Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease
2018-05-05YaRuWenJunHuaYangXiaoWangZhiBinYao
Ya-Ru Wen , Jun-Hua Yang , Xiao Wang Zhi-Bin Yao
1 Department of Anatomy and Neurobiology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, Guangdong Province, China
2 Guangdong Provincial Key Laboratory of Brain Function and Disease, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou,Guangdong Province, China
Introduction
Alzheimer’s disease (AD), the most common cause of dementia, causes severe impairments to AD patients and heavy financial and social burden to their families and society(Reitz and Mayeux, 2014). Accumulation and deposition of amyloid-β (Aβ) is regarded as the main causative pathology in AD development, and a variety of strategies to promote Aβ clearance have been developed and tested both in experimental and clinical studies (Wisniewski and Drummond,2016; Bate, 2017; Wang et al., 2017). However, the therapeutic effects of current Aβ clearance-based pharmacotherapies are unsatisfactory and can cause severe side effects including encephalitis and meningitis (Cash et al., 2014). In general,these therapeutic methods involve active immunization, pas-sive immunization or other mechanisms to directly increase the clearance of Aβ from the brain. Nevertheless, there has been no report of a method to promote the lymphatic clearance of Aβ from the cerebrospinal fluid (CSF). This might be because the structure and function of the lymphatic system of the central nervous system (CNS) were not described in detail until 2015 (Aspelund et al., 2015; Louveau et al., 2015).Before 2015, it was widely thought that no classical lymphatic vessels were present in the CNS, including the brain and meninges. Therefore, few studies argued that the lymphatic clearance of Aβ from the CNS mattered (Shibata et al., 2000;Abbott, 2004), although several studies reported the presence of Aβ in cervical lymphatic nodes (Tsai et al., 2003; Pappolla et al., 2014). In 2015, Aspelund et al. (2015) and Louveau et al. (2015) verified that cells and solutes in the CSF drained into the deep cervical lymphatic nodes through classical lymphatic vessels located in the dura. Therefore, the transport of Aβ from the brain into the CSF indicates another potentially important route for brain-produced Aβ clearance.
We hypothesized that an increase in dural lymphangiogenesis improves the lymphatic clearance of Aβ from the CSF and thus would have therapeutic effects on AD pathology concerning the accumulation and deposition of Aβ in the brain. To the best of our knowledge, no reports to date have addressed this issue.
Vascular endothelial growth factor (VEGF)-C plays an important role in lymphangiogenesis and angiogenesis in embryos and tumors (Kukk et al., 1996; Karkkainen et al.,2004; Hirakawa et al., 2007; Gore et al., 2011). Recombinant human VEGF (rhVEGF)-C is a human recombinant protein specific to VEGF receptor-3 (VEGFR-3); these are critical factors for lymphatic growth (Iwami et al., 2015),and VEGF-C/VEGFR-3 signaling is involved in maintaining tissue electrolyte balance (Machnik et al., 2009; Wiig et al.,2013). The current study applied rhVEGF-C to a transgenic mouse model of AD to investigate the effect on Aβ clearance from the brain and CSF and the underlying mechanism.
Materials and Methods
Human lymphatic endothelial cell (hLEC) culture
hLECs were purchased from ScienCell Research Laboratories (Catalog #2500, Carlsbad, CA, USA) and maintained in Dulbecco’s modified Eagle’s medium (Life Technologies,Shanghai, China) containing 10% fetal bovine serum (Bio-Ind, Israel) and 1% penicillin/streptomycin solution (Life Technologies, Shanghai, China) and were seeded in culture bottles (Corning, New York City, NY, USA), and then incubated at 37°C in a humidified incubator with an atmosphere of 5% CO2. hLECs were digested with 0.25% trypsin-ethylenediamine tetraacetic acid (Life Technologies, Burlington, Canada) for 1 minute, and complete medium was added to terminate digestion. hLECs were then treated with rhVEGF-C protein (100 ng/mL) (Cys156Ser, R&D Systems,Minneapolis, MN, USA) at the indicated time points.
hLEC tube formation assay
Tube formation assay was performed to examine the effects of rhVEGF-C on the tube formation of hLECsin vitro. Ten μL/well liquid Matrigel (Corning, Cat. #356243, Bedford,MA, USA) was coated on the inner walls of 15-well Ibidi μ-slides for angiogenesis (Ibidi, Martinsried, Germany) (Gagliostro et al., 2016), and polymerized for 1 hour at 37°C.hLECs were synchronized by serum starvation for 12 hours prior to treatment with equal amounts of purified factor (100 ng/mL) or 10% fetal bovine serum for 48 hours (Joukov et al., 1998; Chien et al., 2009). hLECs (1 × 104/well) in 50 μL media were seeded into the upper well and incubated for 12 hours at 37°C to allow tube formation. Images of tube formation of each chamber were captured using an inverted phase contrast microscope (Leica DMI 4000B, Wetzlar,Germany) at 5× magnification. Total lengths of tube-like structures (exceeding 20 mm in diameter) were measured and quantified using ImageJ (NIH, Bethesda, MD, USA)(Kazenwadel et al., 2012; Shin et al., 2015).
Animals
Nine-month-old female APPswe/PS1dE9 (APP/PS1)mice were purchased from the Guangdong Medical Laboratory Animal Center in China (Foshan, China) (No.44007200040575). All animals were housed in a specific-pathogen-free facility and maintained on a 12 hourlight/12 hour-dark cycle with a standard diet. This study was approved by the Institute Research Ethics Committee of Sun Yat-sen University of China (approval No. 2017-002) and performed in strict accordance with the UK Animals (Scientific Procedures) Act, 1986. The mice were randomly divided into rhVEGF-C and control groups (n= 16 per group, including 6 mice for morphological analysis and 10 for behavioral tests).
In vivo VEGFR-3 activation
Mice were anesthetized with an intraperitoneal injection of chloral hydrate (10% solution) and placed into a stereotactic frame. The neck muscles were bluntly dissected to expose the dura mater overlying the cisterna magna through a small midline incision. Cannulae (outer diameter 0.3 × inner diameter 0.14 mm, depth 1 mm, custom-made by RWD,Shenzhen, China) were implanted above the cisterna magna and affixed with dental acrylic to maintain their position(de Lange et al., 1997). On day 5 after cannulae implantation, mice were intracerebroventricularly injected (into the cisterna magna) with 5 μL of rhVEGF-C (Cys156Ser, R&D Systems, 200 μg/ mL) or PBS with a 5 μL microliter syringe(RWD, Cat. #79004, Shenzhen, China) at a rate of 1 μL/min over 5 minutes (Kajiya et al., 2009; Heishi et al., 2010; Rangroo Thrane et al., 2013). A total of four injections were performed once every 2 days. Meninges were harvested 7 days after the last injection for immunofluorescence staining. The experimental timeline is shown in Figure 1.
Immunofluorescence staining
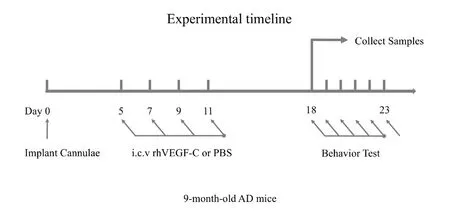
Figure 1 Experimental timeline.
Mice were euthanized with an intraperitoneal injection of chloral hydrate (10% solution) 7 days after the last injection.Approximately 5–10 μL CSF was obtained from the cisterna magna per mouse according to the protocol described previously for enzyme-linked immunosorbent assay (ELISA)(Liu and Duff, 2008). After perfusion with 0.1 M of ice-cold phosphate-buffered saline (PBS) for 5 minutes, surgery was performed as previously described (Louveau et al., 2015).After removal of the skin and muscle from the skull, the skull cap was removed with surgical scissors. Then, the brain was immediately removed and bisected sagittally. The right hemisphere was snap frozen for ELISA, and the left hemisphere for Thioflavine-S staining. Deep cervical lymph nodes were isolated by forceps. Whole-mount meninges, brains and deep cervical lymph nodes were immediately fixed in PBS with 4% paraformaldehyde overnight at 4°C. The meninges (dura mater/arachnoid) were then carefully stripped from the skull cap, washed in PBS three times and processed for staining. Brains and deep cervical lymph nodes were dehydrated in a graded series of sucrose solutions at 4°C. At the level of the hippocampus (bregma −1.58 mm to −2.46 mm), serial coronal sections (30 μm) were obtained from each brain with a freezing microtome (Leica Microsystems,Inc., Exton, PA, USA), and stored in PBS at 4°C prior to immunostaining.
Dura and deep cervical lymph node sections were washed twice with PBS for 5 minutes each and blocked in PBS containing 1% bovine serum albumin and 0.25% Triton X-100(Sigma-Aldrich, St Louis, MO, USA) for 1 hour at 37°C and subsequently incubated overnight at 4°C with appropriate dilutions of primary antibodies: goat anti-Lyve-1 (1:600;R&D Systems) and rabbit anti-Prox1 (1:500; Abcam). Specimens were washed three times in PBS followed by incubation with the appropriate secondary antibody at 37°C for 2 hours. The secondary antibodies were: Alexa 555-conjugated donkey anti-goat, Alexa Fluor 488 goat anti-rabbit, (1:400;both from Invitrogen). Subsequently, the samples were washed three times in PBS and mounted on glass slides with fluorescence mounting media. Brain sections were washed twice with PBS for 5 minutes each and stained with 1% thioflavine-S (Li et al., 2017).
Confocal micrographs were obtained using an LSM 780 confocal laser-scanning microscope (Zeiss, Heidelberg,Germany). For images of the whole mount of the meninges,brain sections and lymphatic nodes, images were acquired with a 10× objective using the Tile Scan function. Images of the meninges were acquired at 512 × 512 pixel resolution and with a z-step of 4 μm. Lymphatic vessels were stained by Lyve-1 antibody (red), and the nuclei of hLECs were stained by Prox-1 antibody (green). The numbers of Prox-1+nuclei colocalized with Lyve-1+vessel were counted using Stereo Investigator (MicroBrightField, Williston, VT, USA). The superficial area of dural lymphatic vessels was quantified with Imaris 8.4 software (Bitplane AG, Zurich, Switzerland).Prox1-positive cell density was determined by dividing the number of Prox1+nuclei by the superficial area of lymphatic vessels. The length of lymphatic vessels of the meninges was quantified with ImageJ. The meningeal lymphatic vessel diameter of dural lymphatic vessels was determined by dividing the superficial area by the length of lymphatic and π. Relative areas of Lyve-1+lymphatic vessels of the deep cervical lymph nodes were measured by ImageJ. For the quantitative image analysis of Aβ plaques, six 30 μm coronal sections (150 μm apart) were selected per mouse. The hippocampus and cerebral cortex of each sample were chosen for quantitative analysis using ImageJ. Data are reported as the percentage of immunolabeled area captured relative to the total demarcated brain region.
ELISA
Briefly, brain samples were weighed and radioimmune precipitation assay buffer (Beyotime, Shanghai, China) containing 1% phenylmethyl sulfonyl fluoride (Beyotime) was added and then sonicated with a homogenizer (Qiagen, Hilden,Germany) for 20 seconds on ice. Samples were then centrifuged for 5 minutes at 12,000 ×gat 4°C, and supernatants were transferred into a new tube and stored at −80°C. The total protein concentration of each sample was adjusted to 4.5 mg/mL using the bovine serum albumin protein assay kit(Beyotime). The levels of human soluble Aβ1–40 and Aβ1–42 in brain, CSF and deep cervical lymph nodes were analyzed by ELISA kits (Cusabio, Wuhan, China). Brains were also assayed for oligomeric Aβ using ELISA kits (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Morris water maze test
The Morris water maze test was performed to assess the hippocampus-dependent spatial learning and memory of transgenic mice 7 days after the last injection (Vorhees and Williams, 2006). Mice were trained to find the escape platform in white non-toxic colored water at 23°C in a 0.8-m diameter pool (Yang et al., 2016). The behavioral test was administered for 6 consecutive days. The acquisition phase was from the first to the fifth day, and the spatial probe occurred on day 6. In each trial, mice were given 60 seconds to find the hidden platform. The escape latency is the mean time required to find the platform of four trials starting from different quadrants, recorded up to 60 seconds. On day 6, the platform was removed from the pool and each mouse was tested by a probe trial for 60 seconds (Counts and Lahiri, 2014). Data were re-corded using a MT-200 Morris image motion system (Chengdu Technology & Market Corp., Chengdu, China). Mice were dried and warmed after every trial.
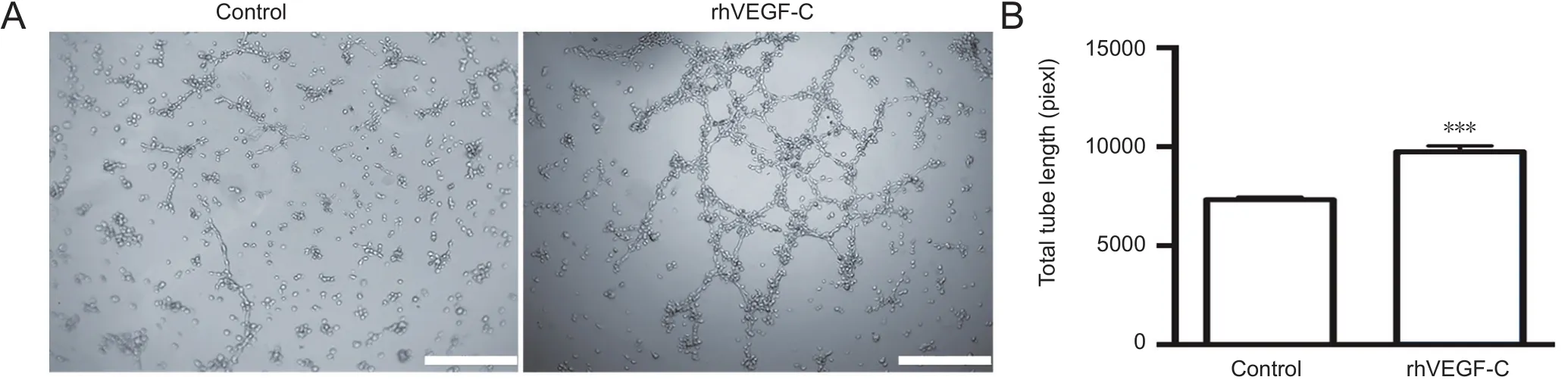
Figure 2 Tube formation of hLECs following rhVEGF-C treatment.
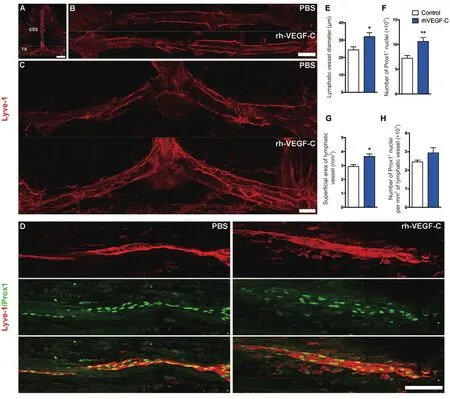
Figure 3 Elevated dural lymphangiogenesis in transgenic mice injected with rhVEGF-C.
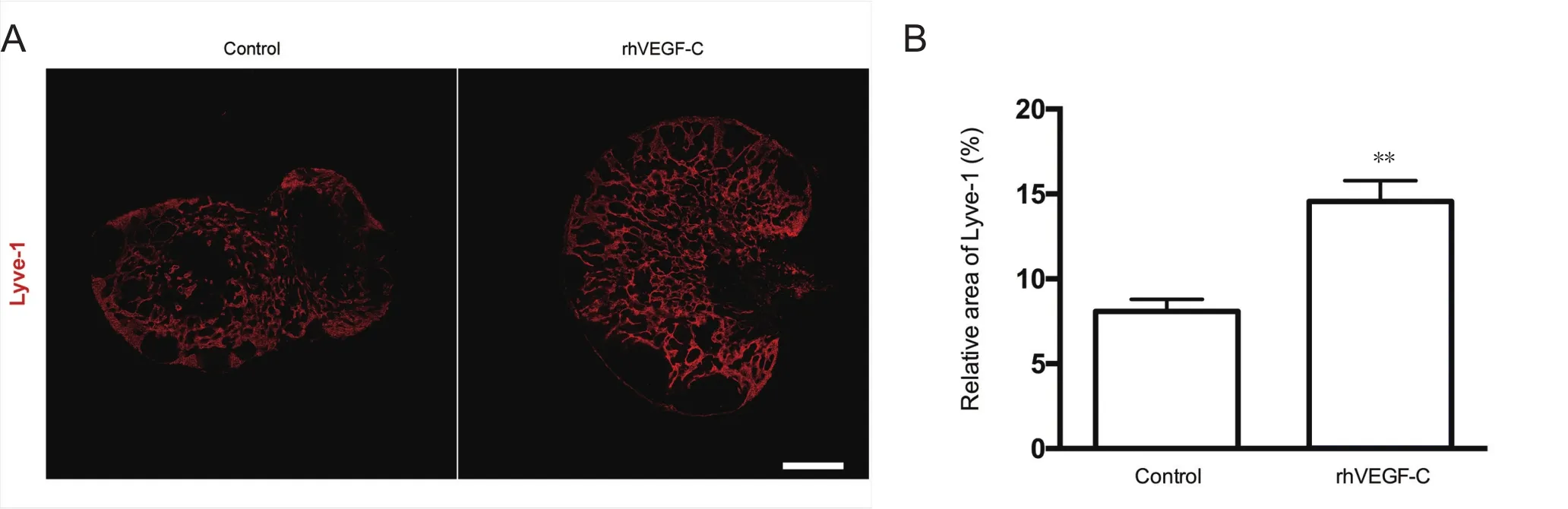
Figure 4 Elevated lymphangiogenesis in dcLNs in APP/PS1 mice treated with rhVEGF-C.
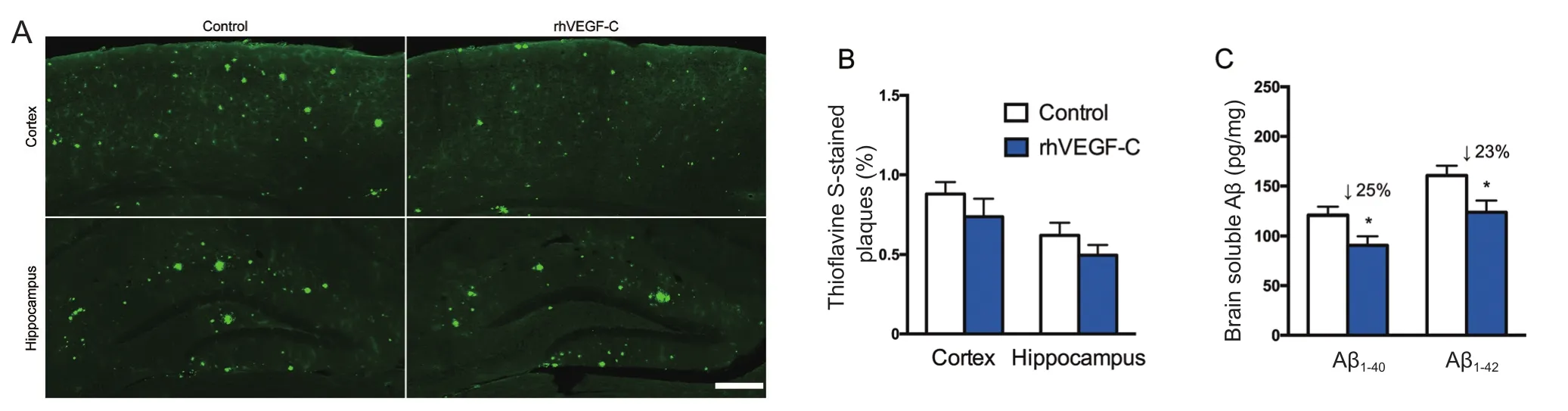
Figure 5 Reduction of cerebral Aβ pathology in APP/PS1 mice treated with rhVEGF-C 7 days after the last injection.
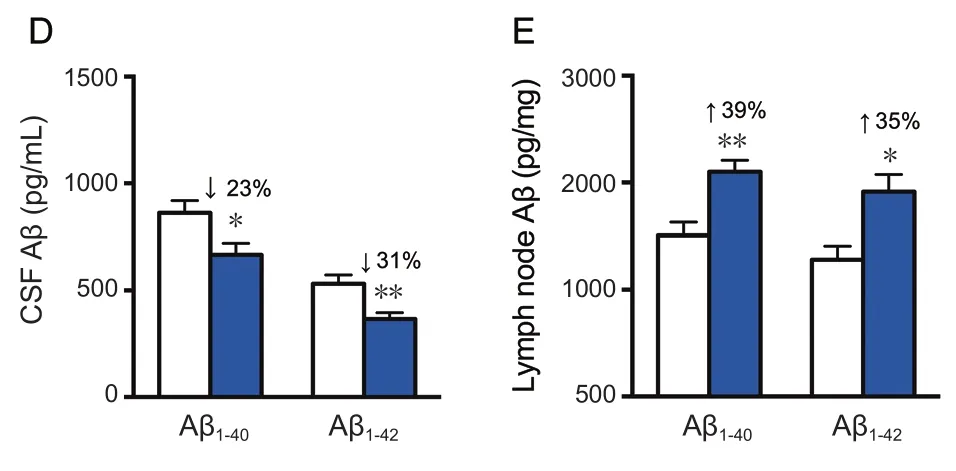
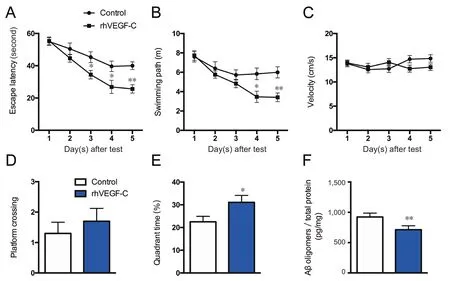
Figure 6 rhVEGF-C improved memory deficits in APP/PS1 transgenic mice.
Statistical analysis
All data, expressed as the mean ± SEM, were statistically analyzed by SPSS 21.0 software (IBM Company, Armonk,NY, USA), according to the principle of random and double-blind testing. Data from the Morris water maze test were analyzed by two-way repeated measures analysis of variance followed by the Bonferronipost-hoctest. The remaining data were analyzed by two-tailed Student’st-test. The statistically significant level was set at α = 0.05.
Results
rhVEGF-C significantly promoted tube formation of hLECs in vitro
Injection of rhVEGF-C, a VEGFR3-specific recombinant protein, into the cisterna magna of C57BL/6 mice increased the mean diameter of lymphatic vessels in the dura mater(Louveau et al., 2015). This alteration continued for no more than 2 weeks but suggested the promoting role of rhVEGF-C on dural lymphangiogenesis. We examined the effects of rhVEGF-C on tube formation by hLECsin vitro.Tube formation was observed 12 hours after hLEC planting on Matrigel-coated Ibidi μ-slides under normal growth conditions. The addition of rhVEGF-C significantly promoted tube formation by hLECs (Figure 2).
Elevated dural lymphangiogenesis in transgenic mice treated with rhVEGF-C
Dura from 9-month-old transgenic mice were labeled immunofluorescently to identify the dural lymphatic vessels(Lyve-1+/Prox-1+vessels). Transgenic mice treated with rhVEGF-C showed elevated lymphangiogenesis in the dural lymphatic vessels compared with the control group. The mean diameter of the dural lymphatic vessels (Figure 3AE), total numbers of Prox-1+nuclei, total superficial area of dural lymphatic vessels and Prox-1+nuclei density were significantly elevated in the rhVEGF-C group compared with controls (Figure 3A–D, F–H). These results suggest that rhVEGF-C induces lymphatic vessel remodeling in the dura mater of transgenic mice. The proportion of Lyve-1+lymphatic vessels in deep cervical lymph nodes was higher in the rhVEGF-C group compared with the control group(Figure 4).
rhVEGF-C reduced Aβ deposition in transgenic mice
Treatment of rhVEGF-C on APP/PS1 transgenic mice was started at 9 months of age, at which point Aβ was overexpressed causing severe Aβ deposition in the CNS. The effect of treatment on plaque burden was quantified by representative confocal micrographs of brain sections (Figure 5A).However, no significant decline was detected regarding the level of Aβ plaque burden in the rhVEGF-C group compared with the control group (Figure 5B). Soluble Aβ1–40and Aβ1–42levels in the brain and CSF were quantified by ELISA and significant alterations in APP/PS1 mice treated with rhVEGF-C were observed. Importantly, levels of Aβ1–40and Aβ1–42in brain homogenates were 121 ± 8 pg/mg and 161 ± 10 pg/mg in control group, and levels were 91 ± 9 pg/mg and 124 ± 12 pg/mg in the injected group at 7 days after the final injection. More specifically, soluble Aβ1–40and Aβ1–42levels were reduced by 25% and 23%, respectively(Figure 5C). Similar results were observed in the CSF of rhVEGF-C treated mice, and soluble Aβ1–40and Aβ1–42levels were reduced by 23% and 31%, respectively (Figure 5D). To determine whether the decrease of Aβ1–40and Aβ1–42in the CNS was associated with cerebral lymphatic drainage from the brain parenchyma to deep cervical lymph nodes, the concentration of Aβ in deep cervical lymph nodes was measured by ELISA. Notably, Aβ levels in deep cervical lymph nodes were significantly increased in the rhVEGF-C group compared with the control group: soluble Aβ1–40and Aβ1–42levels were increased by 39% and 35% in the rhVEGF-C group, respectively (Figure 5E).
Application of rhVEGF-C reduced spatial cognition deficits in transgenic mice
The Morris water maze test was used to assess the spatial learning and memory of mice. The test consists of 5 days of hidden platform tests, followed by a probe trial 24 hours after the last test. In the hidden platform test, transgenic mice injected with rhVEGF-C showed significant improvement compared with controls. From days 3 to 5, the escape latency of rhVEGF-C treated mice declined compared with control mice, and the treated mice took less time to find the platform compared with untreated mice (Figure 6). The time spent in the third quadrant was higher in the rhVEGF-C group compared with the control group on day 6 of the test(Figure 6E).
The Morris water maze test was used to estimate hippocampus-dependent spatial learning and memory ability in transgenic mice treated with rhVEGF-C. Increased lymphangiogenesis facilitated the clearance of Aβ from the CNS to peripheral lymph nodes. Aβ oligomers are thought to result in the pathogenesis of AD. Here, a decline in the concentration of Aβ oligomers was detected in the brains of rhVEGF-C treated mice by ELISA (Figure 6F).
Discussion
Meningeal lymphatic vessels were described in detail in 2015 and are regarded as an important pathway for the draining of solutes from the CSF, including Aβ (Aspelund et al., 2015; Louveau et al., 2015). VEGF-C strongly promotes cytokines to stimulate lymphangiogenesis (Bachmann et al., 2008; Nurmi et al., 2015; Tacconi et al., 2015) and the pro-lymphangiogenesis role of rhVEGF-C was reportedin vitro(Savetsky et al., 2015). Data fromin vivoexperiments provided direct evidence that rhVEGF-C increases dural lymphangiogenesis. Furthermore, we revealed that large amounts of Aβ, both soluble and insoluble, were removed from mouse brain by the administration of rhVEGF-C.Furthermore, performances in the Morris water maze were improved significantly by the administration of rhVEGF-C.A notable aspect for the lymphatic clearance of Aβ is how Aβ aggregates in the brain were transported into the dural lymphatic vessels. According to previous reports, CNS lymphatic vessels exist only in the dura, but not in the brain(Aspelund et al., 2015; Louveau et al., 2015). Previous studies showed that these tubes drain substances directly from the CSF but not from the brain (Weller et al., 2009; Aspelund et al., 2015; Louveau et al., 2015). Therefore, there is no direct lymphatic pathway for Aβ produced in the brain to drain into the dural lymphatic vessels. However, the glymphatic system was reported to be a pathway for Aβ transport from the brain to the CSF (Jessen et al., 2015). Glymphatic CSF influx may serve as a constant pathway carrying soluble Aβ from the brain into the CSF (Nedergaard, 2013). Indeed,this route has an important role in Aβ clearance and its failure might worsen AD pathology by increased Aβ deposition in the brain. Such failure often occurs when para-arterial deposits appear in cerebral amyloid angiopathy (Gupta and Iadecola, 2015), and this failure always accompanies a marked decrease in the levels of soluble Aβ in the CSF (Yamada, 2015). In the clinic, reduced levels of soluble Aβ in the CSF are thought to be a risk factor and a predictor for the emergence of AD (Hoscheidt et al., 2016). In addition,dye injected into brain parenchyma can be detected in the deep cervical lymphatic nodes, indicating substances in the brain are transported through the CNS lymphatic system(Dissing-Olesen et al., 2015). Taken together, the brain-CSF-dural lymphatic vessel pathway is a useful route for the lymphatic clearance of brain-produced Aβ.
The failure to clear soluble Aβ and the subsequent deposition of insoluble Aβ is the core pathological process of AD (Takahashi et al., 2017). In this study, the upregulation of dural lymphangiogenesis by the administration of rh-VEGF-C during the on-setting stage of insoluble Aβ deposition in the brain significantly reduced the burden of senile plaques in transgenic mice. This suggests that people with a high risk for AD should receive preventive strategies to improve the lymphatic clearance of Aβ. However, further clinical studies to obtain direct support for this pathway in humans are required.
This study had some limitations. The effects of rhVEGF-C in wild-type animals were not assessed in this study. Moreover, whether a preventive effect of rhVEGF-C on Aβ-related pathology can be found if it is administrated to young APP/PS-1 transgenic mice is unknown. However, data from 9-month-old APP/PS-1 transgenic mice were sufficient to support the conclusion of the present study.
In addition to the drainage of cellular and large molecular components from interstitial fluid or CSF (Murlidharan and Asokan, 2016), lymphatic vessels regulate local immune activity, including immune cell recruitment and activation(Lund et al., 2016). Therefore, the regulation of dural lymphatic vessels might have other potential effects in modifying immune microenvironments in the dura and CSF, which might have complex implications concerning immune activity in the brain. This is a new field of great significance to explore in the future.
Author contributions:ZBY and YRW designed this study. YRW performed primarily cell culture, animal treatments and immunofluorescence, as well as sample preparation. JHY performed primarily morphological observations and ELISA. XW performed primarily behavioral test. JHY, YRW and XW performed data analyses (blinded to groups).ZBY, YRW and JHY wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:The authors have no conflicts of interest to declare.Financial support:The work was supported by the National Natural Science Foundation of China, No. 31371130 and 31600836; the Special Foundation of Education Department of Guangdong Province of China;the Medical Scientific Research Foundation of Guangdong Province of China, No. 2013-159; the Foundation of Medical Science and Technology Research of Guangdong Province of China, No. A2016273. None of the
funding bodies played any role in the study other than to provide funding.
Research ethics:The study was approved by the Institute Research Ethics Committee of Sun Yat-Sen University of China (approval No. 2017-002) and performed in strict accordance with the U.K. Animals (Scientific Procedures) Act, 1986.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Abbott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45:545-552.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212:991-999.
Bachmann BO, Bock F, Wiegand SJ, Maruyama K, Dana MR, Kruse FE, Luetjen-Drecoll E, Cursiefen C (2008) Promotion of graft survival by vascular endothelial growth factor a neutralization after highrisk corneal transplantation. Arch Ophthalmol 126:71-77.
Bate C (2017) Can we switch production of toxic Aβ oligomers to neuroprotective Aβ monomers to allow synapse regeneration? Neural Regen Res12:1437-1438.
Cash DM, Rohrer JD, Ryan NS, Ourselin S, Fox NC (2014) Imaging endpoints for clinical trials in Alzheimer’s disease. Alzheimers ResTher 6:87.
Chien MH, Ku CC, Johansson G, Chen MW, Hsiao M, Su JL, Inoue H,Hua KT, Wei LH, Kuo ML (2009) Vascular endothelial growth factor-C (VEGF-C) promotes angiogenesis by induction of COX-2 in leukemic cells via the VEGF-R3/JNK/AP-1 pathway. Carcinogenesis 30:2005-2013.
Counts SE, Lahiri DK (2014) Overview of immunotherapy in Alzheimer’s disease (AD) and mechanisms of IVIG neuroprotection in preclinical models of AD. Curr Alzheimer Res 11:623-625.
de Lange EC, Danhof M, de Boer AG, Breimer DD (1997) Methodological considerations of intracerebral microdialysis in pharmacokinetic studies on drug transport across the blood-brain barrier. Brain Res Brain Res Rev 25:27-49.
Dissing-Olesen L, Hong S, Stevens B (2015) New brain lymphatic vessels drain old concepts. EBioMedicine 2:776-777.
Gagliostro V, Seeger P, Garrafa E, Salvi V, Bresciani R, Bosisio D, Sozzani S (2016) Pro-lymphangiogenic properties of IFN-gamma-activated human dendritic cells. Immunol Lett 173:26-35.
Gore AV, Swift MR, Cha YR, Lo B, McKinney MC, Li W, Castranova D, Davis A, Mukouyama YS, Weinstein BM (2011) Rspo1/Wnt signaling promotes angiogenesis via Vegfc/Vegfr3. Development 138:4875-4886.
Gupta A, Iadecola C (2015) Impaired Abeta clearance: a potential link between atherosclerosis and Alzheimer’s disease. Front Aging Neurosci 7:115.
Heishi T, Hosaka T, Suzuki Y, Miyashita H, Oike Y, Takahashi T, Nakamura T, Arioka S, Mitsuda Y, Takakura T, Hojo K, Matsumoto M,Yamauchi C, Ohta H, Sonoda H, Sato Y (2010) Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am J Pathol 176:1950-1958.
Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M(2007) VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109:1010-1017.
Hoscheidt SM, Starks EJ, Oh JM, Zetterberg H, Blennow K, Krause RA, Gleason CE, Puglielli L, Atwood CS, Carlsson CM, Asthana S,Johnson SC, Bendlin BB (2016) Insulin resistance is associated with increased levels of cerebrospinal fluid biomarkers of Alzheimer’s disease and reduced memory function in at-risk healthy middle-aged adults. J Alzheimers Dis 52:1373-1383.
Iwami D, Brinkman CC, Bromberg JS (2015) Vascular endothelial growth factor c/vascular endothelial growth factor receptor 3 signaling regulates chemokine gradients and lymphocyte migration from tissues to lymphatics. Transplantation 99:668-677.
Jessen NA, Munk AS, Lundgaard I, Nedergaard M (2015) The Glymphatic System: A Beginner’s Guide. Neurochem Res 40:2583-2599.
Joukov V, Kumar V, Sorsa T, Arighi E, Weich H, Saksela O, Alitalo K(1998) A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J Biol Chem 273:6599-6602.
Kajiya K, Sawane M, Huggenberger R, Detmar M (2009) Activation of the VEGFR-3 pathway by VEGF-C attenuates UVB-induced edema formation and skin in fl ammation by promoting lymphangiogenesis.J Invest Dermatol 129:1292-1298.
Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV,Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K(2004) Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5:74-80.
Kazenwadel J, Secker GA, Betterman KL, Harvey NL (2012) In vitro assays using primary embryonic mouse lymphatic endothelial cells uncover key roles for FGFR1 signalling in lymphangiogenesis. PLoS One 7:e40497.
Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V,Alitalo K (1996) VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development.Development 122:3829-3837.
Li C, Guo XD, Lei M, Wu JY, Jin JZ, Shi XF, Zhu ZY, Rukachaisirikul V,Hu LH, Wen TQ, Shen X (2017) Thamnolia vermicularis extract improves learning ability in APP/PS1 transgenic mice by ameliorating both Abeta and Tau pathologies. Acta Pharmacol Sin 38:9-28.
Liu L, Duff K (2008) A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J Vis Exp:e960.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD,Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J(2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337-341.
Lund AW, Wagner M, Fankhauser M, Steinskog ES, Broggi MA,Spranger S, Gajewski TF, Alitalo K, Eikesdal HP, Wiig H, Swartz MA (2016) Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest 126:3389-3402.
Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A,Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K,Eckardt KU, Luft FC, et al. (2009) Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15:545-552.
Murlidharan G, Asokan A (2016) 55. Aquaporins and CSF fl ux are critical determinants of AAV mediated CNS gene Transfer. Mol Ther 24:S25.
Nedergaard M (2013) Neuroscience. Garbage truck of the brain. Science 340:1529-1530.
Nurmi H, Saharinen P, Zarkada G, Zheng W, Robciuc MR, Alitalo K(2015) VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med 7:1418-1425.
Pappolla M, Sambamurti K, Vidal R, Pacheco-Quinto J, Poeggeler B,Matsubara E (2014) Evidence for lymphatic Abeta clearance in Alzheimer’s transgenic mice. Neurobiol Dis 71:215-219.
Rangroo Thrane V, Thrane AS, Plog BA, Thiyagarajan M, Iliff JJ, Deane R, Nagelhus EA, Nedergaard M (2013) Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep 3:2582.
Reitz C, Mayeux R (2014) Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88:640-651.
Savetsky IL, Albano NJ, Cuzzone DA, Gardenier JC, Torrisi JS, Garcia Nores GD, Nitti MD, Hespe GE, Nelson TS, Kataru RP, Dixon JB,Mehrara BJ (2015) Lymphatic function regulates contact hypersensitivity dermatitis in obesity. J Invest Dermatol 135:2742-2752.
Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B,Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV(2000) Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106:1489-1499.
Shin K, Kataru RP, Park HJ, Kwon BI, Kim TW, Hong YK, Lee SH(2015) TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat Commun 6:6196.
Tacconi C, Correale C, Gandelli A, Spinelli A, Dejana E, D’Alessio S,Danese S (2015) Vascular endothelial growth factor C disrupts the endothelial lymphatic barrier to promote colorectal cancer invasion.Gastroenterology 148:1438-1451.e8.
Takahashi RH, Nagao T, Gouras GK (2017) Plaque formation and the intraneuronal accumulation of beta-amyloid in Alzheimer’s disease.Pathol Int 67:185-193.
Tsai PW, Shiah SG, Lin MT, Wu CW, Kuo ML (2003) Up-regulation of vascular endothelial growth factor C in breast cancer cells by heregulin-beta 1. A critical role of p38/nuclear factor-kappa B signaling pathway. J Biol Chem 278:5750-5759.
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848-858.
Wang N, Jia YM, Zhang B, Xue D, Reeju M, Li Y, Huang SM, Liu XW(2017) Neuroprotective mechanism of Kai Xin San: upregulation of hippocampal insulin-degrading enzyme protein expression and acceleration of amyloid- beta degradation. Neural Regen Res 12:654-659.
Weller RO, Djuanda E, Yow HY, Carare RO (2009) Lymphatic drainage of the brain and the pathophysiology of neurological disease.Acta Neuropathol 117:1-14.
Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV,Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Muller DN, Kerjaschki D, Luft FC, et al. (2013) Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 123:2803-2815.
Wisniewski T, Drummond E (2016) Developing therapeutic vaccines against Alzheimer’s disease. Expert Rev Vaccines 15:401-415.
Yamada M (2015) Cerebral amyloid angiopathy: emerging concepts. J Stroke 17:17-30.
Yang J, Qi F, Gu H, Zou J, Yang Y, Yuan Q, Yao Z (2016) Neonatal BCG vaccination of mice improves neurogenesis and behavior in early life. Brain Res Bull 120:25-33.
杂志排行
中国神经再生研究(英文版)的其它文章
- Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease
- Alpha-7 nicotinic acetylcholine receptor agonist treatment in a rat model of Huntington’s disease and involvement of heme oxygenase-1
- Compound of icariin, astragalus, and puerarin mitigates iron overload in the cerebral cortex of Alzheimer’s disease mice
- Structural neural connectivity of the vestibular nuclei in the human brain: a diffusion tensor imaging study
- Intracerebroventricularly-administered 1-methyl-4-phenylpyridinium ion and brain-derived neurotrophic factor affect catecholaminergic nerve terminals and neurogenesis in the hippocampus, striatum and substantia nigra
- Brain remodeling after chronic median nerve compression in a rat model
