Throm bospondin type-1 domain-containing 7A in idiopathic membranous nephropathy.The New England Journal of Medicine,371(24): 2277–2287.
Tom ino Y. 2014. Pathogenesis and treatm ent of chronic kidney disease: a review of our recent basic and clinical data.Kidney & Blood Pressure Research,39(5): 450–489.
Torres VE, Chapm an AB, Devuyst O, Gansevoort RT, Grantham JJ,Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerw iec FS. 2012.Tolvaptan in patients with autosomal dom inant polycystic kidney disease.The New England Journal of Medicine,367(25): 2407–2418.
Ucero AC, Benito-Martin A, Izquierdo MC, Sanchez-Niño MD, Sanz AB,Ramos AM, Berzal S, Ruiz-Ortega M, Egido J, Ortiz A. 2014. Unilateral ureteral obstruction: beyond obstruction.International Urology and Nephrology,46(4): 765–776.
Ustundag S, Yalcin O, Sen S, Cukur Z, Ciftci S, Dem irkan B. 2008.Experimental m yoglobinuric acute renal failure: the effect of vitam in C.Renal Failure,30(7): 727–735.
Ustundag S, Sen S, Yalcin O, Ciftci S, Dem irkan B, Ture M. 2009. LCarnitine ameliorates g lycerol-induced m yoglobinuric acute rena l failure in rats.Renal Failure,31(2): 124–133.
Venkatachalam MA, Weinberg JM. 2012. The tubu le patho logy of septic acute kidney injury: a neglected area of research comes of age.Kidney International,81(4): 338–340.
Viscom i C, Spinazzo la A, Maggioni M, Fernandez-Vizarra E, Massa V,Pagano C, Vettor R, Mora M, Zeviani M. 2009. Early-onset liver m tDNA dep letion and late-onset proteinuric nephropathy inMpv17 knockoutmice.Human Molecular Genetics,18(1): 12–26.
Wada Y, Abe M, Moritani H, Mitori H, Kondo M, Tanaka-Am ino K, Eguchi M,Imasato A, Inoki Y, Kajiyama H, Mimura T, Tomura Y. 2016. Original Research: Potential of urinary nephrin as a biomarker reflecting podocyte dysfunction in various kidney disease models.Experimental Biology and Medicine,241(16): 1865–1876.
Wang Y, Wang YP, Tay YC, Harris DCH. 2000. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochem ical events.Kidney International,58(4): 1797–1804.
Ware K, Brodsky P, Satoskar AA, Nadasdy T, Nadasdy G, Wu HF, Rovin BH,Bhatt U, Von Visger J, Hebert LA, Brodsky SV. 2011. Warfarin-related nephropathy mode led by nephron reduction and excessive anticoagulation.Journal of the American Society of Nephrology,22(10): 1856–1862.
Wei QQ, Dong Z. 2012. Mouse model of ischem ic acute kidney injury:technical notes and tricks.American journal of physiology: Renal Physiology,303(11): F1487-F1494.
Wen XY, Peng ZY, Li YJ, Wang HZ, Bishop JV, Chedwick LR, Singbartl K,Kellum JA. 2012. One dose of cyclosporine A is protective at initiation of folic acid-induced acute kidney injury in mice.Nephrology Dialysis Transplantation,27(8): 3100–3109.
Woudenberg-Vrenken TE, Bindels RJM, Hoenderop JGJ. 2009. The ro le of transient recep tor potential channels in kidney disease.Nature Reviews Nephrology,5(8): 441–449.
Wu J, Liu XH, Fan JJ, Chen WF, Wang J, Zeng YJ, Feng XR, Yu XQ, Yang X. 2014. Bardoxolone methyl (BARD) ameliorates aristolochic acid (AA)-induced acute kidney injury through Nrf2 pathway.Toxicology,318: 22–31.Xiao LX, Zhou D, Tan RJ, Fu HY, Zhou LL, Hou FF, Liu YH. 2016.Sustained activation of wnt/β-catenin signaling drives AKI to CKD progression.Journal of the American Society of Nephrology,27(6): 1727–1740.
Xu C, Chang A, Hack BK, Eadon MT, Alper SL, Cunningham PN. 2014.TNF-mediated damage to glomerular endothelium is an important determ inant of acute kidney injury in sepsis.Kidney International,85(1):72–81.
Xu YF, Ma HB, Shao J, Wu JF, Zhou LY, Zhang ZR, Wang YZ, Huang Z,Ren JM, Liu SH, Chen XM, Han JH. 2015. A role for tubular necroptosis in cisp latin-induced AKI.Journal of the American Society of Nephrology,26(11): 2647–2658.
Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. 2010.Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury.Nature Medicine,16(5): 535–543.
Zarjou A, Agarwa l A. 2011. Sepsis and acute kidney injury.Journal of the American Society of Nephrology,22(6): 999–1006.
Zhang JD, Rudem iller NP, Patel MB, Wei QQ, Karlovich NS, Jeffs AD, Wu M, Sparks MA, Privratsky JR, Herrera M, Gurley SB, Nedospasov SA,Crow ley SD. 2016. Com peting actions of type 1 angiotensin ii receptors expressed on t lymphocytes and kidney epithelium during cisplatin-induced AKI.Journal of the American Society of Nephrology,27(8): 2257–2264.
Zhang L, Fu P, Wang L, Cai GY, Zhang L, Chen DZ, Guo DY, Sun XF, Chen FQ, Bi WH, Zeng XJ, Li HY, Liu ZH, Wang Y, Huang SM, Chen XM. 2012.The clinical features and outcome of crush patients with acute kidney injury after the Wenchuan earthquake: differences between e lderly and younger adu lts.Injury,43(9): 1470–1475.
Zhong F, Ma llipattu SK, Estrada C, Menon M, Sa lem F, Jain MK, Chen HY, Wang YJ, Lee K, He JC. 2016. Reduced krüppel-like factor 2 aggravates g lom erular endothe lial cell injury and kidney disease in mice with unilateral nephrectom y.The American Journal of Pathology,186(8):2021–2031.
Zhou SQ, Sun YY, Zhuang YG, Zhao W, Chen YZ, Jiang BJ, Guo CF,Zhang ZL, Peng H, Chen YQ. 2015. Effects of kallistatin on oxidative stress and inflamm ation on renal ischem ia-reperfusion injury in mice.Current Vascular Pharmacology,13(2): 265–273.
Zhou XJ, Zhang J, Xu CG, Wang W. 2014. Curcum in ameliorates renal fibrosis by inhibiting local fibroblast proliferation and extracellular matrix deposition.Journal of Pharmacological Sciences,126(4): 344–350.
杂志排行
Zoological Research的其它文章
- In vivo genome editing thrives with diversified CRISPR technologies
- King cobra peptide OH-CATH30 as a potential candidate drug through clinic drug-resistant isolates
- Biogeography of the Shimba Hills ecosystem herpetofauna in Kenya
- Discovery of Japalura chapaensis Bourret, 1937(Reptilia: Squamata: Agam idae) from Southeast Yunnan Province, China
- Stereotypy and variability of socialcalls among clustering female big-footed myotis(Myotismacrodactylus)
- AutoSeqMan:batch assembly of contigs for Sanger sequences
