Structural neural connectivity of the vestibular nuclei in the human brain: a diffusion tensor imaging study
2018-05-05SungHoJangMiYoungLeeSangSeokYeoHyeokGyuKwon
Sung Ho Jang, Mi Young Lee, Sang Seok Yeo, Hyeok Gyu Kwon
1 Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Namku, Daegu, Republic of Korea
2 Department of Physical Therapy, College of Health and Therapy, Daegu Haany University, Gyeongsan, North Gyeongsang, Republic of Korea
3 Department of Physical Therapy, College of Health Sciences, Dankook University, Dandaero, Cheonan, Republic of Korea
4 Department of Physical Therapy, College of Health Sciences, Catholic University of Pusan, Pusan, Republic of Korea
Introduction
The vestibular nuclei (VN), located at the pons and medulla oblongata, consists of four subnuclei: superior nucleus, inferior nucleus, medial nucleus, and lateral nucleus (Barmack,2003; Haines, 2006; Augustine, 2008; Siegel et al., 2010). It receives and carries various types of sensory information,including eye movement, direction or speed of head movement, and body orientation in space to control the movements (Barmack, 2003; Haines, 2006; Augustine, 2008; Siegel et al., 2010; zu Eulenburg et al., 2012). The VN is known to have neural connection with various brain regions including the cerebellum, thalamus, cerebral cortex, oculomotor nucleus, trochlear nucleus, abducens nucleus, insula, and hypothalamus (Henkel and Martin, 1977; Montgomery,1988; Akbarian et al., 1994; Shiroyama et al., 1999; Barmack,2003; Horowitz et al., 2005; Haines, 2006; Augustine, 2008;Markia et al., 2008; Siegel et al., 2010; Kirsch et al., 2016).The connected brain regions and their relation to functions can be summarized as follows: cerebellum - equilibrium;oculomotor nucleus - control of eye movements; thalamus and cerebral cortex - conscious perception of movement and spatial orientation (Barmack, 2003; Haines, 2006; Augustine,2008; Siegel et al., 2010; zu Eulenburg et al., 2012; Hitier et al., 2014). As a result, an injury to VN can result in vertigo and sensorimotor dysfunction, including loss of equilibrium, poor perception of body movement and eye movement(Barmack, 2003; Haines, 2006; Augustine, 2008; Siegel et al.,2010; Hitier et al., 2014).
Electrophysiologic and tracer techniques have been used in many animal studies reporting on the neural connectivity between the VN and various brain regions (Henkel and Martin, 1977; Montgomery, 1988; Faugier-Grimaud and Ventre,1989; Barmack et al., 1993; Akbarian et al., 1994; Shiroyama et al., 1999; Horowitz et al., 2005; Markia et al., 2008). However, these techniques are limited to application in the live human brain, and the connectivity of the VN in the human brain has not been clearly elucidated. Recently developed diffusion tensor tractography (DTT), derived from diffusion tensor imaging (DTI), is a technique used to reveal the structural neural connectivity in three-dimensional visualization by detection of the translational displacement of water molecules (Parker and Alexander, 2005; Behrens et al., 2007). In particular, the probabilistic tracking method enables estimation of local uncertainty in fiber orientation of each voxel(Parker and Alexander, 2005; Behrens et al., 2007). In other words, it considers the distribution of underlying fiber structure. Accordingly, probabilistic DTI tractography has been widely used to investigate the neural connectivity between neural structures in the human brain, including the amygdala, lateral geniculate body and red nucleus (Muthusamy et al., 2007; Nucifora et al., 2007; Jang and Kwon, 2014; Kwon and Jang, 2014). However, few studies are reported on the structural neural connectivity of the VN in the human brain(Kirsch et al., 2016). In this study, we attempted to investi-gate the structural neural connectivity of the VN in normal subjects using DTT.
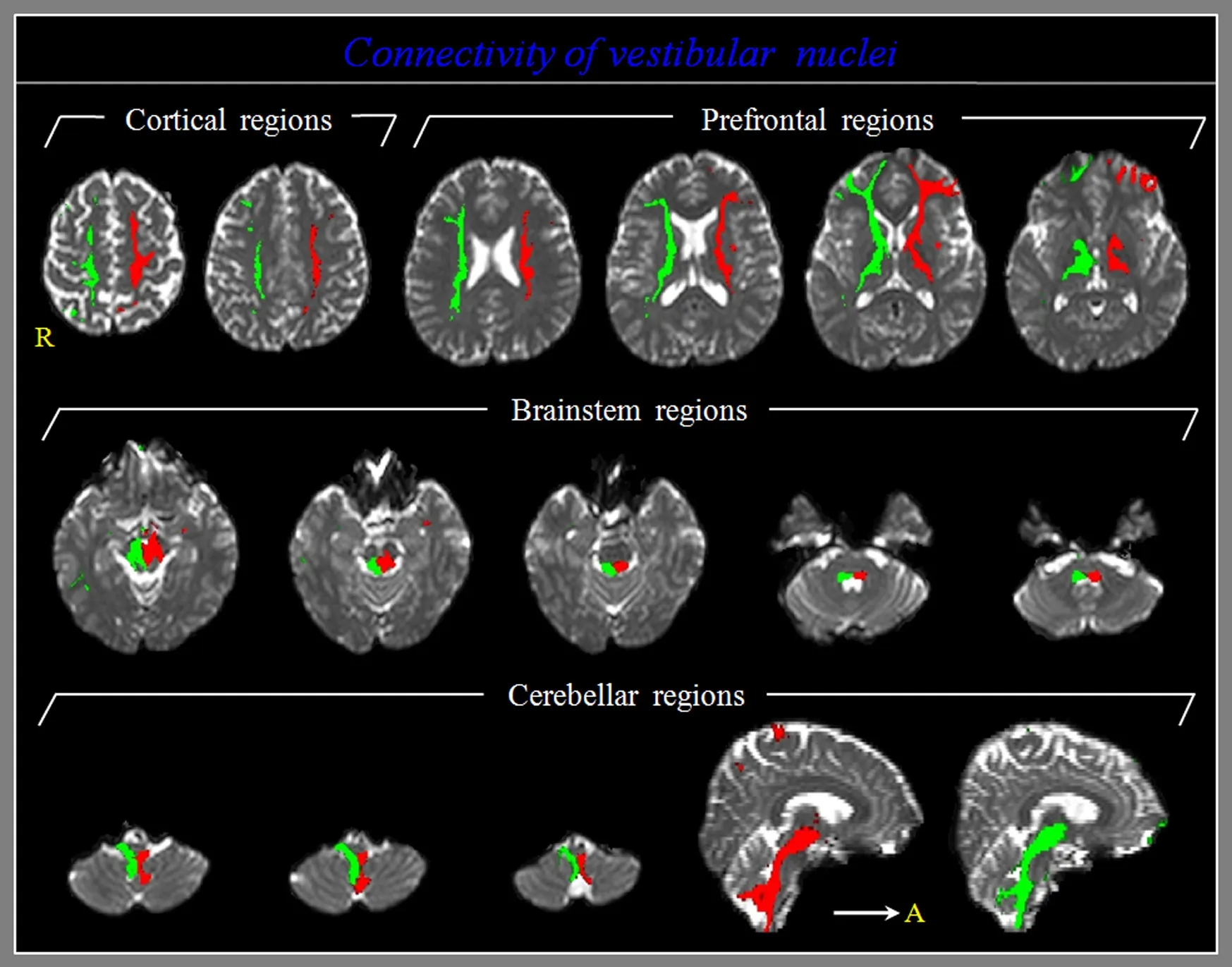
Figure 1 Diffusion tensor tractography for the structural neural connectivity of the vestibular nuclei in a healthy participant.
Participants and Methods
Participants
Thirty-seven healthy participants (22 males and 15 females;mean age of 37.8 years (range 20–56 years) with no previous history of neurological, physical, or psychiatric illness were included in this studyviabulletin board notices. All participants understood the purpose of this study and provided written informed consent prior to participation and the study protocol was approved by the Institutional Review Board of Yeungnam University Hospital, Republic of Korea(YUMC 2017-07-065-011).
Data acquisition
A 1.5 T Philips Gyroscan Intera system (Philips Ltd, Best,The Netherlands) equipped with a 6-channel head coil with a single-shot spin echo planar imaging sequence was used for acquisition of DTT data. For each of the 32 non-collinear, diffusion-sensitizing gradients, 70 contiguous slices were acquired parallel to the anterior commissure-posterior commissure line. Imaging parameters of DTT were as follows:acquisition matrix = 96 × 96; reconstructed to matrix = 192 ×192; field of view = 240 × 240 mm2; repetition time = 10,398 ms; echo time = 72 ms; parallel imaging reduction factor = 2;echo-planar imaging factor = 59;b= 1000 s/mm2; number of excitations = 1; and a slice thickness of 2.5 mm.
Probabilistic fiber tracking
The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl) was used to analyze diffusion-weighted imaging data. Eddy current correction was applied to correct the head motion effect and image distortion. Fiber tracking was performed using probabilistic tractography, and applied in the default tractography option in FMRIB Diffusion Soft-ware (5000 streamline samples, 0.5 mm step lengths, curvature thresholds = 0.2) (Smith et al., 2004; Behrens et al.,2007). The fiber tracking method was used to generate 5000 streamline samples from each seed region of interest with relection of both dominant and non-dominant orientation of diffusion in each voxel in each individual (Smith et al.,2004; Behrens et al., 2007). The fiber tracking method has an advantage in the evaluation of the structural neural connectivities of neural structures. In the seed region of interest, the VN was isolated using the following adjacent structures: the reticular formation (RF, antero-medial boundary), restiform body (lateral boundary), and posterior margin of the medulla (posterior boundary) (Naidich and Duvernoy, 2009). Out of 5,000 streamline samples generated from the seed voxel,results of fiber tracking were visualized at the threshold of 5, 50, and 100 streamlines through each voxel for analysis.Connectivity represented the percentage of connection in the hemispheres of 37 healthy subjects.
Determination of connectivity between the VN and targeted brain regions
Connectivity was defined as the incidence of connection between the VN and each of the following brain regions: the primary motor cortex, premotor cortex, primary somatosensory cortex, posterior parietal cortex, lateral prefrontal cortex,ventromedial prefrontal cortex, orbitofrontal cortex, thalamus,hypothalamus, oculomotor nucleus, trochlear nucleus, abducens nucleus, and reticular formation and cerebellum.
Statistical analysis
SPSS 15.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. The chi-square test was performed to determine the difference in connectivity of the VN between the right and left hemispheres. A level ofP< 0.05 was considered statistically significant.
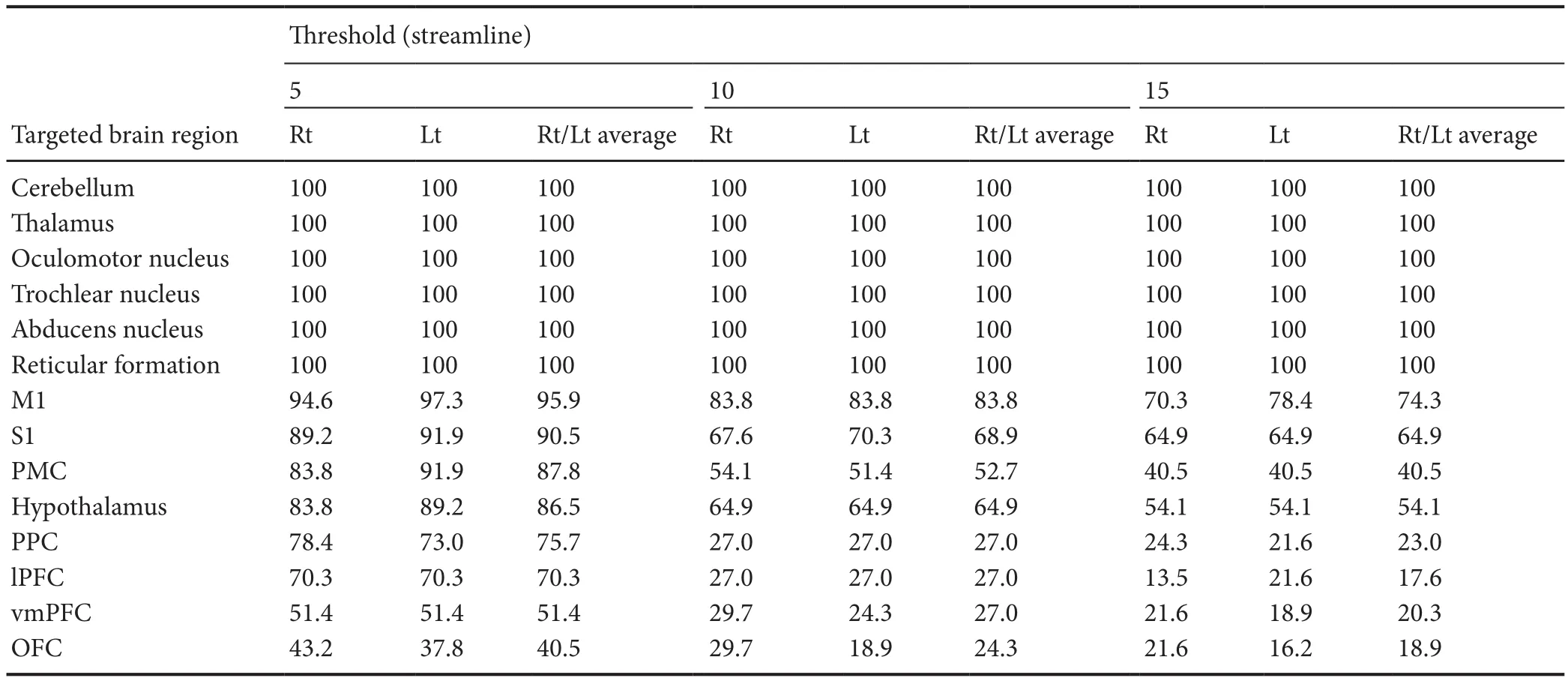
Table 1 Incidence of connectivity (%) between the vestibular nuclei and targeted brain regions in healthy subjects
Results
A summary of the structural neural connectivity of the VN is shown in Table 1 and Figure 1. The VN showed 100%connectivity with the cerebellum, thalamus, oculomotor nucleus, trochlear nucleus, abducens nucleus, and reticular formation, irrespective of thresholds. In contrast, regarding the threshold of 5, 50, and 100 streamlines, the VN showed connectivity with the primary motor cortex (95.9%, 83.8%,and 74.3%), primary somatosensory cortex (90.5%, 68.9%,and 64.9%), premotor cortex (87.8%, 52.7%, and 40.5%),hypothalamus (86.5%, 64.9%, and 54.1%), posterior parietal cortex (75.7%, 27.0%, and 23.0%), lateral prefrontal cortex(70.3%, 27.0%, and 17.6%), ventromedial prefrontal cortex(51.4%, 27.0%, and 20.3%), and orbitofrontal cortex (40.5%,24.3%, and 18.9%), respectively. In all targeted brain regions,no significant difference in connectivity of the VN was observed between the right and left hemispheres (P> 0.05).
Discussion
In this study, probabilistic tracking was used to investigate the structural neural connectivity of the VN in the normal human brain. According to our findings, the VN showed 100% connectivity with brain regions (cerebellum, thalamus,oculomotor nucleus, trochlear nucleus, abducens nucleus,and reticular formation) related to the functions of the VN(equilibrium, control of eye movements, conscious perception of movement, and spatial orientation) irrespective of thresholds, as well as high connectivity (over 70%) with the sensory-motor cortex (primary motor cortex, primary somatosensory cortex, premotor cortex, and posterior parietal cortex), hypothalamus, and lateral prefrontal cortex at the threshold of 5 streamlines.
Tracer technique has been used in many animal studies reporting on the connectivity of the VN (Henkel and Martin, 1977; Montgomery, 1988; Faugier-Grimaud and Ventre,1989; Barmack et al., 1993; Akbarian et al., 1994; Shiroyama et al., 1999; Horowitz et al., 2005; Markia et al., 2008). Using tracer technique, Henkel and Martin (1977) demonstrated connection of the major afferent fibers of the VN with the cerebellum in 300 rats and that the superior VN terminated at the ipsilateral trochlear nucleus and oculomotor nucleus. Subsequently, Akbarian et al. (1994), who reported that the VN connected the premotor and parietal cortex in five monkeys, suggested that these connectivities might affect the vestibulocular reflex. Horowitz et al. (2005) reported connection of the VN with various brain regions, including the thalamic nucleus, hypothalamus, oculomotor nucleus,and cerebellum in 24 hamsters. Markia et al. (2008) recently reported on the connectivity between the VN and paraventricular nucleus of hypothalamus using a monosynaptic retrograde tracer technique in six rats. Our results appear to be consistent with those of the previous studies.
To the best of our knowledge, only one study involving the human brain has been reported on the structural neural connectivity of the VN using DTT (Kirsch et al., 2016 ).Kirsch et al. (2016) examined the functional and structural connectivity between the VN and ipsilateral and contralateral parieto-insular vestibular cortex in 24 normal subjects using DTT and functional MRI. They found two ipsilateral pathways of the VN to the parieto-insular vestibular cortex,a direct pathway without the thalamus and an indirect pathway with the thalamus in either the posterolateral or paramedian nuclei, and with regard to the contralateral pathways, the VN connected with the parieto-insular vestibular cortexviathe posterolateral thalamus (Kirsch et al., 2016). Compared to Kirsch’s study, our study investigated the structural neural connectivity of the VN to almost all brain regions and adopted three different thresholds to determine the potential and tendency of connectivity between the VN and all brain regions. We believe our findings would be helpful for clinicians who are engaged in research on neurological diseases in terms of diagnosis, treatment plan, and prognosis prediction.
In conclusion, in this study, we investigated the structural neural connectivity of the VN in normal human subjects and found that the VN showed high connectivity with brain regions related to the functions including equilibrium, eye movements, conscious perception of movement, and spatial orientation. We believe that the methods used in this study to investigate the structural neural connectivity of the VN in the live human brain, as well as the corresponding results, would provide valuable information for clinicians and researchers studying the VN. However, several limitations of this study should be considered (Parker and Alexander, 2005; Yamada et al., 2009; Fillard et al., 2011). First, although the VN is composed of four subnuclei, we found no connectivity between specific subnuclei of the VN and each brain region. Second,because DTT cannot discern the direction, the afferent and efferent fibers could not be divided between the VN and brain regions. Third, when using probabilistic DTT tractography,crossing fibers in a voxel or partial volume effect throughout the white matter of the brain can cause both false positive and negative results (Yamada et al., 2009; Fillard et al., 2011).Therefore, to overcome these limitations, in-depth studies, as well as studies regarding clinical application of our results for patients with brain injury, are encouraged.
Author contributions:SHJ conceived and designed this study, was responsible for data acquisition, wrote and authorized the paper. MYL participated in research design and data acquisition. SSY participated in research design and provided technical assistance. HGK participated in data acquisition and analysis, was in charge of fundraising, contributed to paper writing, and provided technical support. All authors approved the final version of this paper.
Conflicts of interest:None declared.
Financial support:This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF)funded by the Ministry of Education (2015R1D1A4A01020385).
Research ethics:All participants provided informed consent for participation and the study was approved by the institutional Review Board of Yeungnam University Hospital (YUMC 2017-07-065-011). The study followed the Declaration of Helsinki and relevant ethical principles.
Declaration of participant consent:The authors certify that they will obtain all appropriate participant consent forms. In the form, the participants will give their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Akbarian S, Grusser OJ, Guldin WO (1994) Corticofugal connections between the cerebral cortex and brainstem vestibular nuclei in the macaque monkey. J Comp Neurol 339:421-437.
Augustine JR (2008) Human Neuroanatomy. NJ, USA: John Wiley &Sons.
Barmack NH (2003) Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull 60:511-541.
Barmack NH, Baughman RW, Errico P, Shojaku H (1993) Vestibular primary afferent projection to the cerebellum of the rabbit. J Comp Neurol 327:521-534.
Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007)Probabilistic diffusion tractography with multiple fibre orientations:What can we gain? Neuroimage 34:144-155.
Faugier-Grimaud S, Ventre J (1989) Anatomic connections of inferior parietal cortex (area 7) with subcortical structures related to vestibulo-ocular function in a monkey (Macaca fascicularis). J Comp Neurol 280:1-14.
Fillard P, Descoteaux M, Goh A, Gouttard S, Jeurissen B, Malcolm J,Ramirez-Manzanares A, Reisert M, Sakaie K, Tensaouti F, Yo T, Mangin JF, Poupon C (2011) Quantitative evaluation of 10 tractography algorithms on a realistic diffusion MR phantom. Neuroimage 56:220-234.
Haines DE (2006) Fundamental neuroscience, 3rdEdition. Philadelphia:Churchill Livingstone.
Henkel CK, Martin GF (1977) The vestibular complex of the American opossum didelphis virginiana. II. Afferent and efferent connections. J Comp Neurol 172:321-348.
Hitier M, Besnard S, Smith PF (2014) Vestibular pathways involved in cognition. Front Integr Neurosci 8:59.
Horowitz SS, Blanchard J, Morin LP (2005) Medial vestibular connections with the hypocretin (orexin) system. J Comp Neurol 487:127-146.
Jang SH, Kwon HG (2014) Neural connectivity of the amygdala in the human brain: a diffusion tensor imaging study. Neural Netw World 24:591-599.
Kirsch V, Keeser D, Hergenroeder T, Erat O, Ertl-Wagner B, Brandt T, Dieterich M (2016) Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct Funct 221:1291-308.
Kwon HG, Jang SH (2014) Neural connectivity of the lateral geniculate body in the human brain: diffusion tensor imaging study. Neurosci Lett 578:66-70.
Markia B, Kovacs ZI, Palkovits M (2008) Projections from the vestibular nuclei to the hypothalamic paraventricular nucleus: morphological evidence for the existence of a vestibular stress pathway in the rat brain.Brain Struct Funct 213:239-245.
Montgomery NM (1988) Projections of the vestibular and cerebellar nuclei in Rana pipiens. Brain Behav Evol 31:82-95.
Muthusamy KA, Aravamuthan BR, Kringelbach ML, Jenkinson N, Voets NL, Johansen-Berg H, Stein JF, Aziz TZ (2007) Connectivity of the human pedunculopontine nucleus region and diffusion tensor imaging in surgical targeting. J Neurosurg 107:814-820.
Naidich TP, Duvernoy HM (2009) Duvernoy’s atlas of the human brain stem and cerebellum: high- field MRI: surface anatomy, internal structure, vascularization and 3D sectional anatomy. Wien; New York:Springer.
Nucifora PG, Verma R, Lee SK, Melhem ER (2007) Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology 245:367-384.
Parker GJ, Alexander DC (2005) Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci 360:893-902.
Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K (1999) Projections of the vestibular nuclei to the thalamus in the rat: a Phaseolus vulgaris leucoagglutinin study. J Comp Neurol 407:318-332.
Siegel A, Sapru HN, Siegel H (2010) Essential neuroscience, 3th ed. Edition: Lippincott Williams & Wilkins.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE,Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE,Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM,Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208-219.
Yamada K, Sakai K, Akazawa K, Yuen S, Nishimura T (2009) MR tractography: a review of its clinical applications. Magn Reson Med Sci 8:165-174.
zu Eulenburg P, Caspers S, Roski C, Eickhoff SB (2012) Meta-analytical definition and functional connectivity of the human vestibular cortex.Neuroimage 60:162-169.
杂志排行
中国神经再生研究(英文版)的其它文章
- Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease
- Alpha-7 nicotinic acetylcholine receptor agonist treatment in a rat model of Huntington’s disease and involvement of heme oxygenase-1
- Compound of icariin, astragalus, and puerarin mitigates iron overload in the cerebral cortex of Alzheimer’s disease mice
- Intracerebroventricularly-administered 1-methyl-4-phenylpyridinium ion and brain-derived neurotrophic factor affect catecholaminergic nerve terminals and neurogenesis in the hippocampus, striatum and substantia nigra
- Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease
- Brain remodeling after chronic median nerve compression in a rat model
