缺血性卒中基础与临床研究进展
2018-05-07马媛媛杨国源
马媛媛 杨国源
脑卒中系各种原因引起的脑血管病变致局灶性神经功能缺损或中枢神经系统损伤[1]。脑卒中是目前导致全球人类死亡的第2位疾病,是导致成人永久性病残的首要病因[2⁃3]。美国每40秒即有1例罹患脑卒中[4]。2013年,全球有 103×106例发生脑卒中,65×106例死于脑卒中,达11.30×106/残疾调整生命年(DALY)[5]。由于不发达国家脑卒中危险因素增加,其造成的经济负担仍有增加趋势。预计截至2030年,脑卒中病死例数将达120×106例,达0.20×109/残疾调整生命年[6]。我国是脑卒中负担最重的国家之一,过去30年间脑卒中负担增加,特别是农村地区[7⁃8],2013 年我国脑卒中患病率、发病率和病死率分别为1114.80/10万、246.80/10万和114.80/10 万[9]。
脑卒中包括缺血性卒中(约占87%[10])和出血性卒中。既往数十年,脑卒中的临床治疗取得新的进展,特别是血管内机械取栓的出现,开启缺血性卒中治疗的新篇章。对于梗死灶小且有良好侧支循环的患者,血管内机械取栓可以显著改善临床症状[11⁃12]。随机对照临床试验显示,缺血性卒中 8 小时内予血管内机械取栓治疗可以有效改善临床症状[13]。英国国家卫生与临床优化研究所(NICE)制定指南,只要条件允许,静脉溶栓和血管内机械取栓是缺血性卒中治疗的“金标准”[1]。目前,缺血性卒中的治疗策略主要分为三步:建立卒中单元;对符合条件的患者进行静脉溶栓;血管内机械取栓重新开通堵塞的血管[14⁃18]。但是由于严格的治疗时间窗(脑卒中后4.50小时内)或存在严重的并发症风险,仅不足5%的患者予以重组组织型纤溶酶原激活物(rt⁃PA)静脉溶栓,部分行rt⁃PA 静脉溶栓治疗的患者可同时行血管内机械取栓治疗,大多数缺血性卒中患者仅行对症支持治疗[19]。有研究显示,行rt⁃PA静脉溶栓的患者中仅10%临床预后较好,行血管内机械取栓的患者中71%实现血管再通、仅50%实现缺血⁃再灌注[20⁃22]。因此,积极寻找改善缺血性卒中预后的方法即显得尤为重要。尽管缺血性卒中病理生理学机制复杂,临床治疗进展缓慢,尚待大量动物实验和临床前试验以加快临床转化进程,但是目前已有众多临床前或临床治疗策略显示出较好的治疗潜能。本文拟就我国国民经济和社会发展第十二个五年规划(以下简称“十二五”)时期取得的缺血性卒中基础与临床研究进展进行概述。
一、组学技术进展
无论是静脉溶栓,还是快速有效促进组织修复或减少损伤均需要有特定方向,多组学技术的出现为缺血性卒中提供新的特异性治疗靶点。多组学技术包括基因组学、二代基因测序、转录组学、蛋白质组学和代谢组学。通过上述组学技术,不仅可以发现脑卒中损伤或修复相关病理生理学机制,同时还可以为临床药物试验提供快速评价药物毒性和治疗效果的分子学标志物。全基因组相关性研究(GWAS)业已发现众多与脑卒中发生与发展相关的重要遗传学标志物,如定位于12q24的ALDH2基因与所有类型缺血性卒中密切相关,而某些基因如HDAC9基因和定位于1p13.2的TSPAN2基因与大动脉粥样硬化型(LAA型)密切相关,PITX2和ZFHX3基因与心源性栓塞型(CE型)密切相关[23],某种单核苷酸多态性(SNP)与冠心病密切相关[24]。最近研究显示,髓过氧化物酶(MPO)基因表达上调与腔隙性梗死密切相关,由于该基因不受环境因素的影响,提示MPO基因是脑血管病的致病基因[25]。基因组学的进展为早期采取有效措施干预脑卒中高危人群生活方式和针对性治疗提供可能。
蛋白质组学和代谢组学的快速发展有利于脑卒中的诊断、治疗和预后评价。蛋白质组学技术发现,rt⁃PA静脉溶栓可以引起脑卒中患者血浆蛋白酶如基质金属蛋白酶(MMPs)表达变化,提示rt⁃PA可能通过改变相关蛋白质表达水平或调节蛋白酶活性发挥治疗作用或引起颅内出血等并发症[26]。蛋白质组学技术有助于发现脑卒中后血⁃脑屏障(BBB)破坏相关关键靶分子,从而拓宽rt⁃PA静脉溶栓的治疗范围。蛋白质组学技术显示,血清淀粉样蛋白A(SAA)水平升高与脑卒中后感染密切相关,提示可以通过检测血清淀粉样蛋白A表达变化以预测并预防脑卒中后感染的发生[27]。临床前实验方面,通过蛋白质组学技术发现,与缺血性卒中组和缺血预处理组小鼠相比,缺血性卒中耐受组小鼠脑组织转录抑制蛋白特别是多梳家族(PcG)水平升高,介导脑卒中后神经保护的表观遗传学机制[28]。亦有研究显示,缺血性卒中患者移植骨髓间充质干细胞(BMSCs)后,一些蛋白质如Abca1、Grb2、Ptgds等水平升高,提示其可能是骨髓间充质干细胞发挥治疗作用的关键[29]。因此,蛋白质组学技术可以提供更多有价值的信息,有助于研究人员更好地探讨缺血性卒中的病理生理学机制,为临床治疗缺血性卒中提供新的靶点。代谢组学通过检测体液中小分子代谢物,一方面可以增加影像学检查的敏感性,另一方面可以有助于预测短暂性脑缺血发作(TIA)患者发生脑卒中的可能[30⁃31]。有学者采集脑卒中患者体液包括血浆、尿液和粪便进行代谢组学分析,发现血浆和尿液同型半胱氨酸(Hcy)、叶酸、支链氨基酸和脂质代谢物水平可以预测脑卒中复发风险[32]。此外,血浆支链氨基酸和脂质代谢物水平降低与脑卒中后抑郁(PSD)和脑卒中后认知功能障碍(PSCI)相关[33]。根据脑卒中特殊病理生理学变化,联合代谢组学技术可以将脑卒中分为不同亚组,以进行更加精准的治疗[34]。更重要的是,代谢组学技术不仅可以弥补蛋白质组学不能快速诊断的缺陷,而且可以将蛋白质组学和基因组学信息相联系。有学者采用代谢组学技术研究白藜芦醇治疗缺血性卒中的作用机制,发现神经元沉默信息调节因子1(SIRT1)基因可以调节葡萄糖代谢和糖酵解过程,影响实验动物对缺血性卒中的耐受程度,提示代谢组学可以作为研究缺血性卒中发病机制和药物作用机制的新方法[35]。未来有望通过上述组学技术、多学科合作,更好地研究脑卒中发病机制和损伤机制[1]。
二、基因治疗进展
脑卒中后数小时至数周,脑组织损伤仍在继续,为基因治疗提供可能。目前,基因治疗的主要措施是通过外源性导入具有抗凋亡、增加细胞存活、靶向引导干细胞、促进血管再生和神经再生等作用的基因,最大程度地促进组织恢复、减轻脑组织损伤[36]。抗凋亡基因主要是B细胞淋巴瘤/白血病⁃2(Bcl⁃2)家族成员,包括 Bcl⁃2、Bcl⁃xL 和 Bcl⁃2l2或 Bcl⁃w,可以调节细胞凋亡、促进神经再生[37]。基础研究显示,多种病毒载体,如单纯疱疹病毒(HSV)[38]、腺病毒[39]、腺相关病毒(AAV)[40]和慢病毒[41]等均可介导缺血性卒中后 Bcl⁃2 过表达,且发挥良好治疗作用。通过单纯疱疹病毒载体在脑组织中过表达Bcl⁃2,可以减少梗死灶体积、减轻神经胶质细胞增生,可能是通过抑制骨形态发生蛋白4(BMP⁃4)表达上调以促进脑缺血后纹状体区神经再生[42]。过表达Bcl⁃2可以抑制脑卒中后脑组织凋亡诱导因子(AIF)的核转位,增加神经元存活[43]。亦有研究显示,缺血性卒中患者脑组织过表达热休克蛋白(HSP)家族成员HSP72和谷胱甘肽过氧化物酶(GSH⁃Px),通过上调 Bcl⁃2表达以发挥减少脑缺血后神经元损伤、促进长期功能恢复的作用[44⁃45]。
神经营养因子具有调节神经细胞生长、发育、增殖、分化,并在生理和病理环境下促进神经细胞存活,维持神经细胞稳态的作用。目前,多项研究采用不同病毒载体在脑组织过表达神经营养因子相关基因,如血管内皮生长因子(VEGF)[46]、脑源性神经营养因子(BDNF)[47]、胶质细胞源性神经营养因子(GDNF)[48⁃50]、睫状神经营养因子(CNTF)[48]、脑多巴胺源性神经营养因子(CDNF)[51]和类肝素结合样表皮生长因子(HB⁃EGF)[52],可以不同程度减小梗死灶体积和抑制凋亡通路激活,从而减轻脑组织损伤。除直接过表达神经营养因子相关基因以发挥神经保护作用外,亦可以过表达增加内源性干细胞活性的基因和促内源性修复的基因,如趋化因子CXCL12,又称基质细胞衍生因子⁃1(SDF⁃1),于缺血性卒中后表达上调[53]。研究显示,缺血性卒中前3天预过表达或脑卒中后1周过表达CXCL12,可以减轻脑萎缩,促进血管新生和神经再生,促进少突前体细胞迁移、分化、髓鞘再生,从而加速脑组织修复[54⁃56]。另一种神经生长因子——轴突导向因子⁃1(netrin⁃1)在生理状态下可以促进神经轴突生长、发育和成熟,发生缺血性卒中时表达上调。研究显示,缺血性卒中小鼠脑组织过表达轴突导向因子⁃1,可以促进脑缺血后少突胶质前体细胞增殖和成熟,减轻白质损害,改善脑卒中后运动功能、肢体协调能力和探索能力[57⁃58]。在既往研究中,过表达基因的时间点从预处理至脑卒中后即刻、数小时、1周,治疗时间窗广泛,为不同类型脑卒中的治疗提供理论基础。
三、微小RNA治疗进展
微小RNA(miRNA)是小序列非编码RNA,转录后可以调节多种蛋白质表达。1993年,Lee等[59]首先在秀丽线虫体内发现miRNA。迄今已在200余种物种中发现 30 × 103余种 miRNA[60]。过去 10 年间,关于miRNA结构、功能、生物学特性和病理生理学作用机制的研究发展迅速[61]。在病理条件下,血浆和体液miRNA含量迅速变化,通过调节下游靶蛋白表达加速或延缓疾病进程[62]。多种miRNA均与缺血性卒中高危因素如高血压、糖尿病和动脉粥样硬化等相关(表1),研究显示,与正常对照者相比,高血压患者血清miRNA⁃7⁃5p 和 miRNA⁃26b⁃5p 水平升高[63],推测miRNA可能与高血压的发生与发展相关;过表达miRNA⁃21可以通过抑制Bcl⁃2表达而诱导胰腺β细胞凋亡[64],提示miRNA⁃21高表达可能加速糖尿病的发生;人动脉粥样硬化血管组织中,miRNA⁃206 水平降低[65];体外实验结果显示,过表达miRNA⁃206可以抑制血管平滑肌增殖,诱导其凋亡[66],提示 miRNA⁃206可能是动脉粥硬化的治疗靶点。缺血性卒中急性期,miRNA表达变化与脑卒中病理生理学机制如兴奋性毒性、炎症反应、氧化应激、细胞凋亡、血⁃脑屏障破坏等相关(表1),例如,缺血性卒中急性期血浆miRNA⁃181c水平降低,与血小板计数呈正相关[67];缺血性卒中模型小鼠急性期脑组织过表达miRNA⁃181c,可以增加神经元和小胶质细胞凋亡及梗死灶体积[67];缺血性卒中恢复期,miRNA通过调节神经再生和血管再生促进脑组织修复[61](表 1)。鉴于 miRNA 在缺血性卒中病理生理学机制中的重要作用,多项研究采用miRNA治疗缺血性卒中,侧脑室过表达miRNA⁃148b可通过调节 Wnt/β⁃连环蛋白(β⁃catenin)信号转导通路,促进缺血性卒中后神经干细胞(NSCs)分化为成熟神经元和星形胶质细胞,从而促进神经功能恢复[68];脑组织过表达miRNA⁃1906可通过下调Toll样受体4(TLR⁃4)水平以发挥减小梗死灶体积、促进功能恢复的作用[69];过表达 miRNA⁃29b 可减轻血⁃脑屏障破坏、减小梗死灶体积,可能与抑制水通道蛋白4(AQP4)表达有关[70]。由于miRNA在体液中稳定存在且具有组织特异性,有多项研究检测血浆miRNA表达变化,旨在探讨可以有效预测缺血性卒中和评价预后的新型miRNA,以填补临床尚无针对缺血性卒中血浆分子生物学标志物的空白。最新研究显示,脑卒中后抑郁患者血浆miRNA⁃92a⁃3p水平升高,与抑郁程度和前脑白质高信号相关[71],提示miRNA⁃92a⁃3p可能介导脑卒中后抑郁。另一项研究对缺血性卒中患者和正常对照者进行miRNA组学分析,证实缺血性卒中后血浆miRNA⁃125a⁃5p、miRNA⁃125b⁃5p和 miRNA⁃143⁃3p水平升高,提示这3种miRNA有望成为缺血性卒中早期诊断的分子标志物。尽管关于miRNA作为缺血性卒中分子标志物的研究较多,但目前临床进展仍较缓慢,主要是由于既往miRNA检测存在异质性,且后续验证缺乏科学严谨的实验设计[72],尚待更多研究加速miRNA作为缺血性卒中分子标志物的临床转化过程[73]。

表1 脑卒中相关miRNATable 1. miRNAs associated with ischemic stroke
四、干细胞治疗进展
目前,除rt⁃PA静脉溶栓和血管内机械取栓外,干细胞治疗是最有可能从根本上促进缺血性卒中治疗进展的方法[14]。干细胞治疗包括胚胎干细胞(ESCs)[74⁃75]、诱导型多能干细胞(iPSCs)[76]、神经干细 胞[77⁃79]、骨 髓 间 充 质 干 细 胞[80⁃83]、造 血 干 细 胞(HSCs)[84]、内皮祖细胞(EPCs)[85⁃86]、脂肪间充质干细胞(ADSCs)[87],可以改善缺血性卒中预后(表2)。具体作用机制尚待进一步研究,但目前实验研究结果显示,干细胞通过抑制炎症反应、调节免疫应答、分化为成熟神经元或内皮细胞、促进神经突触重塑、促进神经再生和血管再生、分泌神经营养因子等多种途径发挥治疗作用[88⁃91]。干细胞治疗缺血性卒中的基础研究成果显著,过去15年间有多项干细胞治疗缺血性卒中的早期临床试验,其中一项多中心随机双盲Ⅱ期临床试验显示,缺血性卒中急性期(24~48小时)静脉移植异体多能干细胞(MultiStem®,美国Athersys公司)安全,能够改善脑卒中后1年的神经功能[91]。干细胞治疗的最常见不良反应是头痛和发热,但多呈自限性,主要与移植途径有关,头痛主要出现在接受立体定位注射移植的患者[82,92⁃93]。最新的研究采用新的移植方式——经鼻内途径移植。研究显示,经鼻内途径移植骨髓间充质干细胞治疗缺血性卒中可以促进神经血管再生,改善神经功能[94⁃95]。与其他移植方式如动脉注射移植、静脉注射移植和立体定位注射移植相比,经鼻内途径移植的优势在于简单、无创且移植细胞可以快速通过鼻黏膜进入脑实质。因此,经鼻内途径移植有可能成为临床干细胞移植途径的新选择[91,96]。多项研究证实,干细胞治疗缺血性卒中安全、有效,关于其最佳治疗时间窗和最佳移植途径的临床试验仍在进行中[91,97]。移植的干细胞主要是外源性神经干细胞、自体骨髓来源的间充质干细胞(MSCs)、单核细胞和CD34+干细胞;移植途径包括静脉注射移植、动脉注射移植、鞘内注射移植和立体定位注射移植;治疗效果评价的最短时间是移植后24小时(https://www.clinicaltrials.gov/ct2/results?cond=&term=NCT00859014&cntry1=&state1=&recrs=,试验编号:NCT00859014)、最长时间是移植 后 5年(https://www.clinicaltrials.gov/ct2/results?cond=&term=NCT01151124&cntry1=&state1=&Search=Search,试验编号:NCT01151124)。干细胞治疗缺血性卒中业已展现出巨大潜能,未来需要解决的问题主要有:(1)移植干细胞的来源。(2)干细胞治疗的最佳时间窗。(3)最大程度发挥和维持移植干细胞促组织修复的功能特性有待突破。(4)移植时导致肺栓塞或脑栓塞及移植后成瘤(胚胎干细胞或诱导型多能干细胞)[92,98]有待解决。因此,尚待更多试验设计科学、合理的大规模随机对照临床试验以验证干细胞治疗的安全性和有效性,以及实验设计科学、合理的基础研究,以阐明干细胞生物学特性、提高干细胞治疗效果。

表2 干细胞治疗缺血性卒中临床试验Table 2. Clinical trials of stem cell therapy for ischemic stroke
五、药物治疗进展
脑卒中是一种由包括环境因素和遗传因素在内的多因素共同导致的疾病,其病理生理学过程复杂,多种生物学分子、细胞和信号转导通路激活,给临床治疗带来巨大挑战,也同时提供多个可干预的治疗靶点。缺血性卒中治疗的首要目标是恢复脑组织灌注且不加重脑组织损伤,同时对加重缺血⁃再灌注损伤的因素进行调节,促进脑组织修复[1]。过去20年间有450种药物应用于缺血性卒中的治疗研究。迄今仅40种药物进入临床试验阶段,19种药物在不同国家批准上市。目前,处于缺血性卒中临床试验阶段或已应用于临床的药物主要包括溶栓药、抗血小板药、神经保护药和促组织修复药[99⁃100](表3)。
1.溶栓药 主要包括rt⁃PA、瑞替普酶和替奈普酶[101]。一项I期临床试验结果显示,急性缺血性卒中3~6小时联合应用瑞替普酶和阿昔单抗可以改善脑缺血症状[102]。另一项Ⅱ期临床试验比较瑞替普酶与替奈普酶的治疗效果,结果显示,急性缺血性卒中3~4小时,替奈普酶组患者脑组织再灌注和神经功能均优于瑞替普酶组,认为替奈普酶有望进入Ⅲ期临床试验阶段[103]。最近一项临床试验比较瑞替普酶和替奈普酶(相同时间点和相同治疗剂量)的治疗效果,结果显示,二者对挽救缺血脑组织并无明显差异[104],提示两种药物的治疗效果尚待大规模随机对照临床试验的验证。
2.抗血小板药 主要包括阿司匹林、氯吡格雷和西洛他唑。阿司匹林通过抑制环氧合酶、氯吡格雷通过抑制ADP和嘌呤受体抗血小板聚集。阿司匹林是目前最主要的缺血性卒中二级预防药物,氯吡格雷可用于阿司匹林过敏的脑卒中患者二级预防。由于可以增加颅内出血风险,不推荐长期联合应用阿司匹林和氯吡格雷[105]。研究显示,缺血性卒中初期,与单纯应用阿司匹林相比,更换另一种抗血小板药或联合应用其他抗血小板药,发生心血管并发症和脑卒中复发风险降低[106]。因此,阿司匹林和氯吡格雷的最佳临床治疗策略尚待进一步基础与临床研究。西洛他唑是新一代抗血小板药,通过抑制磷酸二酯酶3(PDE3)抗血小板聚集。动物实验显示,在全脑缺血模型小鼠中,西洛他唑通过抑制C⁃Jun氨基末端激酶3(JNK3)/Caspase⁃3信号转导通路以发挥减少神经元凋亡和预防脑卒中后认知功能障碍的作用[107]。此外,西洛他唑还可以通过抑制细胞间粘附分子⁃1(ICAM⁃1)表达和激活小胶质细胞,改善长期预后[108]。目前,西洛他唑主要用于治疗血栓闭塞性脉管炎,未推荐用于缺血性卒中的治疗,尚待进一步在缺血性卒中动物模型中进行研究,以为西洛他唑治疗缺血性卒中提供理论证据。
3.神经保护药 包括中成药银杏叶和芹菜提取物丁苯酞(NBP)、甲磺酸法舒地尔、胞二磷胆碱、尤瑞克林、洛伐他汀、多奈哌齐、依达拉奉[109]、格列本脲、重组人活化蛋白C、Caffeinol等。临床药物主要针对缺血性卒中后介导脑组织损伤的不同病理生理学应激过程发挥作用,包括扩血管和增加脑血流量(CBF)[110⁃113]、抗氧化应激[114]、抗细胞凋亡[115⁃117]、抑制兴奋性毒性[118⁃119]和抑制炎症反应[99⁃100,120⁃121]等,其中,丁苯酞已进入Ⅳ期临床试验[99]。尽管临床药物治疗研究取得一定进展,但某些药物的临床效果仍缺乏足够证据,尚待高质量的大规模随机对照临床试验进一步验证[122⁃124]。
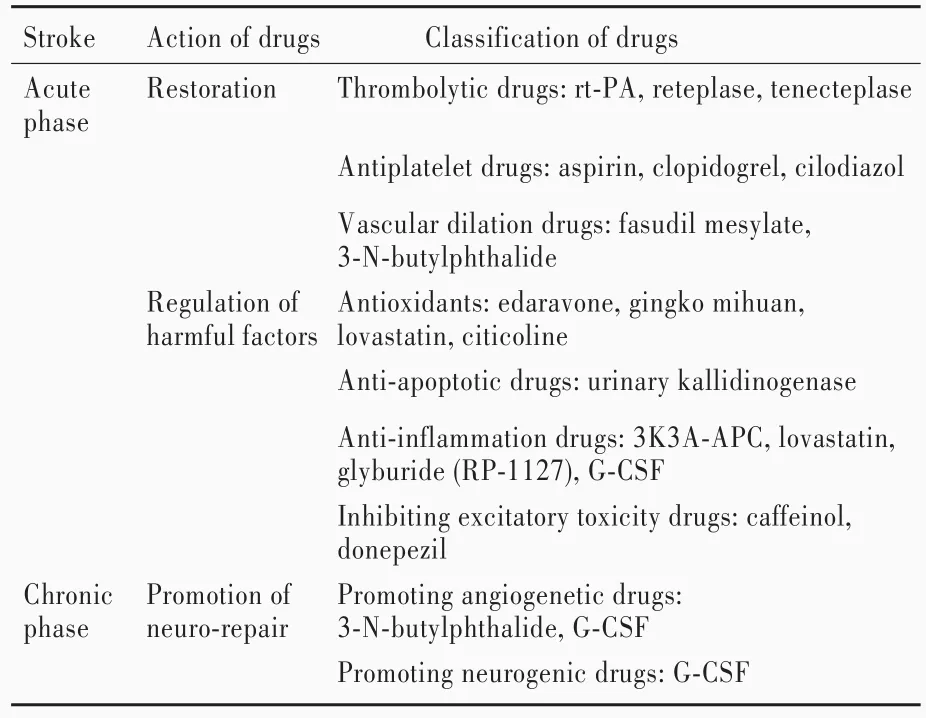
表3 缺血性卒中治疗药物分类及作用机制Table 3. Classification and therapeutic mechanism of drugs for clinical treatment of ischemic stroke
4.促组织修复药 目前临床应用相对较少,基础和临床研究较多的主要是粒细胞集落刺激因子(G⁃CSF),可以促进造血干细胞动员和分化[125⁃126],广泛应用于遗传性或获得性粒细胞减少症以及促进移植干细胞增殖[127]。近年动物实验显示,除用于造血系统疾病的治疗外,粒细胞集落刺激因子还可以通过抑制细胞凋亡和炎症反应,促进血管新生和神经再生,促进脑组织修复,改善缺血性卒中小鼠长期预后[128⁃131]。小规模临床试验显示,缺血性卒中24~48小时内应用粒细胞集落刺激因子是安全的,但并未起到改善神经功能的效果[132⁃135]。因此,粒细胞集落刺激因子治疗缺血性卒中的效果尚待进一步基础研究和大规模随机对照临床试验的验证。
六、展望
脑卒中是全球性疾病,病理生理学机制复杂,多种基础研究有效的治疗方法在临床实践中效果欠佳,提示基础研究策略与临床实际应用仍然存在较大差距,需要科研工作者和广大临床医师之间加强交流与合作。一方面,科研工作者在设计实验时尽可能遵循脑卒中临床发作规律,如选择高龄、有多个基础疾病的实验动物,选择患者易接受的治疗方法如静脉滴注或口服,不仅应重视急性期治疗效果,而且应重视长期预后;另一方面,临床医师应对进入临床试验阶段的药物进行科学严谨的大规模随机对照临床试验,以明确药物治疗效果。国家“十二五”时期,我们课题组通过药物治疗、基因治疗、miRNA治疗、干细胞治疗等方法对缺血性卒中治疗效果及作用机制进行较为深入的研究,主要在调节缺血性卒中后血管功能、促进血管新生和神经再生方面取得一些成果。未来在我国国民经济和社会发展第十三个五年规划(简称“十三五”)时期,我们课题组将加强再生医学的前沿研究,注重学科交叉与转化,在基因治疗、组织工程、干细胞技术、生物医学材料等方面进行新理论指导下的技术提升。此外,将重点针对缺血性卒中病理生理学过程中涉及炎症反应调控机制、神经免疫调节机制及移植干细胞和其他神经细胞的相互调节机制进行研究,以期为个体化精准治疗提供新方法和新策略。总之,脑卒中基础研究与临床治疗是一项艰巨且需长期坚持的任务,只有加强多方面人才、多学科交叉与合作,方能加快基础研究向临床转化的进展。
[1]Neuhaus AA, Couch Y, Hadley G, Buchan AM.Neuroprotection in stroke:the importance of collaboration and reproducibility[J].Brain,2017,140:2079⁃2092.
[2]Murray CJ,Lopez AD.Measuring the global burden of disease[J].N Engl J Med,2013,369:448⁃457.
[3]Lozano R,Naghavi M,Foreman K,Lim S,Shibuya K,Aboyans V,Abraham J,Adair T,Aggarwal R,Ahn SY,Alvarado M,Anderson HR,Anderson LM,AndrewsKG,Atkinson C,Baddour LM,Barker⁃Collo S,Bartels DH,Bell ML,Benjamin EJ,Bennett D,Bhalla K,Bikbov B,Bin Abdulhak A,Birbeck G,Blyth F,Bolliger I,Boufous S,Bucello C,Burch M,Burney P,Carapetis J,Chen H,Chou D,Chugh SS,Coffeng LE,Colan SD,Colquhoun S,Colson KE,Condon J,Connor MD,Cooper LT,Corriere M,Cortinovis M,de Vaccaro KC,Couser W,Cowie BC,Criqui MH,Cross M,Dabhadkar KC,Dahodwala N,De Leo D,Degenhardt L,Delossantos A,Denenberg J,Des Jarlais DC,Dharmaratne SD,Dorsey ER,Driscoll T,Duber H,Ebel B,Erwin PJ,Espindola P,Ezzati M,Feigin V,Flaxman AD,Forouzanfar MH,Fowkes FG,Franklin R,Fransen M,Freeman MK,Gabriel SE,Gakidou E,Gaspari F,Gillum RF,Gonzalez⁃Medina D,Halasa YA,Haring D,Harrison JE,Havmoeller R,Hay RJ,Hoen B,Hotez PJ,Hoy D,Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N,Karthikeyan G,Kassebaum N,Keren A,Khoo JP,Knowlton LM,Kobusingye O,Koranteng A,Krishnamurthi R,Lipnick M,Lipshultz SE,Ohno SL,Mabweijano J,MacIntyre MF,Mallinger L,March L,Marks GB,Marks R,Matsumori A,Matzopoulos R,Mayosi BM,McAnulty JH,McDermott MM,McGrath J,Mensah GA,Merriman TR,Michaud C,Miller M,Miller TR,Mock C,Mocumbi AO,Mokdad AA,Moran A,Mulholland K,Nair MN,Naldi L,Narayan KM,Nasseri K,Norman P,O'Donnell M,Omer SB,Ortblad K,Osborne R,Ozgediz D,Pahari B,Pandian JD,Rivero AP,Padilla RP,Perez⁃Ruiz F,Perico N,Phillips D,Pierce K,Pope CA 3rd,Porrini E,Pourmalek F,Raju M,Ranganathan D,Rehm JT,Rein DB,Remuzzi G,Rivara FP,Roberts T,De Leon FR,Rosenfeld LC,Rushton L,Sacco RL,Salomon JA,Sampson U,Sanman E,SchwebelDC,Segui⁃GomezM,Shepard DS,Singh D,Singleton J,Sliwa K,Smith E,Steer A,Taylor JA,Thomas B,Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA,Venketasubramanian N,Vijayakumar L,Vos T,Wagner GR,Wang M,Wang W,Watt K,Weinstock MA,Weintraub R,Wilkinson JD,Woolf AD,Wulf S,Yeh PH,Yip P,Zabetian A,Zheng ZJ,Lopez AD,Murray CJ,AlMazroa MA,Memish ZA.Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010:a systematic analysis for the Global Burden of Disease Study 2010[J].Lancet,2012,380:2095⁃2128.
[4]Mozaffarian D,Benjamin EJ,Go AS,Arnett DK,Blaha MJ,Cushman M,Das SR,de Ferranti S,Després JP,Fullerton HJ,Howard VJ,Huffman MD,Isasi CR,Jiménez MC,Judd SE,Kissela BM,Lichtman JH,Lisabeth LD,Liu S,Mackey RH,Magid DJ,McGuire DK,Mohler ER 3rd,Moy CS,Muntner P,Mussolino ME,Nasir K,Neumar RW,Nichol G,Palaniappan L,Pandey DK,Reeves MJ,Rodriguez CJ,Rosamond W,Sorlie PD,Stein J,Towfighi A,Turan TN,Virani SS,Woo D,Yeh RW,Turner MB;American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Executive summary:heart disease and stroke statistics.2016 update:a report from the American Heart Association[J].Circulation,2016,133:447⁃454.
[5]Feigin VL,Krishnamurthi RV,Parmar P,Norrving B,Mensah GA,Bennett DA,Barker⁃Collo S,Moran AE,Sacco RL,Truelsen T,Davis S,Pandian JD,Naghavi M,Forouzanfar MH,Nguyen G,Johnson CO,Vos T,Meretoja A,Murray CJ,Roth GA;GBD 2013WritingGroup,GBD 2013StrokePanel Experts Group.Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013:the GBD 2013 study[J].Neuroepidemiology,2015,45:161⁃176.
[6]Feigin VL,Forouzanfar MH,Krishnamurthi R,Mensah GA,Connor M,Bennett DA,Moran AE,Sacco RL,Anderson L,Truelsen T,O'Donnell M,Venketasubramanian N,Barker⁃Collo S,Lawes CM,Wang W,Shinohara Y,Witt E,Ezzati M,Naghavi M,Murray C;Global Burden of Diseases,Injuries,and Risk Factors Study 2010(GBD 2010)and the GBD Stroke Experts Group.Global and regional burden of stroke during 1990-2010:findings from the Global Burden of Disease Study 2010[J].Lancet,2014,383:245⁃254.
[7]Bailey EL,Smith C,Sudlow CL,Wardlaw JM.Pathology of lacunar ischemic stroke in humans:a systematic review[J].Brain Pathol,2012,22:583⁃591.
[8]Warlow C,Sudlow C,Dennis M,Wardlaw J,Sandercock P.Stroke[J].Lancet,2003,362:1211⁃1224.
[9]Wang W,Jiang B,Sun H,Ru X,Sun D,Wang L,Wang L,Jiang Y,Li Y,Wang Y,Chen Z,Wu S,Zhang Y,Wang D,Wang Y,Feigin VL;NESS⁃China Investigators.Prevalence,incidence,and mortality of stroke in China:results from a nationwide population ⁃based survey of 480 687 adults[J].Circulation,2017,135:759⁃771.
[10]Mozaffarian D,Benjamin EJ,Go AS,Arnett DK,Blaha MJ,Cushman M,Das SR,de Ferranti S,Despres JP,Fullerton HJ,Howard VJ,Huffman MD,Isasi CR,Jiménez MC,Judd SE,Kissela BM,Lichtman JH,Lisabeth LD,Liu S,Mackey RH,Magid DJ,McGuire DK,Mohler ER 3rd,Moy CS,Muntner P,Mussolino ME,Nasir K,Neumar RW,Nichol G,Palaniappan L,Pandey DK,Reeves MJ,Rodriguez CJ,Rosamond W,Sorlie PD,Stein J,Towfighi A,Turan TN,Virani SS,Woo D,Yeh RW,Turner MB;American Heart Association Statistics Committee,Stroke Statistics Subcommittee.Heart disease and stroke statistics:2016 update.A report from the American Heart Association[J].Circulation,2016,133:E38⁃360.
[11]Goyal M,Demchuk AM,Menon BK,Eesa M,Rempel JL,Thornton J,Roy D,Jovin TG,Willinsky RA,Sapkota BL,Dowlatshahi D,Frei DF,Kamal NR,Montanera WJ,Poppe AY,Ryckborst KJ,Silver FL,Shuaib A,Tampieri D,Williams D,Bang OY,Baxter BW,Burns PA,Choe H,Heo JH,Holmstedt CA,Jankowitz B,Kelly M,Linares G,Mandzia JL,Shankar J,Sohn SI,Swartz RH,Barber PA,Coutts SB,Smith EE,Morrish WF,Weill A,Subramaniam S,Mitha AP,Wong JH,Lowerison MW,Sajobi TT, Hill MD;ESCAPE Trial Investigators.Randomized assessment of rapid endovascular treatment of ischemic stroke[J].N Engl J Med,2015,372:1019⁃1030.
[12]Saver JL,Goyal M,Bonafe A,Diener HC,Levy EI,Pereira VM,Albers GW,Cognard C,Cohen DJ,Hacke W,Jansen O,Jovin TG,Mattle HP,Nogueira RG,Siddiqui AH,Yavagal DR,Baxter BW,Devlin TG,Lopes DK,Reddy VK,du Mesnil de Rochemont R,Singer OC,Jahan R;SWIFT PRIME Investigators.Stent⁃retriever thrombectomy after intravenous t⁃PA vs.t⁃PA alone in stroke[J].N Engl J Med,2015,372:2285⁃2295.
[13]BalamiJS,Sutherland BA,Edmunds LD,Grunwald IQ,Neuhaus AA,Hadley G,Karbalai H,Metcalf KA,DeLuca GC,Buchan AM.A systematic review and meta⁃analysis of randomized controlled trials of endovascular thrombectomy compared with best medical treatment for acute ischemic stroke[J].Int J Stroke,2015,10:1168⁃1178.
[14]Doeppner TR,Bähr M,Hermann DM,Giebel B.Concise review:extracellular vesicles overcoming limitations of cell therapies in ischemic stroke[J].Stem Cells Transl Med,2017,6:2044⁃2052.
[15]Campbell BC,Mitchell PJ,Kleinig TJ,Dewey HM,Churilov L,Yassi N,Yan B,Dowling RJ,Parsons MW,Oxley TJ,Wu TY,BrooksM,Simpson MA,Miteff F,Levi CR,Krause M,Harrington TJ,Faulder KC,Steinfort BS,Priglinger M,Ang T,Scroop R,Barber PA,McGuinness B,Wijeratne T,Phan TG,Chong W,Chandra RV,Bladin CF,Badve M,Rice H,de Villiers L,Ma H,Desmond PM,Donnan GA,Davis SM;EXTEND⁃IA Investigators.Endovascular therapy for ischemic stroke with perfusion ⁃imaging selection[J].N Engl J Med,2015,372:1009⁃1018.
[16]National Institute of Neurological Disorders and Stroke rt⁃PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke[J].N Engl J Med,1995,333:1581⁃1587.
[17]Hsu PS,Lin HH,Li CR,Chung WS.Increased risk of stroke in patients with osteoarthritis:a population⁃based cohort study[J].Osteoarthritis Cartilage,2017,25:1026⁃1031.
[18]Hacke W,Kaste M,Bluhmki E,Brozman M,Davalos A,Guidetti D,Larrue V,Lees KR,Medeghri Z,Machnig T,Schneider D,von Kummer R,Wahlgren N,Toni D;ECASS Investigators.Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke[J].N Engl J Med,2008,359:1317⁃1329.
[19]Fonarow GC,Smith EE,Saver JL,Reeves MJ,Hernandez AF,Peterson ED,Sacco RL,Schwamm LH.Improving door⁃to⁃needle times in acute ischemic stroke:the design and rationale for the American Heart Association/American Stroke Association's Target.Stroke Initiative[J].Stroke,2011,42:2983⁃2989.
[20]Paciaroni M,Balucani C,Agnelli G,Caso V,Silvestrelli G,Grotta JC,Demchuk AM,Sohn SI,Orlandi G,Leys D,Pezzini A,Alexandrov AV,Silvestrini M,Fofi L,Barlinn K,Inzitari D,Ferrarese C,Tassi R,Tsivgoulis G,Consoli D,Baldi A,Bovi P,Luda E,Galletti G,Invernizzi P,DeLodovici ML,Corea F,Del Sette M,Monaco S,Marcheselli S,Alberti A,Venti M,Acciarresi M,D'Amore C,Macellari F,Lanari A,Previdi P,Gonzales NR,Pandurengan RK,Vahidy FS,Sline M,Bal SS,Chiti A,Gialdini G,Dumont F,Cordonnier C,Debette S,Padovani A,Cerqua R,Bodechtel U,Kepplinger J,Nesi M,Nencini P,Beretta S,Trentini C,Martini G,Piperidou C,Heliopoulos I,D'Anna S,Cappellari M,Donati E,Bono G,Traverso E,Toni D.Systemic thrombolysis in patients with acute ischemic stroke and Internal Carotid ARtery Occlusion:the ICARO study[J].Stroke,2012,43:125⁃130.
[21]Emberson J,Lees KR,Lyden P,Blackwell L,Albers G,Bluhmki E,Brott T,Cohen G,Davis S,Donnan G,Grotta J,Howard G,Kaste M,Koga M,von Kummer R,Lansberg M,Lindley RI,Murray G,Olivot JM,Parsons M,Tilley B,Toni D,Toyoda K,Wahlgren N,Wardlaw J,Whiteley W,del Zoppo GJ,Baigent C,Sandercock P,Hacke W;Stroke Thrombolysis Trialists' Collaborative Group.Effect of treatment delay,age,and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke:a meta⁃analysis of individual patient data from randomised trials[J].Lancet,2014,384:1929⁃1935.
[22]Goyal M,Menon BK,van Zwam WH,Dippel DW,Mitchell PJ,Demchuk AM,Dávalos A,Majoie CB,van der Lugt A,de Miquel MA,Donnan GA,Roos YB,Bonafe A,Jahan R,Diener HC,van den Berg LA,Levy EI,Berkhemer OA,Pereira VM,Rempel J,Millán M,Davis SM,Roy D,Thornton J,Román LS,Ribó M,Beumer D,Stouch B,Brown S,Campbell BC,van Oostenbrugge RJ,Saver JL,Hill MD,Jovin TG;HERMES collaborators.Endovascular thrombectomy after large⁃vessel ischaemic stroke:a meta⁃analysis of individual patient data from five randomised trials[J].Lancet,2016,387:1723⁃1731.
[23]NINDS Stroke Genetics Network(SiGN),International Stroke Genetics Consortium (ISGC).Loci associated with ischaemic stroke and its subtypes(SiGN):a genome⁃wide association study[J].Lancet Neurol,2016,15:174⁃184.
[24]Black M,Wang W,Wang W.Ischemic stroke:from next generation sequencing and GWAS to community genomics[J]?OMICS,2015,19:451⁃460.
[25]Phuah CL,Dave T,Malik R,Raffeld MR,Ayres AM,Goldstein JN,Viswanathan A,Greenberg SM,Jagiella JM,Hansen BM,Norrving B,Jimenez⁃Conde J,Roquer J,Pichler A,Enzinger C,Montaner J,Fernandez⁃Cadenas I,Lindgren A,Slowik A,Schmidt R,Biffi A,Rost N,Langefeld CD,Markus HS,Mitchell BD,Worrall BB,Kittner SJ,Woo D,Dichgans M,Rosand J,Anderson CD;METASTROKE,NINDS⁃SiGN Consortium,International Stroke Genetics Consortium.Genetic variants influencing elevated myeloperoxidase levels increase risk of stroke[J].Brain,2017,140:2663⁃2672.
[26]Ning M,Sarracino DA,Buonanno FS,Krastins B,Chou S,McMullin D,Wang X,Lopez M,Lo EH.Proteomic protease substrate profiling of tPA treatment in acute ischemic stroke patients:a step toward individualizing thrombolytic therapy at the bedside[J].Transl Stroke Res,2010,1:268⁃275.
[27]Azurmendi L,Lapierre⁃Fetaud V,Schneider J,Montaner J,Katan M,Sanchez JC.Proteomic discovery and verification of serum amyloid A as a predictor marker of patients at risk of post⁃stroke infection:a pilot study[J].Clin Proteomics,2017,14:27.
[28]Stapels M,Piper C,Yang T,Li M,Stowell C,Xiong ZG,Saugstad J,Simon RP,Geromanos S,Langridge J,Lan JQ,Zhou A.Polycomb group proteins as epigenetic mediators of neuroprotection in ischemic tolerance[J].Sci Signal,2010,3:ra15.
[29]Dongsheng H,Zhuo Z,Jiamin L,Hailan M,Lijuan H,Fan C,Dan Y,He Z,Yun X.Proteomic analysis of the peri⁃infarct area after human umbilical cord mesenchymal stem cell transplantation in experimental stroke[J].Aging Dis,2016,7:623⁃634.
[30]Laskowitz DT,Kasner SE,Saver J,Remmel KS,Jauch EC;BRAIN Study Group.Clinical usefulness of a biomarker⁃based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury(BRAIN)study[J].Stroke,2009,40:77⁃85.
[31]Jove M,Mauri⁃Capdevila G,Suarez I,Cambray S,Sanahuja J,Quílez A,Farré J,Benabdelhak I,Pamplona R,Portero⁃Otin M,Purroy F.Metabolomics predicts stroke recurrence after transient ischemic attack[J].Neurology,2015,84:36⁃45.
[32]Qureshi MI,Vorkas PA,Coupland AP,Jenkins IH,Holmes E,Davies AH.Lessons from metabonomics on the neurobiology of stroke[J].Neuroscientist,2016[.Epub ahead of print]
[33]Ding X,Liu R,Li W,Ni H,Liu Y,Wu D,Yang S,Liu J,Xiao B,Liu S.A metabonomic investigation on the biochemical perturbation in post⁃stroke patients with depressive disorder(PSD[)J].Metab Brain Dis,2016,31:279⁃287.
[34]Dickens AM,Larkin JR,Davis BG,Griffin JL,Claridge TD,Sibson NR,Anthony DC.NMR⁃based metabolomics separates the distinct stages of disease in a chronic relapsing model of multiple sclerosis[J].J Neuroimmune Pharmacol,2015,10:435⁃444.
[35]Koronowski KB,Khoury N,Saul I,Loris ZB,Cohan CH,Stradecki⁃Cohan HM,Dave KR,Young JI,Perez⁃Pinzon MA.Neuronal SIRT1(Silent Information Regulator 2 Homologue 1)regulates glycolysis and mediates resveratrol⁃induced ischemic tolerance[J].Stroke,2017,48:3117⁃3125.
[36]Craig AJ,Housley GD.Evaluation of gene therapy as an intervention strategy to treat brain injury from stroke[J].Front Mol Neurosci,2016,9:34.
[37]Czabotar PE,Lessene G,Strasser A,Adams JM.Control of apoptosis by the BCL⁃2 protein family:implications for physiology and therapy[J].Nat Rev Mol Cell Biol,2014,15:49⁃63.
[38]Yenari MA,Dumas TC,Sapolsky RM,Steinberg GK.Gene therapy for treatment of cerebral ischemia using defective herpes simplex viral vectors[J].Ann NY Acad Sci,2001,939:340⁃357.
[39]Kilic E,Hermann DM,Kügler S,Kilic U,Holzmüller H,Schmeer C,Bähr M.Adenovirus⁃mediated Bcl⁃X(L)expression using a neuron⁃specific synapsin⁃1 promoter protects against disseminated neuronal injury and brain infarction following focal cerebral ischemia in mice[J].Neurobiol Dis,2002,11:275⁃284.
[40]Sun Y,Jin K,Clark KR,Peel A,Mao XO,Chang Q,Simon RP,Greenberg DA.Adeno⁃associated virus⁃mediated delivery of BCL⁃w gene improves outcome after transient focal cerebral ischemia[J].Gene Ther,2003,10:115⁃122.
[41]Wong LF,Ralph GS,Walmsley LE,Bienemann AS,Parham S,Kingsman SM,Uney JB,Mazarakis ND.Lentiviral⁃mediated delivery of Bcl⁃2 or GDNF protects against excitotoxicity in the rat hippocampus[J].Mol Ther,2005,11:89⁃95.
[42]Lei ZN,Liu F,Zhang LM,Huang YL,Sun FY.Bcl⁃2 increases stroke⁃induced striatal neurogenesis in adult brains by inhibiting BMP⁃4 function via activation of beta⁃catenin signaling[J].Neurochem Int,2012,61:34⁃42.
[43]Zhao H,Yenari MA,Cheng D,Barreto⁃Chang OL,Sapolsky RM,Steinberg GK.Bcl⁃2 transfection via herpes simplex virus blocks apoptosis⁃inducing factor translocation after focal ischemia in the rat[J].J Cereb Blood Flow Metab,2004,24:681⁃692.
[44]Hoehn B,Ringer TM,Xu L,Giffard RG,Sapolsky RM,Steinberg GK,Yenari MA.Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage[J].J Cereb Blood Flow Metab,2001,21:1303⁃1309.
[45]Xu L,Xiong X,Ouyang Y,Barreto G,Giffard R.Heat shock protein 72(Hsp72)improves long term recovery after focal cerebral ischemia in mice[J].Neurosci Lett,2011,488:279 ⁃282.
[46]Shen F,Su H,Fan Y,Chen Y,Zhu Y,Liu W,Young WL,Yang GY.Adeno⁃associated viral⁃vector⁃mediated hypoxia⁃inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice[J].Stroke,2006,37:2601⁃2606.
[47]Zhang J,Yu Z,Yu Z,Yang Z,Zhao H,Liu L,Zhao J.rAAV⁃mediated delivery of brain⁃derived neurotrophic factor promotes neurite outgrowth and protects neurodegeneration in focal ischemic model[J].Int J Clin Exp Pathol,2011,4:496 ⁃504.
[48]Hermann DM,Kilic E,Kugler S,Isenmann S,Bahr M.Adenovirus⁃mediated GDNF and CNTF pretreatment protects against striatal injury following transient middle cerebral artery occlusion in mice[J].Neurobiol Dis,2001,8:655⁃666.
[49]Tsai TH,Chen SL,Chiang YH,Lin SZ,Ma HI,Kuo SW,Tsao YP.Recombinant adeno⁃associated virus vector expressing glial cell line⁃derived neurotrophic factor reduces ischemia⁃induced damage[J].Exp Neurol,2000,166:266⁃275.
[50]Harvey BK,Chang CF,Chiang YH,Bowers WJ,Morales M,Hoffer BJ,Wang Y,Federoff HJ.HSV amplicon delivery of glial cell line⁃derived neurotrophic factor is neuroprotective against ischemic injury[J].Exp Neurol,2003,183:47⁃55.
[51]Matlik K,Abo⁃Ramadan U,Harvey BK,Arumäe U,Airavaara M.AAV ⁃mediated targeting of gene expression to the peri⁃infarct region in rat cortical stroke model[J].J Neurosci Methods,2014,236:107⁃113.
[52]Sugiura S,Kitagawa K,Tanaka S,Todo K,Omura⁃Matsuoka E,Sasaki T,Mabuchi T,Matsushita K,Yagita Y,Hori M.Adenovirus⁃mediated gene transfer of heparin⁃binding epidermal growth factor⁃like growth factor enhances neurogenesis and angiogenesis after focal cerebral ischemia in rats[J].Stroke,2005,36:859⁃864.
[53]Wang Y,Huang J,Li Y,Yang GY.Roles of chemokine CXCL12 and its receptors in ischemic stroke[J].Curr Drug Targets,2012,13:166⁃172.
[54]Yoo J,Seo JJ,Eom JH,Hwang DY.Effects of stromal cell⁃derived factor 1 α delivered at different phases of transient focal ischemia in rats[J].Neuroscience,2012,209:171⁃186.
[55]Li Y,Huang J,He X,Tang G,Tang YH,Liu Y,Lin X,Lu Y,Yang GY,Wang Y.Postacute stromal cell⁃derived factor⁃1 α expression promotes neurovascular recovery in ischemic mice[J].Stroke,2014,45:1822⁃1829.
[56]Li Y,Tang G,Liu Y,He X,Huang J,Lin X,Zhang Z,Yang GY,Wang Y.CXCL12 gene therapy ameliorates ischemia⁃induced white matter injury in mouse brain[J].Stem Cells Transl Med,2015,4:1122⁃1130.
[57]Sun H,Le T,Chang TT,Habib A,Wu S,Shen F,Young WL,Su H,Liu J.AAV⁃mediated netrin⁃1 overexpression increases peri⁃infarct blood vessel density and improves motor function recovery after experimental stroke[J].Neurobiol Dis,2011,44:73⁃83.
[58]He X,Li Y,Lu H,Zhang Z,Wang Y,Yang GY.Netrin⁃1 overexpression promotes white matter repairing and remodeling after focal cerebral ischemia in mice[J].J Cereb Blood Flow Metab,2013,33:1921⁃1927.
[59]Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin⁃4 encodes small RNAs with antisense complementarity to lin⁃14[J].Cell,1993,75:843⁃854.
[60]Kozomara A,Griffiths⁃Jones S.miRBase:annotating high confidence microRNAs using deep sequencing data [J].Nucleic Acids Res,2014,42:D68⁃73.
[61]Li G,Morris⁃Blanco KC,Lopez MS,Yang T,Zhao H,Vemuganti R,Luo Y.Impact of microRNAs on ischemic stroke:from pre⁃to post⁃disease[J].Prog Neurobiol,2017.[Epub ahead of print]
[62]Williams MR,Stedtfeld RD,Stedtfeld TM,Tiedje JM,Hashsham SA.Quantification of microRNAs directly from body fluids using a base⁃stacking isothermal amplification method in a point⁃of⁃care device[J].Biomed Microdevices,2017,19:45.
[63]Kaneto CM,Nascimento JS,Moreira MC,Ludovico ND,Santana AP,Silva RA,Silva⁃Jardim I,Santos JL,Sousa SMB,Lima PS.MicroRNA profiling identifies miR⁃7⁃5p and miR⁃26b⁃5p as differentially expressed in hypertensive patients with left ventricular hypertrophy[J].Braz J Med Biol Res,2017,50:E6211.
[64]Sims EK,Lakhter AJ,Anderson⁃Baucum E,Kono T,Tong X,Evans⁃Molina C.MicroRNA 21targetsBCL2mRNA to increase apoptosis in rat and human beta cells [J].Diabetologia,2017,60:1057⁃1065.
[65]Xing T,Du L,Zhuang X,Zhang L,Hao J,Wang J.Upregulation of microRNA⁃206 induces apoptosis of vascular smooth muscle cells and decreases risk of atherosclerosis through modulating FOXP1[J].Exp Ther Med,2017,14:4097⁃4103.
[66]Li H,Xiang Y,Fan LJ,Zhang XY,Li JP,Yu CX,Bao LY,Cao DS,Xing WB,Liao XH,Zhang TC.Myocardin inhibited the gap protein connexin 43 via promoted miR⁃206 to regulate vascular smooth muscle cell phenotypic switch[J].Gene,2017,616:22⁃30.
[67]Ma Q,Zhao H,Tao Z,Wang R,Liu P,Han Z,Ma S,Luo Y,Jia J.MicroRNA⁃181c exacerbates brain injury in acute ischemic stroke[J].Aging Dis,2016,7:705⁃714.
[68]Wang J,Chen T,Shan G.miR⁃148b regulates proliferation and differentiation of neuralstem cells via Wnt/beta⁃catenin signaling in rat ischemic stroke mode[lJ].Front Cell Neurosci,2017,11:329.
[69]Xu X,Wen Z,Zhao N,Xu X,Wang F,Gao J,Jiang Y,Liu X.MicroRNA ⁃1906,a novel regulator of toll⁃like receptor 4,ameliorates ischemic injury after experimental stroke in mice[J].J Neurosci,2017,37:10498⁃10515.
[70]Wang Y,Huang J,Ma Y,Tang G,Liu Y,Chen X,Zhang Z,Zeng L,Wang Y,Ouyang YB,Yang GY.MicroRNA⁃29b is a therapeutic target in cerebral ischemia associated with aquaporin 4[J].J Cereb Blood Flow Metab,2015,35:1977 ⁃1984.
[71]He JR,Zhang Y,Lu WJ,Liang HB,Tu XQ,Ma FY,Yang GY,Zeng LL.Age⁃related frontal periventricular white matter hyperintensities and miR ⁃92a⁃3p are associated with early⁃onset post⁃stroke depression[J].Front Aging Neurosci,2017,9:328.
[72]Keller A,Meese E.Can circulating miRNAs live up to the promise of being minimal invasive biomarkers in clinical settings[J]?Wiley Interdiscip Rev RNA,2016,7:148⁃156.
[73]Karakas M, Zeller T. A biomarker ocular: circulating MicroRNAs toward diagnostics for acute ischemic stroke[J].Circ Res,2017,121:905⁃907.
[74]Bühnemann C,Scholz A,Bernreuther C,Malik CY,Braun H,Schachner M,Reymann KG,Dihné M.Neuronal differentiation of transplanted embryonic stem cell⁃derived precursors in stroke lesions of adult rats[J].Brain,2006,129(Pt 12):3238 ⁃3248.
[75]Drury⁃Stewart D,Song M,Mohamad O,Guo Y,Gu X,Chen D,Wei L.Highly efficient differentiation of neural precursors from human embryonic stem cells and benefits of transplantation after ischemic stroke in mice[J].Stem Cell Res Ther,2013,4:93.
[76]Chau MJ,Deveau TC,Song M,Gu X,Chen D,Wei L.iPSC transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats[J].Stem Cells,2014,32:3075⁃3087.
[77]Tang Y,Wang J,Lin X,Wang L,Shao B,Jin K,Wang Y,Yang GY.Neural stem cell protects aged ratbrain from ischemia⁃reperfusion injury through neurogenesis and angiogenesis[J].J Cereb Blood Flow Metab,2014,34:1138 ⁃1147.
[78]Abeysinghe HC,Bokhari L,Quigley A,Choolani M,Chan J,Dusting GJ,Crook JM,Kobayashi NR,Roulston CL.Pre⁃differentiation of human neural stem cells into GABAergic neurons prior to transplant results in greater repopulation of the damaged brain and accelerates functional recovery after transient ischemic stroke[J].Stem Cell Res Ther,2015,6:186.
[79]Chu K,Park KI,Lee ST,Jung KH,Ko SY,Kang L,Sinn DI,Lee YS,Kim SU,Kim M,Roh JK.Combined treatment of vascular endothelial growth factor and human neural stem cells in experimental focal cerebral ischemia[J].Neurosci Res,2005,53:384⁃390.
[80]Nakazaki M,Sasaki M,Kataoka⁃Sasaki Y,Oka S,Namioka T,Namioka A,Onodera R,Suzuki J,Sasaki Y,Nagahama H,Mikami T,Wanibuchi M,Kocsis JD,Honmou O.Intravenous infusion of mesenchymal stem cells inhibits intracranial hemorrhage after recombinant tissue plasminogen activator therapy for transient middle cerebral artery occlusion in rats[J].J Neurosurg,2017,127:917⁃926.
[81]Fukuda Y,Horie N,Satoh K,Yamaguchi S,Morofuji Y,Hiu T,Izumo T,Hayashi K,Nishida N,Nagata I.Intra⁃arterial transplantation of low⁃dose stem cells provides functional recovery without adverse effects after stroke[J].Cell Mol Neurobiol,2015,35:399⁃406.
[82]Chen L,Zhang G,Khan AA,Guo X,Gu Y.Clinical efficacy and meta⁃analysis of stem cell therapies for patients with brain ischemia[J].Stem Cells Int,2016:ID6129579.
[83]Zhang H,Sun F,Wang J,Xie L,Yang C,Pan M,Shao B,Yang GY,Yang SH,Zhuge Q,Jin K.Combining injectable plasma scaffold with mesenchymal stem/stromalcells for repairing infarct cavity after ischemic stroke[J].Aging Dis,2017,8:203⁃214.
[84]Felfly H,Haddad GG.Hematopoietic stem cells:potential new applications for translational medicine[J].J Stem Cells,2014,9:163⁃197.
[85]Fan Y,Shen F,Frenzel T,Zhu W,Ye J,Liu J,Chen Y,Su H,Young WL, Yang GY. Endothelial progenitor cell transplantation improves long⁃term stroke outcome in mice[J].Ann Neurol,2010,67:488⁃497.
[86]Chen C,Lin X,Wang J,Tang G,Mu Z,Chen X,Xu J,Wang Y,Zhang Z,Yang GY.Effect of HMGB1 on the paracrine action of EPC promotes post⁃ischemic neovascularization in mice[J].Stem Cells,2014,32:2679⁃2689.
[87]Huang H,Lin F,Jiang J,Chen Y,Mei A,Zhu P.Effects of intra⁃arterial transplantation of adipose⁃derived stem cells on the expression of netrin ⁃1 and its receptor DCC in the peri⁃infarct cortex after experimental stroke[J].Stem Cell Res Ther,2017,8:223.
[88]Leong WK,Lewis MD,Koblar SA.Concise review.Preclinical studies on human cell⁃based therapy in rodent ischemic stroke models:where are we now after a decade[J]?Stem Cells,2013,31:1040⁃1043.
[89]Vu Q,Xie K,Eckert M,Zhao W,Cramer SC.Meta⁃analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke[J].Neurology,2014,82:1277⁃1286.
[90]Gervois P,Wolfs E,Ratajczak J,Dillen Y,Vangansewinkel T,Hilkens P,Bronckaers A,Lambrichts I,Struys T.Stem cell⁃based therapies for ischemic stroke:preclinical results and the potential of imaging⁃assisted evaluation of donor cell fate and mechanisms of brain regeneration[J].Med Res Rev,2016,36:1080⁃1126.
[91]Wei L,Wei ZZ,Jiang MQ,Mohamad O,Yu SP.Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke[J].Prog Neurobiol,2017,157:49⁃78.
[92]Bang OY,Kim EH,Cha JM,Moon GJ.Adult stem cell therapy for stroke:challenges and progress[J].J Stroke,2016,18:256⁃266.
[93]Nagpal A,Choy FC,Howell S,Hillier S,Chan F,Hamilton⁃Bruce MA,Koblar SA.Safety and effectiveness of stem cell therapies in early⁃phase clinical trials in stroke:a systematic review and meta⁃analysis[J].Stem Cell Res Ther,2017,8:191.
[94]Wei N,Yu SP,Gu X,Taylor TM,Song D,Liu XF,Wei L.Delayed intranasal delivery of hypoxic⁃preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice[J].Cell Transplant,2013,22:977⁃991.
[95]Wei ZZ,Gu X,Ferdinand A,Lee JH,Ji X,Ji XM,Yu SP,Wei L.Intranasal delivery of bone marrow mesenchymal stem cells improved neurovascular regeneration and rescued neuropsychiatric deficits after neonatal stroke in rats[J].Cell Transplant,2015,24:391⁃402.
[96]Danielyan L,Schäfer R,von Ameln⁃Mayerhofer A,Buadze M,Geisler J,Klopfer T,Burkhardt U,Proksch B,Verleysdonk S,Ayturan M,Buniatian GH,Gleiter CH,Frey WH 2nd.Intranasal delivery of cells to the brain[J].Eur J Cell Biol,2009,88:315⁃324.
[97]Venkat P,Shen Y,Chopp M,Chen J.Cell⁃based and pharmacological neurorestorative therapies for ischemic stroke[J].Neuropharmacology,2017[.Epub ahead of print]
[98]Janowski M,Wagner DC,Boltze J.Stem cell⁃based tissue replacement after stroke:factual necessity or notorious fiction[J]?Stroke,2015,46:2354⁃2363.
[99]Chen X,Wang K.The fate of medications evaluated for ischemic stroke pharmacotherapy over the period 1995-2015[J].Acta Pharm Sin B,2016,6:522⁃530.
[100]Reis C,Akyol O,Ho WM,Araujo C,Huang L,Applegate RⅡ,Zhang JH.Phase Ⅰ and phase Ⅱ therapies for acute ischemic stroke:an update on currently studied drugs in clinical research[J].Biomed Res Int,2017:ID4863079.
[101]Verstraete M.Third ⁃generation thrombolytic drugs[J].Am J Med,2000,109:52⁃58.
[102]Qureshi AI,Harris⁃Lane P,Kirmani JF,Janjua N,Divani AA,Mohammad YM,Suarez JI,Montgomery MO.Intra⁃arterial reteplase and intravenous abciximab in patients with acute ischemic stroke:an open⁃label,dose⁃ranging,phase Ⅰ study[J].Neurosurgery,2006,59:789⁃796.
[103]Parsons M,Spratt N,Bivard A,Campbell B,Chung K,Miteff F,O'Brien B,Bladin C,McElduff P,Allen C,Bateman G,Donnan G,Davis S,Levi C.A randomized trial of tenecteplase versus alteplase for acute ischemic stroke[J].N Engl J Med,2012,366:1099⁃1107.
[104]Huang X,Cheripelli BK,Lloyd SM,Kalladka D,Moreton FC,Siddiqui A,Ford I,Muir KW.Alteplase versus tenecteplase for thrombolysis after ischaemic stroke(ATTEST):a phase 2,randomised,open ⁃label,blinded endpoint study[J].Lancet Neurol,2015,14:368⁃376.
[105]Oza R,Rundell K,Garcellano M.Recurrent ischemic stroke:strategies for prevention[J].Am Fam Physician,2017,96:436⁃440.
[106]Lee M,Saver JL,Hong KS,Rao NM,Wu YL,Ovbiagele B.Antiplatelet regimen for patients with breakthrough strokes while on aspirin:a systematic review and Meta ⁃analysis[J].Stroke,2017,48:2610⁃2613.
[107]Qi DS,Tao JH,Zhang LQ,Li M,Wang M,Qu R,Zhang SC,Liu P,Liu F,Miu JC,Ma JY,Mei XY,Zhang F.Neuroprotection of cilostazol against ischemia/reperfusion⁃induced cognitive deficits through inhibiting JNK3/caspase⁃3 by enhancing Akt1[J].Brain Res,2016,1653:67⁃74.
[108]Kitamura A,Manso Y,Duncombe J,Searcy J,Koudelka J,Binnie M,Webster S,Lennen R,Jansen M,Marshall I,Ihara M,Kalaria RN,Horsburgh K.Long⁃term cilostazol treatment reduces gliovascular damage and memory impairment in a mouse model of chronic cerebral hypoperfusion[J].Sci Rep,2017,7:4299.
[109]Wang Y,Zhao Z,Chow N,Rajput PS,Griffin JH,Lyden PD,Zlokovic BV. Activated protein C analog protects from ischemic stroke and extends the therapeutic window of tissue⁃type plasminogen activator in aged female mice and hypertensive rats[J].Stroke,2013,44:3529⁃3536.
[110]Okamura N,Saito M,Mori A,Sakamoto K,Kametaka S,Nakahara T,Ishii K.Vasodilator effects of fasudil,a Rho⁃kinase inhibitor, on retinal arterioles in stroke⁃prone spontaneously hypertensive rats[J].J Ocul Pharmacol Ther,2007,23:207⁃212.
[111]Mu ZH,Jiang Z,Lin XJ,Wang LP,Xi Y,Zhang ZJ,Wang YT,Yang GY.Vessel dilation attenuates endothelial dysfunction following middle cerebral artery occlusion in hyperglycemic rats[J].CNS Neurosci Ther,2016,22:316⁃324.
[112]Ji XC,Zhao WH,Cao DX,Shi QQ,Wang XL.Novel neuroprotectant chiral 3⁃N⁃butylphthalide inhibits tandem⁃pore⁃domain potassium channel TREK ⁃1[J].Acta Pharmacol Sin,2011,32:182⁃187.
[113]Zhang T,Yan W,Li Q,Fu J,Liu K,Jia W,Sun X,Liu X.3⁃N⁃Butylphthalide (NBP) attenuated neuronal autophagy and amyloid⁃beta expression in diabetic mice subjected to brain ischemia[J].Neurol Res,2011,33:396⁃404.
[114]Rao AM, Hatcher JF, Dempsey RJ. CDP⁃choline:neuroprotection in transient forebrain ischemia of gerbils[J].J Neurosci Res,1999,58:697⁃705.
[115]Xia CF,Yin H,Yao YY,Borlongan CV,Chao L,Chao J.Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis[J].Hum Gene Ther,2006,17:206⁃219.
[116]Guo H,Singh I,Wang Y,Deane R,Barrett T,Fernández JA,Chow N,Griffin JH,Zlokovic BV.Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity[J].Eur J Neurosci,2009,29:1119⁃1130.
[117]Cheng T,Liu D,Griffin JH,Fernández JA,Castellino F,Rosen ED,Fukudome K,Zlokovic BV.Activated protein C blocks p53⁃mediated apoptosis in ischemic human brain endothelium and is neuroprotective[J].Nat Med,2003,9:338⁃342.
[118]Zhao X,Strong R,Piriyawat P,Palusinski R,Grotta JC,Aronowski J.Caffeinol at the receptor level:anti⁃ischemic effect of N⁃methyl⁃D⁃aspartate receptor blockade is potentiated by caffeine[J].Stroke,2010,41:363⁃367.
[119]Belayev L,Khoutorova L,Zhang Y,Belayev A,Zhao W,Busto R,Ginsberg MD.Caffeinol confers cortical but not subcortical neuroprotection after transient focal cerebral ischemia in rats[J].Brain Res,2004,1008:278⁃283.
[120]Elkind MS,Sacco RL,Macarthur RB,Peerschke E,Neils G,Andrews H,Stillman J,Corporan T,Leifer D,Liu R,Cheung K.High⁃dose lovastatin for acute ischemic stroke:results of the phase Ⅰ dose escalation neuroprotection with statin therapy for acute recovery trial(NeuSTART)[J].Cerebrovasc Dis,2009,28:266⁃275.
[121]Abdallah DM,Nassar NN,Abd⁃El⁃Salam RM.Glibenclamide ameliorates ischemia⁃reperfusion injury via modulating oxidative stress and inflammatory mediators in the rat hippocampus[J].Brain Res,2011,1385:257⁃262.
[122]Ziganshina LE, Abakumova T. Cerebrolysin for acute ischaemic stroke[J].Cochrane Database Syst Rev,2015,(6):CD007026.
[123]Ziganshina LE,Abakumova T,Kuchaeva A.Cerebrolysin for acute ischaemic stroke[J].Cochrane Database Syst Rev,2010,(4):CD007026.
[124]Dávalos A,Alvarez⁃Sabín J,Castillo J,Díez⁃Tejedor E,Ferro J,Martínez⁃Vila E,Serena J,Segura T,Cruz VT,Masjuan J,Cobo E,Secades JJ;International Citicoline Trial on acUte Stroke(ICTUS)trial investigators.Citicoline in the treatment of acute ischaemic stroke: an international, randomised,multicentre,placebo⁃controlled study(ICTUS trial)[J].Lancet,2012,380:349⁃357.
[125]Welte K,Platzer E,Lu L,Gabrilove JL,Levi E,Mertelsmann R,Moore MA.Purification and biochemical characterization of human pluripotent hematopoietic colony⁃stimulating factor[J].Proc Natl Acad Sci USA,1985,82:1526⁃1530.
[126]Demetri GD,Griffin JD.Granulocyte colony⁃stimulating factor and its receptor[J].Blood,1991,78:2791⁃2808.
[127]Mehta HM,Malandra M,Corey SJ.G⁃CSF and GM⁃CSF in neutropenia[J].J Immunol,2015,195:1341⁃1349.
[128]Solaroglu I,Cahill J,Tsubokawa T,Beskonakli E,Zhang JH.Granulocyte colony⁃stimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation[J].Neurol Res,2009,31:167⁃172.
[129]Lee ST,Chu K,Jung KH,Ko SY,Kim EH,Sinn DI,Lee YS,Lo EH,Kim M,Roh JK.Granulocyte colony⁃stimulating factor enhances angiogenesis after focal cerebral ischemia[J].Brain Res,2005,1058(1/2):120⁃128.
[130]Schneider A,Kruger C,Steigleder T,Weber D,Pitzer C,Laage R,Aronowski J,Maurer MH,Gassler N,Mier W,Hasselblatt M,Kollmar R,Schwab S,Sommer C,Bach A,Kuhn HG,Schabitz WR.The hematopoietic factor G⁃CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis[J].J Clin Invest,2005,115:2083⁃2098.
[131]Solaroglu I,Digicaylioglu M,Keles GE,Zhang JH.New missions for an old agent:granulocyte⁃colony stimulating factor in the treatment of stroke patients[J].Curr Med Chem,2015,22:1302⁃1309.
[132]Alasheev AM,Belkin AA,Leiderman IN,Ivanov RA,Isakova TM.Granulocyte⁃colony⁃stimulating factor for acute ischemic stroke:a randomized controlled trial(STEMTHER)[J].Transl Stroke Res,2011,2:358⁃365.
[133]Shyu WC,Lin SZ,Lee CC,Liu DD,Li H.Granulocyte colony⁃stimulating factor for acute ischemicstroke:a randomized controlled tria[lJ].CMAJ,2006,174:927⁃933.
[134]Boy S,Sauerbruch S,Kraemer M,Schormann T,Schlachetzki F,Schuierer G,Luerding R,Hennemann B,Orso E,Dabringhaus A,Winkler J,Bogdahn U;RAIS(Regeneration in Acute Ischemic Stroke) Study Group. Mobilisation of hematopoietic CD34+precursor cells in patients with acute stroke is safe:results of an open⁃labeled non randomized phase Ⅰ/Ⅱ tria[lJ].PLoS One,2011,6:E23099.
[135]Ringelstein EB,Thijs V,Norrving B,Chamorro A,Aichner F,Grond M,Saver J,Laage R,Schneider A,Rathgeb F,Vogt G,Charissé G,Fiebach JB,Schwab S,Schäbitz WR,Kollmar R,Fisher M,Brozman M,Skoloudik D,Gruber F,Serena Leal J,Veltkamp R, Köhrmann M, Berrouschot J; AXIS 2 Investigators.Granulocyte colony⁃stimulating factor in patients with acute ischemic stroke:results of the AX200 for Ischemic Stroke tria[lJ].Stroke,2013,44:2681⁃2687.
