Astrocytic Kir4.1 potassium channels as a novel therapeutic target for epilepsy and mood disorders
2018-05-05YukihiroOhno
Astrocytic Kir4.1 channels and spatial potassium buffering: Astrocytes play a crucial role in maintaining the structural and functional integrity of the brain, which includes formation of the blood-brain barrier, maintenance of water and ion homeostasis, metabolism of neurotransmitters and secretion of various neuroactive molecules.Among these functions, spatial potassium (K+) buffering by astrocytes is an essential system for controlling extracellular K+concentration ([K+]o) and neuronal excitability (Figure 1) (Kofuji and Newman, 2004; Ohno et al., 2015). Neurons normally release considerable amounts of K+during the repolarization phase of an action potential. At tripartite synapses, a single action potential elevates the local [K+]olevel by about 1 mM and, if uncorrected, [K+]oreaches 10 mM or more, causing abnormal discharges and finally spreading depression. Spatial K+buffering by astrocytes is a K+-clearance system which removes an excess of extracellular K+and transports it to the regions of low [K+]osuch as capillary vessels (Figure 1). In addition,the spatial K+buffering system is coupled to astrocytic glutamate uptake by glutamate transporters (e.g., EAAT1 and EAAT2) and water transport by aquaporin-4 (AQP4) (Kofuji and Newman, 2004; Ohno et al., 2015).
Spatial K+buffering is mainly mediated by two types of inwardly rectifying K+(Kir) channels, Kir4.1 channels (homo-tetramer of Kir4.1 subunits) and Kir4.1/5.1 channels (hetero-tetramer of Kir4.1 and Kir5.1 subunits)(Figure 1). Kir4.1 and Kir5.1 subunits consist of 379 and 418 amino acids, respectively, and possess two transmembrane-spanning (TM) structures, which form the pore–region of the Kir4.1-containing channels (Ohno et al., 2015). Kir4.1 and Kir4.1/5.1 channels constitutively allow large inward K+currents at potentials negative to K+equilibrium potential (EK) and small, but significant,outward K+currents at those positive to EK(Kofuji and Newman,2004). Therefore, they can absorb excessive extracellular K+locally elevated at synapses and transport it to sites with low K+concentrations, depending on the difference between local EKand the membrane potential of astrocytes. Thus, Kir4.1 and Kir4.1/5.1 channels directly influence the spatial K+buffering capacity of astrocytes and dysfunction of these channels causes an excitation of neurons by elevating the [K+]oand extracellular glutamate ([glutamate]o) levels(Figure 1) (Djukic et al., 2007; Ohno et al., 2015).
Role of Kir4.1 channels in modulating brain-derived neurotrophic factor (BDNF) expression in astrocytes:In response to various pathophysiological stimuli and/or under certain disease conditions,astrocytes activate diverse signal transduction pathways (e.g., Ca2+signaling and multiple 2ndmessenger-protein kinase cascades) and secrete various neuroactive molecules such as gliotransmitters (e.g.,glutamate, ATP, and D-serine), cytokines (e.g., tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β)) and neurotrophic factors (e.g., BDNF and glia-derived neurotrophic factor (GDNF))(Devinsky et al., 2013). We explored the role of astrocytic Kir4.1 channels in modulating the expression of neurotrophic factors (i.e.,BDNF, nerve growth factor (NGF), GDNF and ciliary neurotrophic factor (CNTF)) and demonstrated that inhibition (pharmacological blockade and expressional knockdown) of Kir4.1 channels markedly facilitated the mRNA and protein expression of BDNF in astrocytes(Kinboshi et al., 2017). Antidepressant agents, including sertraline,fluoxetine and imipramine, specifically bind to the two amino acids(i.e., Thr128 and Glu158) located on the pore and the 2ndTM (TM-2)domain of Kir4.1, respectively, and reversibly inhibit Kir4.1 channel activity in a subunit-dependent manner (Ohno et al., 2007; Su et al.,2007; Furutani et al., 2009). Treatments with these agents enhanced BDNF expression in astrocytes according to their relative potencies for Kir4.1 channel (Kinboshi et al., 2017). In addition, knockdown of Kir4.1 expression by the transfection of small interfering RNA (siRNA) targeting Kir4.1 significantly elevated the mRNA and protein levels of BDNF. Knockdown of Kir4.1 expression by Kir4.1 siRNA also weakly increased the GDNF expression, but negligibly affected the expression of NGF or CNTF. Furthermore, the BDNF induction by Kir4.1 siRNA transfection was suppressed by the MEK1/2 inhibitor, but not by the p38 MAPK or the JNK inhibitor (Kinboshi et al.,2017). These results strongly suggest that inhibition of Kir4.1 channels facilitates BDNF expression in astrocytes, probably by activating the Ras/Raf/MEK/ERK pathway, illustrating a novel role of Kir4.1 channels in regulating BDNF synthesis and secretion in astrocytes(Figure 1). Indeed, immunofluorescent double staining analysis revealed that Kir4.1 was co-expressed with BDNF in most astrocytes, where BDNF were densely stained in numerous fine filaments(processes) surrounding astrocyte somata, reflecting the secretory process of synthetized BDNF (Kinboshi et al., 2017).
Therapeutic target for epilepsy and mood disorders:It is now known that Kir4.1 channels are closely involved in the pathogenesis of epileptic disorders. Studies using conditional knockout (cKO)techniques targeted to astroglial Kir4.1 demonstrated a clear linkage between dysfunction of Kir4.1 channels and induction of convulsive seizures (Djukic et al., 2007). These animals developed pronounced ataxia and hypersusceptibility of the induction of generalized tonic-clonic seizures (GTCS) to external stimuli (i.e., sudden touch),suggesting that dysfunction of Kir4.1 channels facilitate seizure generation by disrupting K+buffering and the glutamate reuptake functions of astrocytes. In addition, previous studies showed that brain Kir4.1 expression was significantly reduced in animal models of GTCS such as DBA/2 mice and Noda epileptic rats (NER) (Ohno et al., 2015). Furthermore, clinical studies have shown that loss-offunction mutations in the human geneKCNJ10encoding Kir4.1 caused the epileptic disorder so-called “EAST” (Epilepsy, Ataxia,Sensorineural deafness and Tubulopathy) or “SeSAME” (Seizures,Sensorineural deafness, Ataxia, Mental retardation, and Electrolyte imbalance) syndrome (Bockenhauer et al., 2009; Scholl et al., 2009).Frequent mutations ofKCNJ10include R65P at the cytoplasmic end of the 1stTM (TM-1) region, G77R (TM-1), C140R (extracellular loop between TM-1 and TM-2), T164I, A167V (cytoplasmic end of TM-2), R175Q, R199X and R297C (C-terminal domain). All these mutations of Kir4.1 drastically impair the function of both Kir4.1 channels and Kir4/1/5.1 channels. Furthermore, the down-regulation and/or impaired functioning of Kir4.1 channels were also reported in brain specimens from patients with temporal lobe epilepsy (Ohno et al., 2015). All this evidence strongly suggests that astrocytic Kir4.1 channels are involved in the development of chronic epilepsy (epileptogenesis).
Although the precise mechanisms of Kir4.1 channels underlying the control of epileptogenesis remain to be elucidated, disruption of astrocytic spatial K+buffering by Kir4.1 channel dysfunction elevates[K+]oand [glutamate]olevels at tripartite synapses, which causes hyperexcitation of neurons (Figure 1). In addition, enhancement of astrocytic BDNF expression by Kir4.1 channel dysfunction seems to facilitate the development of chronic epilepsy. Indeed, BDNF is known as a key modulator of epileptogenesis, eliciting synaptic plasticity, neural sprouting, neurogenesis and reactive gliosis (Jankowsky and Patterson, 2001). Numerous studies showed that BDNF expression was up-regulated in various animal models of epilepsy and in human epileptic disorders, and that inhibition of BDNF/TrkB function prevented the development of epilepsy. It is therefore likely that astrocytic Kir4.1 plays a crucial role in modulating both ictogenesis (acute seizure generation) and epileptogenesis (development of chronic epilepsy) and that enhancement of the Kir4.1 channel expression or the Kir4.1 channel activity may cure or prevent epileptic disorders.
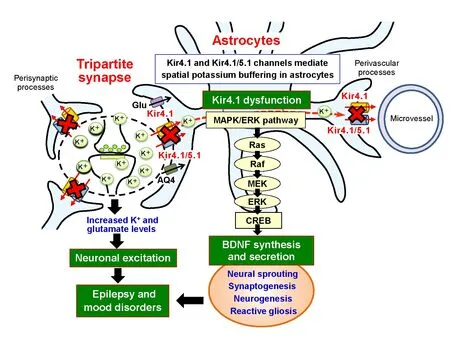
Figure 1 Schematic diagram showing the effects of the Kir4.1 dysfunction on neuronal excitability and astrocytic brain-derived neurotrophic factor (BDNF) expression.
Besides epileptic disorders, BDNF is also implicated in the pathogenesis and treatment of neuropsychiatric disorders such as major depression, schizophrenia and addiction (Autry and Monteggia,2012). Specifically, the evidence that antidepressants enhanced the BDNF expression in astrocytes by inhibiting Kir4.1 channels (Kinboshi et al., 2017) yields a novel hypothesis that astrocytic Kir4.1 channels can serve as a novel therapeutic target for mood disorders(e.g., major depression and bipolar disease). Thus, the Kir4.1-BDNF system in astrocytes may be important as a non-monoaminergic mechanism underlying antidepressant action. Furthermore, since pathophysiological alterations of astrocytic Kir4.1 channels were also demonstrated in Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, autism spectrum disorder patients, Huntington’s disease and multiple sclerosis (Ohno et al., 2015; Kinboshi et al.,2017), the astrocytic Kir4.1-BDNF system may modify the induction and development of such central nervous system disorders.
Conclusions:Spatial K+buffering by astrocytes has long been recognized as an essential system for controlling [K+]oand neuronal excitability in the brain. In addition, evidence is now accumulating that astrocytic K+buffering is closely associated with the pathophysiological basis of neurological and psychiatric disorders such as epilepsy and mood disorders. Specifically, gene mutations of Kir4.1 evoke epileptic disorders in patients (e.g., EAST/SeSAME syndrome and temporal lobe epilepsy). Furthermore, recent evidence illustrates that Kir4.1 plays an important role in modulating the expression of BDNF in astrocytes. Since BDNF causes a variety of influences on brain functions (e.g., synaptic plasticity, neural sprouting, neurogenesis and reactive gliosis), astrocytic Kir4.1 may serve as a novel target for the treatment of epilepsy, major depression and other neuropsychiatric disorders.
This work was supported in part by a Grant from AMED(17ek0109120h0003) and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (17K08324 and 15H04892).
Yukihiro Ohno*Laboratory of Pharmacology, Osaka University of Pharmaceutical Sciences, Osaka, Japan
orcid:0000-0001-5349-7135 (Yukihiro Ohno)
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Eva M Jimenez-Mateos, Royal College of Surgeons in Ireland, Ireland.
Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64:238-258.
Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T,Thompson D, Cross JH, van’t Hoff W, Al Masri O, Tullus K, Yeung S,Anikster Y, Klootwijk E, et al. (2009) Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360:1960-1970.
Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA (2013)Glia and epilepsy: excitability and inflammation. Trends Neurosci 36:174-184.
Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD (2007) Conditional knock-out of Kir4.1 leads to glial membrane depolarization,inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27:11354-11365.
Furutani K, Ohno Y, Inanobe A, Hibino H, Kurachi Y (2009) Mutational and in silico analyses for antidepressant block of astroglial inward-recti fier Kir4.1 channel. Mol Pharmacol 75:1287-1295.
Jankowsky JL, Patterson PH (2001) The role of cytokines and growth factors in seizures and their sequelae. Prog Neurobiol 63:125-149.
Kinboshi M, Mukai T, Nagao Y, Matsuba Y, Tsuji Y, Tanaka S, Tokudome K,Shimizu S, Ito H, Ikeda A, Inanobe A, Kurachi Y, Inoue S, Ohno Y (2017)Inhibition of inwardly rectifying potassium (Kir) 4.1 channels facilitates brain-derived neurotrophic factor (BDNF) expression in astrocytes.Front Mol Neurosci 10:408.
Kofuji P, Newman EA (2004) Potassium buffering in the central nervous system. Neuroscience 129:1045-1056.
Ohno Y, Hibino H, Lossin C, Inanobe A, Kurachi Y (2007) Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res 1178:44-51.
Ohno Y, Tokudome K, Kunisawa N, Iha HA, Kinboshi M, Mukai T, Serikawa T, Shimizu S (2015) Role of astroglial Kir4.1 channels in the pathogenesis and treatment of epilepsy. Ther Targets Neurol Dis 2:e476.
Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP (2009) Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A 106:5842-5847.
Su S, Ohno Y, Lossin C, Hibino H, Inanobe A, Kurachi Y (2007) Inhibition of astroglial inwardly rectifying Kir4.1 channels by a tricyclic antidepressant, nortriptyline. J Pharmacol Exp Ther 320:573-580.
杂志排行
中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration
