Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
2018-05-05BarakatWalterKraftsik
I. Barakat-Walter, R. Kraftsik
Department of Fundamental Neurosciences, Faculty of Biology and Medicine, University of Lausanne, Lausanne, Switzerland
The Need to Stimulate Regeneration of Severed Peripheral Nerves
Peripheral nerve lesions are common and serious injuries affecting about 3% of trauma patients annually and generally lead to considerable motor and sensory disability (Noble et al., 1998). Despite the capacity for endogenous axonal regeneration in the peripheral nervous system, it is commonly found that functional recovery is poor. It is clinically recognized that functional recovery of hand muscles after brachial plexus injury is negligible, poor at best or rather absent (Gordon et al., 2003). Therefore, several techniques of surgical repair have been used to improve regeneration after severe peripheral nerve lesion. The classical method involves mobilization of the nerve to overcome the gap created between the sectioned nerve stumps. However, in some traumatic nerve injuries, nerve loss leaves a significant gap between the cut ends of the nerve that cannot be closed by end-to-end repair. Then, the standard clinical technique for repairing a peripheral nerve when both ends cannot be sutured without tension is to bridge the gap with autologous nerve grafts taken from another nerve of lesser functional importance. Although autografting provides optimal regeneration (Lundborg, 2004), it has several disadvantages, such as the need for a second surgical step, loss of donor nerve function and the most important the limited supply of donor nerves and mismatch between nerve and graft dimensions (Mackinnon and Dellon, 1988). All these factors pushed researchers to find new conduits that could lead regrowing axons from the proximal to the distal stump of severed nerves. Various repair alternatives to the autograft procedure were investigated and the tubulization technique was quickly accepted by many research groups. Tubulization is the introduction of the proximal and distal ends of the inured nerve into a tube to guide the outgrowing nerve fibers towards the distal nerve end.Among experimental models of tubular structures, silicon and other synthetic conduits have been frequently used (Lundborg, 1987;Chen et al., 1989; Fields et al., 1989). Such conduits have the potential to constitute an external guidance channel whereby factors to promote healing could be introduced (Danielsen et al., 1988; Aebischer et al., 1989). Several investigators have reported success using impermeable silicon tubes to promote regeneration across a gap.In fact, studies showed that when the gap is bridged, the degree of recovery is higher and the beginning of reinnervation occurs sooner compared to unrepaired nerve (Buti et al., 1996; Doolabh et al.,1996).
The Role of Thyroid Hormones (THs) in the Nervous System
The TH, 3,3′,5-triiodothyronine (T3) and its prohormone, thyroxine (T4), are secreted into the blood stream by the thyroid gland in response to thyroid-stimulating hormone (TSH). T4, the least active form of TH is more abundant in the blood plasma than T3which is the active form (Hulbert, 2000). Within the target cell,the transcriptionally active metabolite T3is generated from T4by deiodination (stepwise enzymatic removal of iodine) by the iodothyronine deiodinase enzyme D2. Generally neuronal T3is largely derived from D2-dependent metabolism of T4in glial cells. In adult peripheral nerve D2is not expressed, however nerve lesions induce D2in peripheral nerve sheath compartments containing Schwann cells (Courtin et al., 2005). Therefore, the expression of deiodinases changes according to TH availability, in a kind of feedback system(Li et al., 2001; Bianco and Kim, 2006).
Physiologically, THs are primarily responsible for regulation of metabolism in the human body. Over the last two centuries, clinical observations have revealed that the lack of adequate levels of THs during critical periods of nervous system (NS) development results in cretinism, a syndrome of severe mental deficiency that may be accompanied by retarded growth and neurological deficiency (Kilby, 2003; Glinoer, 2007; Moleti et al., 2016; van Gucht et al., 2016).Moreover, several clinical and experimental data reported that the lack of adequate levels of THs in a critical periods of development leads to histologicals, biochemicals and behavioural abnormalities in the CNS (Porterfield and Hendrich, 1993; Bernal and Nunez,1995; Ahmed et al., 2008; Mayerl et al., 2012)
Although numerous studies have been devoted to investigate the role of THs in the central nervous system (CNS), only a handful of studies investigated the role of THs in peripheral nervous system(PNS) development. In 1970, interesting studies revealed that in rat, the major consequence of thyroid deficiency in hypothyroid neonatal rats is a reduction in the number of myelinated fibers and a delay in the increase of axon diameter of sciatic nerves (Clos and Legrand, 1970). Furthermore, neonatal hypothyroidism in mice affects the growth of myelinated and non-myelinated axons and their associated Schwann cells (Reier and Hughes, 1972). The extent of remyelination carried out by Schwann cells was decreased in hypothyroid animals compared to normal controls (Franklin et al., 1996). Further evidence accumulated over the last decades supports that developmental myelination is a TH dependent process. In particular, studies in genetically modified animals provided abundant evidence that TH plays an important part in regulating oligodendrocyte or Schwann cell lineages and maturationin vivo(Bernal, 2002). In rodent models of inflammatory-demyelinating diseases, TH administration has a positive effect on clinical outcome and demyelination/remyelination balance (D’Intino et al.,2011). Collectively these finding provide evidence demonstrating the important role of THs in the normal development of peripheral nerves.
Olds Studies Reported Contradictory Results on the Role of THs in the Regeneration of Peripheral Nerves
Between 1910 and 1932, some investigators noticed that regeneration following peripheral nerves injury was retarded in thyroidectomized dogs, cats, rabbit and rats (Marinesco and Minea,1910; Isenschmid, 1932). Accordingly, between 1970–1980, several studies were carried out to investigate the effect of treatment with exogenous triiodothyronine (T3) on peripheral nerve regeneration.Experimentally the sciatic nerve was crushed/or transected in adult animals, then animals were treated with daily intraperitoneal or subcutaneous injection with an active form of TH (3,3′,5-triiodo-L-thyronine dissolved in 0.01 N NaOH solution). The results of most studies demonstrated that treatment of adult rats with T3accelerated the elongation of axons regenerating within peripheral nerves and produced an immediate increase in the synthesis of protein in the soma of injured neurons (Cockett and Kiernan,1973; Stelmack and Kiernan, 1977) The increase in the rate of regeneration in animals receiving T3is associated with a more rapid reinnervation of muscles end-plate and skin (McIsaac and Kiernan,1975) and with acceleration of the recovery of motor and sensory functions (Berenberg et al., 1977). Idem, the injection of the less active form of THs (T4) also significantly increased the regeneration of sciatic nerves in adult rats (Danielsen et al., 1986) and stimulated the growth of regenerating hypoglossal nerve and its rate of muscle reinnervation in adult rats (Yu and Srinivasan, 1981). Moreover,positive clinical effects of TH were reported, such as accelerated outgrowth of new T3axons and recovery of sensory conduction in a patient with a transected ulnar nerve (McQuarrie, 1975). Nevertheless, at about the same times conflicting results are began to emerge indicating that the exogenous TH injection did not produce detectable changes in either the rate of growth of regenerating sensory and motor axons (Allpress and Pollock, 1986; Papakostas et al., 2009), or in muscle reinnervation (Cotrufo et al., 1979) and axonal transport (Forman et al., 1977; Frizell and McLean, 1979).Also adding T3to the drinking water to produce hyperthyroidism in old rats, did not show that T3treatment has a beneficial effect on muscle reinnervation (Finkelstein et al., 1993). The interpretation of these contradictory results is very likely due to the different techniques and methods used at the time. In fact, the daily subcutaneous or intraperitoneal injections of a variable amount of TH (5–30 μg/kg per day) probably induces a high concentration of T3in the whole body, which may induce physiological complications in the animal (Yu and Srinivasan, 1981). This was supported by a study that indicated that excess THs in mice induced a decrease in the mean number of end-plates and in cholinesterase concentrations.There is also a possibility that excess TH may interfere with axonal transport or neuromuscular interaction (Kazakov, 1992). Granted addition of T3to the drinking water cannot ensure a defined and controlled concentration of the hormone in the animals. As a result the question of whether or not THs improves peripheral nerve regeneration remains open.
To avoid increases in the amount of TH throughout the body of an animal due to repetitive daily injection of T3, we need to find a technique that allows local administration of hormones at the level of the injured nerve. The tubulization technique which began to be used in the 1980s provided a good tool to study the effect of local administration of THs at the level of transected peripheral nerves.
Nuclear Receptors of THs are Expressed by Neurons and Glial Cells
From 1970 to 1980, a large amount of information emerged demonstrating that the actions of THs are necessarily mediated through a signal to nuclear TH receptor (TR). The interaction complex of THTR stimulates formation of heterodimers that modulates binding to specific DNA sequence to effect then the expression of specific genes in target cells (Oppenheimer et al., 1972; Ruel et al., 1985; Yokota et al., 1986).However, apart from nuclear TR, THs can exert non-genomic effects through transmembrane integrin receptor, αVβ3as well as cytoplasmic TR variants (Bhumika and Darras, 2014). In 1988, the production of monoclonal antibodies (mAb 2B3, 4B II)raised against the nuclear TRs of the rat liver, made it possible to localize the presence of TR using immunocytochemistry (Luo et al.,1988; Tagami et al., 1990).
Anatomically sciatic nerves contain motor and sensory fibers derived respectively of the motor neurons in the spinal cord of the CNS and sensory neurons in the dorsal root ganglia (DRG)of the PNS at vertebral levels L4–5. Using the mAb 2B3, immunocytochemistry demonstrate that motor and sensory neurons permanently express TR. Receptor expression starts in the third week of embryonic life and continue throughout the whole life of the animal (Barakat-Walter et al., 1992, 1993). While motor and sensory neurons express TR in a similar way, a clear difference in the expression of TR was detected between glial cells of the CNS and Schwann and satellite glial cells of the PNS. Like neurons,astrocytes and oligodendrocytes permanently express TR during embryonic and adult life, whereas Schwann and satellite glial cells transiently express TR only for limited periods of development and regeneration. TR expression by Schwann and satellite glial cells extends from the third weeks of embryonic life up to the end of the second postnatal week, a period characterized firstly by a high rate of Schwann cell proliferation and then by the formation of myelination sheaths (Peters and Muir, 1959; Asbury, 1967). After two postnatal weeks, in young and adult rats, the nuclei of Schwann cells no longer express TR immunoreactivity (Barakat-Walter et al.,1992, 1993) unless the sciatic nerve is injured, whereby Schwann cells and fibrocytes re-express TRs first in the proximal and distal stumps adjacent to the lesion, and then extending along the entire distal segment. However, when regenerating axons growing from the proximal stump invade the distal segments the re-expressed TRs again disappear from the Schwann cells. The disappearance of TR expression is detected first in the proximal nerve segment and then in the distal branches of the sciatic nerve; hence, the loss of TR immunoreactivity in these cells seems to spread proximo-distally along the sciatic nerve as the centrifugal process of myelination observed along growing peripheral nerves (Speidel, 1964).
Initially in the seventies, it was believed that TH function occurred through a single high affinity nuclear receptor. However,thereafter two isoforms of TRs (TRα and TRβ) encode by two separated genes were isolated from different tissues. At least three distinct mRNA species were generated by differential processing of the TRα transcript and two distinct mRNA species of TR β (Benbrook and Pfahl, 1987; Thompson et al., 1987; Mitsuhashi et al.,1988). TRα-1 is the only one of the TRα isoforms binds T3, while isoforms TRα-2 and TRα-2v fail to activate transcription in T3responsive cells (Koenig et al., 1989; Nakai et al., 1990; Kalyanaraman et al., 2014). On the other side, both TRβ isoforms (β1 and β2)bind T3, in addition, several reports indicated that TRα-1, TRβ1 and TRβ2 bind T3with similar affinity. Using a panel of antibodies specific for each isoform of TRs, differential expression of TR isoforms was observed between neurons and glial cells. In PNS of adult rats, sensory neurons expressed essentially TR β-1 and little of TRα-common isoforms (TRα-1 and α-2) while Schwann cells exhibited primarily TRα-1 isoform (Glauser and Barakat Walter,1997). Equally in CNS, TRβ2 immunorectivity was distributed throughout the gray matter in the rat forebrain, in the brainstem in regions of the telencephalon and diencephalon, but no immunostaining was present in white matter fiber tracts or choroid plexus(Lechan et al., 1993). The differential expression of TRα and β by neurons and glial cells indicates that the feedback regulation of circulating TH could occur by binding to either α or β TR isoforms.The TRs detected in neurons and Schwann cells by immunocytochemistry are functional receptors able to bind T3and which function as ligand-activated transcription factors. This was demonstrated by autoradiographic technique based on the incubation of tissue sections with125I-labeled T3, to visualize the T3binding sites(Barakat-Walter and Droz, 1995). The ability of sensory neurons and Schwann cells to express TRs is also preserved in tissue culture(Barakat-Walter et al., 1992).
Since the ontogeny of TRs can provide information as to the timing of nervous system sensitivity to THs at the genomic level,the results mentioned above suggest that THs may have a role in Schwann cell proliferation and also in the regulation of Schwann cell gene expression during nerve fiber regeneration and myelination (Barakat-Walter et al., 1992, 1993; Walter, 1993; Thi et al.,1998).
THs Exert a Trophic Action on Sensory
Neurons in vitro
Before the examination of the effect of THs on peripheral nerve regeneration, it made sense to investigate the effect of THsin vitrousing neuron cell cultures first is to detect whether THs have a trophic effect on neurons and to establish if THs act directly/or indirectly on neurons. The addition of T3, at physiological concentrations in culture medium promoted the survival of sensory neurons in mixed DRG cell cultures, where neurons are intermingled with non-neuronal cells as well as in neuron-enriched cultures, where non-neuronal cells are eliminated at the outset (Barakat-Walter,1996). Since T3stimulates sensory neuron survival in neuron-enriched cultures to the same extent as in mixed DRG cell cultures,that suggest that the stimulating effect of T3on neuronal survival is mediated directly through neurons (Barakat-Walter, 1996). In explant DRG cultures, in which the architecture of the ganglion is intact and the interaction of axons with Schwann cells is preserved, the adding of exogenous T3stimulated the outgrowth of neurites from sensory neurons. Moreover, if exogenous T3and nerve growth factor(NGF) were added together to explant DRG, they induced a synergic rather than additive effect on neurite outgrowth (Barakat-Walter,1996). Another important effect observed in DRG cultures is that as well as the presence of NGF in the medium culture the presence of T3is also essential to induce the expression of microtubule-associated proteins (MAPs) (Barakat-Walter and Riederer, 1996). These findings suggest that the interaction between THs and trophic factors is required for MAP expression, thought to be involved in the regulation of microtubule assembly and retrograde transport mechanisms. In the CNS the interaction between TH and NGF regulates the ontogeny of a number of neuronal structures in the brain, (Clos and Legrand, 1970; Legrand and Clos, 1991), and plays a specific role in the growth and maintenance of cholinergic neurons in the basal forebrain of neonatal rats (Hayashi and Patel, 1987; Patel et al.,1988).
Local Administration of THs In Silicon Tubes is Sufficient to Set Off Several Mechanisms to
Improve the regeneration of injured sciatic nerve
Because daily exogenous subcutaneous or intraperitoneal injections of T3in adult animals produced conflicting results, the silicone nerve guides permit investigating the effect of local administration of THs on the regeneration of injured peripheral nerves. With this in mind, our group performed studies in model whereby the right sciatic nerve in adult rats was first surgically transected and then silicone tubes implanted filled with either T3solution or vehicle as control. The combination of morphological and morphometric analysis of nerve regeneration revealed that animals treated with T3had more complete nerve regeneration, demonstrated by a significant increase in the number and diameter of axons that grew into the mid-and distal end of the silicone tube, and also by the thicker myelin sheaths (Voinesco et al., 1998). The stimulatory effect of T3on nerve regeneration was not only acceleration, but also a real improvement in regeneration, with increased number of regenerated axons and improvement of myelination sustained over time after the operation (Voinesco et al., 1998). A beneficial effect of T3administration within silicone tubes was also observed on the regeneration of injured rat facial nerve (Oble et al., 2004) and cavernous nerves (Bessede et al., 2010).
The fact that T3has a short half-life (Garcia et al., 1976) and therefore the concentration of T3within the silicone tube decreases with time, this suggests that T3acts rapidly soon after administration. Therefore, it is probable that the administration of T3is suffi-cient to set off several mechanisms which in turn enhance neuronal survival and axonal regeneration in the long term. This could explain the greater effect of T3on nerve regeneration compared to the effect of only one growth factor or matrix molecules (Dubuisson et al., 1993; Laird et al., 1995; Seckel et al., 1995; Newman et al., 1996;Utley et al., 1996; Walter et al., 1996; Chen et al., 2000; Moir et al.,2000; Xu et al., 2003).
THs Rescue Axotomized Sensory and Motor Neurons from Death
Generally after a peripheral nerve lesion in adult rats, a percentage(30–60%) of sensory neurons in DRG undergo a series of reactive changes leading to cell death, while other neurons survive the injury and are able to regenerate (Himes and Tessler, 1989; Melville et al., 1989; Vestergaard et al., 1997). However, survival depends on several factors including the type of neuron, age of the animal, and the degree and proximity of the injury to the cell body. Unlike the vulnerability of sensory neurons, spinal motor neurons are less susceptible to injury-induced cell death, far fewer spinal motor neurons die after peripheral nerve transection in adult mammals (Himes and Tessler, 1989; Melville et al., 1989; Liuzzi and Tedeschi, 1991).Although the number of sensory and motor neurons that die after axotomy is greater in young neonatal animals (Schmalbruch, 1987;Himes and Tessler, 1989; Snider and Thanedar, 1989; Sendtner et al.,1990) there is still a loss of neurons following peripheral nerve injury in adults.
As we already found that T3treatment increased significantly the number of regenerated axons it is necessary to investigate whether this increase is due to a real increase in the number of surviving axotomised neurons allowing them to grow new axons, or to an artificial arborisation of regenerated axons. It is well demonstrated that the total neuron number is a superior parameter to evaluate neuron loss.Since the physical dissector method provides an accurate estimation of cell number (Popken and Farel, 1997; Delaloye et al., 2009) this method was used to estimate the total number of surviving sensory neurons in adult rat lumbar DRG following sciatic nerve injury. The statistical analysis of estimated neuron numbers in DRG revealed that the transection of right sciatic nerves in control rats in which the silicone nerve guide filled with phosphate buffer saline (PBS),leads to a significant loss (34%) in the number of surviving sensory neurons in ipsilateral DRG (Schenker et al., 2003), this result corroborates the finding of other studies mentioned above. However, administration of T3in the silicone nerve guide immediately after sciatic nerve transection significantly reduces the loss of sensory neurons in L4or L5ganglia; only 10% of sensory neurons die in T3treated rats(Schenker et al., 2003). This result suggests that T3administrated at the site of the transected nerve was retrogradely transported in the axotomized neuron’s soma that protected them from death. This suggestion is supported by the fact that when we transected the right sciatic nerve in adult rats and125I-labeled T3administrated in nerve guide, the125I labeling was observed only in the ipsilateral sensory neurons located in DRG at the level of T4and T5, indicating that T3was retrogradely transported in the soma of axotomized sensory neurons and this likely saves them from death.
Further evidence obtainedin vitroshowed that the addition of T3at physiological concentrations to DRG neuron-enriched cultures significantly increased the number of surviving sensory neurons as compared to control cultures (Barakat-Walter, 1996). The local administration of T3in nerve guides following sciatic nerve transection does not only save sensory neurons from death but also motor neurons. The counting of regenerated myelinated motor axons double immunostained with anti-choline-acetyl-transferase and anti-neurofilament on semi-thin sections taken from the midpoint of regenerated nerves showes that the percentage of regenerated myelinated motor axons is significantly higher in T3treated rats than in control rats (Panaite and Barakat-Walter, 2010). The protective effect of THs against neuronal loss was also demonstrated in the CNS by interesting studies. A reduction of hippocampal neuronal damage after ischemia by repeated daily T4administration was shown by Rami and Krieglstein (Rami and Krieglstein, 1992),with an approximately 50% increase in neuronal density attributable to hormone treatment. In experimental traumatic brain injury in mice, T3treatment 1 hour after injury results in a significant improvement in motor and cognitive recovery revealed by control cortical impact injury, as well as a marked reduction of lesion volume (Crupi et al., 2013). Together these results indicate that T3has a neuroprotective effect and promote the growth of new axons.
For Clinical Human Nerve Repair the Use of Biodegradable Nerve Guides is Essential
Because of its inert and elastic properties, silicon tubes were one of the first and most frequently used to bridge transected nerves(Wang-Bennett and Coker, 1990; Lundborg et al., 1997; Chamberlain et al., 1998). However, the many experiments done in animals shows that all non-biodegradable nerve conduits present various problems and cannot be used in humans (Bardou et al., 2013).The main disadvantage of non-biodegradable conduits is that they remain in situ as foreign bodies and therefore cause a chronic inflammatory response that may be deleterious for the function of the regenerated nerve (Merle et al., 1989). Silicone or other non-degradable tubes cannot be used for clinical application and it was necessary to overcome having permanent guides. In the search for novel materials that satisfy nerve regeneration and the best functional recovery, large attention was paid to biodegradable guides(Mackinnon and Dellon, 1988; Den Dunnen et al., 1993; Keeley et al., 1993; Bini et al., 2004; Nakamura et al., 2004; Yang et al., 2004;Ciardelli and Chiono, 2006; Mohammadi et al., 2013). It is generally accepted that an ideal nerve guide for clinical application should: 1)have flexibility allowing the implantation of the tube and surgical manipulation (Robinson et al., 1989); 2) allow the axons to regenerate in favourable natural environment (Brunelli et al., 1994) 3) be no immunogenic, no allergenic, no cytotoxic and no carcinogenic, and;4) prevent neuroma formation and the growth of fibrous tissue, 5)degrade at a defined rate in accordance with the axonal growth rates and therefore maintain mechanical continuity until the gap has been bridged.
Combination of Biodegradable Nerve Guide and Local Administration of THs is a Potential Therapeutic Approach for Human Peripheral Nerve Repair
Amongst the most promising synthetic biocompatible biodegradable nerve guides are tubes composed of an amorphous copolymer of 50% DL-lactide and 50% ε caprolactone [poly (DLLA-ε CL);developed by Polyganics (B.V., Groningen, the Netherlands). The Polyganics biodegradable tubes have been shown to be useful functional nerve guides, allowing fast axonal regeneration with good recovery and, generally, they degrade in approximately 3 months and induce less inflammation during degradation (den Dunnen et al., 1995; Petersen et al., 1996; Meek et al., 2001; Varejao et al., 2003; Ijkema-Paassen et al., 2004). However, it is not known whether these guides facilitate the administration of a factor capable of stimulating neuronal regeneration and whether the products of the degradation of the guides do not prevent the stimulating effect of the molecules administered in them. To develop clinical trials using TH in human peripheral nerve injuries, it is necessary to test whether the biodegradable nerve guides allow a single and local administration of T3to improve the regeneration of severed peripheral nerves, as silicone tubes did. The comparative study between the nerves regenerated within Polyganics biodegradable P(DLLA-e-CL) nerve guides with those that are regenerated within silicon guides showed that the regeneration of transected nerve within biodegradable nerve guides is just as good as that obtained using silicon tubes. In addition, the morphological and statistical analyses of nerve regeneration within these guides revealed that the biodegradable nerve guides allow the T3to stimulate the regeneration of transected nerves as well as the silicon guides, as demonstrated by a significant increase in the number of myelinated regenerated axons and the skewed diameter of myelinated axons towards larger values compared to controls (Barakat-Walter et al.,2007). Generally, following nerve injury, recovery is estimated by the sum of the number of regenerated axons and reinnervation of denervated peripheral targets and eventually by the return of motor and sensory function. The analysis of neuromuscular junctions of gastrocnemius and plantar muscle clearly showed that T3enhanced the reinnervation of denervated end-plates (EPs) as demonstrated by significantly higher recovery of the size and shape complexity of reinnervated EPs and by increased acetylcholine receptor (AChR)density on post synaptic membranes compared to controls (Panaite and Barakat-Walter, 2010). In addition, the enhancement of muscle reinnervation by T3is accompanied by an increase in the compound muscle action potential (CMAP) of the flexor and extensor muscles seen by electrophysiological recordings (Barakat-Walter et al., 2007; Panaite and Barakat-Walter, 2010). The behavior monitoring of operated rats confirmed the stimulation effect of T3, as treated rats recovered faster their motor and sensory functions compared to control rats. All these data confirmed that the T3stimulation in biodegradable guides was equal to that obtained using silicone guides. It is worth mentioning that the use of P(DLLA-ε-CL) biodegradable guides revealed positive aspects such as the degradation of the tubes does not induce acidification of the intra-luminal environment which could prevent the stimulating action of T3, and tube- degradation products are not associated with significant inflammation as there is no a significant presence of monocytes or macrophages on histological sections of regenerated nerves with biodegradable guides (Barakat-Walter et al., 2007).However, two minor handicaps were observed: 1) approximately 15% of the biodegradable P(DLLA-ε-CL) tubes collapsed and prevented regeneration of the sciatic nerves, 2) the nerve guides are not completely degraded even 4 months post-surgery which in our opinion is a little bit long compared to the duration of nerve regeneration in rats. Therefore, further improvement in biodegradable tube production is necessary to prevent the small level of collapse observed and to adapt their degradation to the speed of axonal regeneration.
Mechanisms by which T3 Might Stimulate Nerve Regeneration
The ability of the PNS to regenerate is due to the capacity of injured neurons to survive and grow new axons and also to the ability of non-neuronal cells in peripheral nerves to support axonal growth. As exogenous administration of TH clearly accelerates and improves nerve regeneration and functional recovery it is essential to understand how T3could stimulate regeneration of severed nerves. Generally the biological activity of THs circulating in the blood is initiated by TH transporters across the plasma membrane,then mediate by the nuclear binding of T3to a class of high-affinity,low-capacity binding sites which modulating transcription factors(Cheng et al., 2010; Bhumika and Darras, 2014).
Since both axotomized sensory and motor neurons as well as non-neuronal cells possess T3receptors (Barakat-Walter et al.,1992; Barakat-Walter, 1999), the T3administrated in nerve guide can act on both axotomized neurons and Schwann cells to set offseveral mechanisms which enhance neuronal survival and axonal regeneration (Figure 1).
To understand the cellular and molecular mechanisms by which THs enhance peripheral nerve regeneration, several efforts have focused on the changes in the growth factor or protein expression rescuing neurons from death and allowing them to grow new axons. Direct or indirect data revealed that neurotrophins such brain derived neurotrophic factor (BDNF), neurotrophic factor-3 (NT3)or NGF are crucial for neuron survival and axon outgrowth (Barde,1989; Kaselis et al., 2014). Both BDNF and NT3are TH-dependent,they are reduced in hypothyroid and increased in hyperthyroid developing nervous system (Camboni et al., 2003; Shulga et al.,2009; Gilbert and Lasley, 2013)) Interesting studies reported that in adult rat, the prenatal exposure to drugs and chemicals that interfere with thyroid function significantly reduced hippocampal BDNF levels (Chakraborty et al., 2012). In humans during the first few days after traumatic injury of the adult nervous system, BDNF mRNA levels are reduced by 50% in CNS as well as in PNS, indicating that BDNF plays a role in survival of neurons (Lipska et al., 2001;Gonzalez et al., 2005). In adult male mice, treatment with T31 hour after traumatic brain injury resulted in a significant improvement in motor and cognitive recovery as well as a marked reduction in lesion volumes. Also T3significantly enhanced the post traumatic brain injury expression of neuroprotective neurotrophins (BDNF,and GDNF) compared to vehicle (Crupi et al., 2013). T3not only regulates the expression of the neurotrophic factors, but also the expression of their receptors. Two studies have shown that hypothyroidism strongly reduces NGF receptor immunoreactivity in the rat cerebellar cortex (Legrand and Clos, 1991) and TrkA mRNA levels in the rat striatum (Alvarez-Dolado et al., 1994)
Another mechanism by which T3prevents neuronal cell death may be the regulation of expression of cytoskeletal proteins which provide the structural framework of neurons and are essential for the transport of organelles. Neurofilament (NF) proteins provide structural support for axons and regulate axon diameter. while tubulin and microtubules are involved in axonal growth and supply the basis for fast axonal transport (Nixon and Casey, 2004).Different studies reported that the expression of these proteins is regulated by T3in both the PNS and CNS. A Western blot analysis of the levels of cytoskeletal proteins in the different segments of operated sciatic nerves of rats revealed that TH up regulated synthesis, axonal transport and post-translational modification of NF and tubulin proteins during nerve regeneration (Schenker et al.,2002). TH also stimulates the expression of neurofilaments genes during brain development in the CNS and hypothyroidism markedly reduced their expression, both at mRNA and protein levels(Ghosh et al., 1999). The administration of T3per osin adult rabbit increased the levels of tubulins α6, β3 and β-actin (Tentes et al.,2003). Other results indicated that THs play a major role in the cytoskeletal transport of tubulin and actin from their site of synthesis to that of assembly, thus facilitating axo-dendritic outgrowth and morphological differentiation (De et al., 1991). Likewise, T3regulates the expression of growth cone protein (SCG10) whose expression is essential for growth cone advance that closely correlates with neurite outgrowth during development and regeneration (Grenningloh et al., 2004). Transection of adult rat sciatic nerve induces an upregulation of SCG10 mRNA in lumbar sensory and motor neurons and a strong increase in SCG10 protein levels in the proximal nerve segment (Mason et al., 2002; Voria et al., 2006). The administration of T3in silicone tubes increased the upregulation of SCG10 mRNA in axotomized sensory neurons in lumbar dorsal root ganglia and also caused a significantly higher increase in SCG10 protein levels,detected along different segments of the regenerating nerve (Voria et al., 2006).
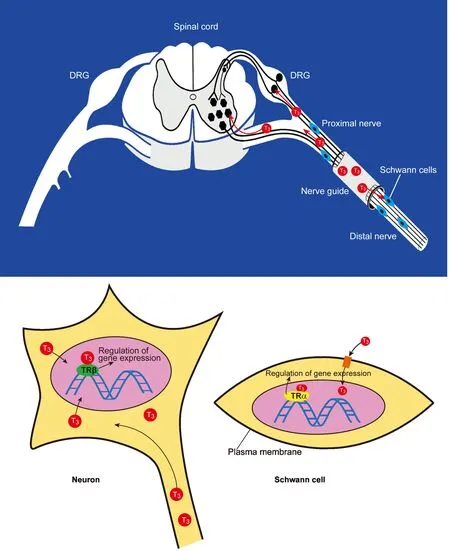
Figure 1 The diagram illustrates how the 3,3′,5-triiodothyronine (T3) administrated in nerve guide acts on both axotomized neurons and Schwann cells.
On intrinsic aspects of neuronal response to injury interesting recent study reported that following peripheral nerve injury, transcriptional responses are orchestrated to regulate the expression of numerous genes in the lesioned nerve thereby activating the intrinsic regeneration program (Li et al., 2015). Three transcriptional phases of peripheral nerve regeneration were identified within the period of 14 days of post nerve injury. During the first phase following injury a few number of genes involved with axon growth was up-regulated. During the second phase an increase in the number of the up-regulated genes associated with axon growth is detected, such as activating transcription factor 3 (ATF3), angiotensin II receptor type 2 (AGTR2), arginine 1 (ARG1), cyclin dependent kinase inhibitor 1A (CDKN1A), fibroblast growth factor 2 (FGF2), interleukin 6(IL-6), insulin-like growth factor 1 (IGF-1), fibroblast growth factor receptor 3 (FGFR3), Runt-related transcription factor 3 (RUNX3),JUN, and suppressor of cytokine signaling 3 (SOCS3). During the third regeneration phase, other up-regulated genes associated with axon growth further were identified, like growth-associated protein 43 kDa (GAP-43), neuropeptide Y (NPY), phospholipase D2(PLD2), gene associated with neurite/axon growth (GNA15), colony stimulating factor 1 (CSF1), Rho-related GTP-binding protein(RHOQ) and proline-rich attachment domain (PRAD),etc.(Li et al., 2015). Other study reported that axonal signaling response to nerve injury induces alterations in cellular signaling, transcription,translation and post –translation modifications. Numerous kinases(MAP kinases ErK1 and ErK2 and JnK) are activated in cell bodies in response to an injury event (Obata et al., 2004). Since T3improves nerve regeneration, it is possible that T3regulates up/down the activity of these transcriptional factors and genes.
The other mechanism by which T3stimulates peripheral nerve regeneration is a direct action on Schwann cells (Figure 1), which play a critical role first in the growth of axotomized axons (Bryan et al.,1996), then in myelination of new regenerated axons. Two distinct phenotypes of Schwann cells were identified, myelinating Schwann cells and non-myelinating Schwann cells which are distinguished by their molecules expression and by their morphologic relationships with axons. In intact normal nerve, a high number of Schwann cells are myelinating cells which express high levels of the components of myelin sheaths, including protein zero (P0), myelin basic protein(MBP), peripheral myelin protein 2 (P2, PMP2; FABP8; M-FABP)and myelin-associated glycoprotein (MAG). Following peripheral nerve injury, Schwann cells switch their function from myelinating to non-myelinating cells which express high levels of L-NCAM, L1 and modest levels of low affinity NGF receptors (NGFR) and the GAP-43(Martini, 1994; Stettner et al., 2018), then they proliferate in response to nerve trauma (Williams, 1988). When cell proliferation has slowed and differentiation ensues, the Schwann cells provide the new regenerating axons with trophic factors, without which the axons are unable to grow beyond a certain distance (Politis et al., 1982; Keynes,1987; Bryan et al., 1996). Results from experimentsin vitrodemon-strated that the addition of T3of physiological concentrations to dissociated sciatic nerve Schwann cell cultures, significantly increased the number of cells incorporating3H-thymindine, indicating that T3increases the cell proliferation (Walter, 1993). Likewise, in DRG explant cultures, the addition of T3promotes neurite outgrowth, if the proliferation of Schwann cells is inhibited by the antimitotic agent cytosine arabinoside, the stimulatory effect of T3on neurite outgrowth is considerably reduced, what provides evidence that the stimulatory effect of T3on neurite outgrowth is mediated through Schwann cells(Barakat-Walter, 1996).
After having facilitated the outgrowth of new axons, Schwann cells again switch their function to myelination in order to remyelinate the newly regenerated axons. It is worth noting that the most important stimulating effect of local T3administration in nerve guides, is the acceleration and improvement of the myelination of regenerating axons and subsequent reinnervating the dennervated muscles (Barakat-Walter, 1996; Voinesco et al., 1998; Panaite and Barakat-Walter, 2010). The stimulatory effect of T3on myelination in the peripheral and central nervous systems was demonstrated by old and recent studies. In PNS, thyroid deficiency in neonatal rats induce a reduction in the number of myelinated fibers and a delay in the increase in axon diameter in sciatic nerves (Clos and Legrand 1970), also affects the growth of non-myelinated axons and their associated Schwann cells (Reier and Hughes 1972). Contrariwise, THs accelerate recovery of motor function after crushing the sciatic nerve in adult hyperthyroid rats (Berenberg et al., 1977). In CNS, several findings indicate that myelin genes are direct targets for THs action, and the expression of some genes such as myelin basic protein, myelin associated glycoproteine, proteolipid protein and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP), is controlled by TH (Munoz et al., 1991; Tosic et al., 1992; Ibarrola and Rodriguez-Pena, 1997; Strait et al., 1997). In adult male rabbits, the subcellular fractionation and electrophoretic analysis of proteins from the regenerating nerve, seven days after nerve injury revealed that T3treatment increases P0, MBP, myelin P2 (PMP-2) and histones H3 and H2A in proximal and distal nerve surgery segments(Tentes et al., 2003). A recent study revealed that PMP-2 may play a role in remyelination of the injured peripheral nervous system,by affecting the nodal and internodal configuration (Stettner et al.,2018). Since T3regulates the expression of PMP-2, suggests that probably THs act on nodes and internodes length.
Still the molecular component by which T3promotes myelination in Schwann cells or oligodendrocytes was poorly characterized. However, recently, Dugas and his colleagues used the genomic expression analyses to identify the genes specifically regulated by T3in purified oligodendrocyte precursor cells. Among the genes identified were four transcription factors, Kruppel-like factor 9(KLF9), basic helix-loop-helix family member e22 (BHLHe22),Hairless (Hr), and albumin D box-binding protein (DBP), all of which were induced in oligodendrocyte precursor by both brief and long term exposure to T3(Dugas et al., 2012). More analyses and identification of other T3-induced genes involved in myelination and remyelination may lead to novel insights into how to enhance the regeneration of myelin.
Generally functional recovery is a sum of the number of regenerated axons and reinnervation of denervated peripheral targets. Interesting old and recent studies reported that the increased number of regenerated new axons and the enhancement of axonal myelination in animals receiving T3is linked with an improvement and more rapid reinnervation of muscle end-plates and skin, synaptic transmission and motor functional recovery (McIsaac and Kiernan, 1975; Panaite and Barakat-Walter, 2010). The enhancement of muscle reinnervation by T3probably involves a general regulation of contractile protein synthesis as well as all members of the myosin heavy chain (MyHC) multigene family. Both the absolute and relative amounts of all four major histocompatibility complexs (MHCs)expressed in the adult plantaris muscle are significantly affected by the separate and combined interventions of functional overload and exogenous T3treatment (Gambke et al., 1983; Kirschbaum et al., 1990; Diffee et al., 1991; Swoap et al., 1994; Yu et al., 2000). On the other hand, T3increased the levels of acetylcholinesterase messenger RNA in the slow soleus muscles close to the levels in the fast extensor digitorum longus (Pregelj et al., 2003) and influence the stabilization of motor innervation of reinnervated muscle (Cuppini et al., 1996).
Conclusion
The injury of human peripheral nerves either in upper or lower limbs has a negative impact socially and economically as functional recovery is often poor and needs a long time, particularly for severe nerves injuries. Therefore, the repair of injured nerves remains a challenging problem for several research groups. THs which are the most important factors for nervous system development and function could improve peripheral nerve regeneration. Recent experimental data showed that a single and local administration of T3within Polyganics biodegradable P(DLLA-ε-CL) tubes, immediately after sciatic nerve transection in adult rats is sufficient to rapidly trigger several mechanisms that in turn enhance neuronal survival,axonal regeneration, muscle reinnervation, improve and accelerate recovery of motor and sensory functions. These data show that this technique provides a serious step towards future clinical application of T3in peripheral nerve damage. The advantage of this model is that T3is administered locally and in single dose which does not cause neither an increase in TH in the animal body nor physiological complications. Moreover, the importance of administration of T3in biodegradable tubes to repair damaged human nerves comes from the fact that the biodegradable tubes resorb in situ after promoting nerve regeneration by T3. In addition, the degradation of these tubes do not produce side effects in the operated animal.
Author contributions:Conceived the idea and wrote the review: IBW. Literature search and finalization of the manuscript: RK and IBW.
Conflicts of interest:None declared.
Financial support:Our grateful thanks to the Swiss National Science Foundation, SUVA foundation and Novartis foundation for their generous support of our research.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review reports:
Reviewer 1:Tetsuro Tamaki, Tokai University School of Medicine, Japan.
Comments to authors:This is the review article described the role of thyroid hormone in the peripheral nerve injury. Basically, this is well and strictly written, particularly about the research history of thyroid hormone and nervous system using enough number of references.
Reviewer 2:Mark A. Yorek, University of Iowa, USA.
Comments to authors:The effects of thyroid hormone as a main intervention for nerve regeneration is probably not a mainstream idea for most but the authors present interesting data in support of this question.
Aebischer P, Salessiotis AN, Winn SR (1989) Basic fibroblast growth factor released from synthetic guidance channels facilitates peripheral nerve regeneration across long nerve gaps. J Neurosci Res 23:282-289.
Ahmed OM, El-Gareib AW, El-Bakry AM, Abd El-Tawab SM, Ahmed RG(2008) Thyroid hormones states and brain development interactions. Int J Dev Neurosci 26:147-209.
Allpress SJ, Pollock M (1986) Morphological and functional effects of triiodothyronine on regenerating peripheral nerve. ExpNeurol 91:382-391.
Alvarez-Dolado M, Iglesias T, Rodriguez-Pena A, Bernal J, Munoz A (1994)Expression of neurotrophins and the trk family of neurotrophin receptors in normal and hypothyroid rat brain. Brain Res Mol Brain Res 27:249-257.
Asbury AK (1967) Schwann cell proliferation in developing mouse sciatic nerve. A radioautographic study. J Cell Biol 34:735-743.
Barakat-Walter I (1996) Triiodothyronine exerts a trophic action on rat sensory neuron survival and neurite outgrowth through different pathways.Eur J Neurosci 8:455-466.
Barakat-Walter I (1999) Role of thyroid hormones and their receptors in peripheral nerve regeneration. J Neurobiol 40:541-559.
Barakat-Walter I, Droz B (1995) Nuclear and cytoplasmic triiodothyronine-binding sites in primary sensory neurons and Schwann cells: radioautographic study during development. JNeuroendocrinol 7:127-136.
Barakat-Walter I, Riederer BM (1996) Triiodothyronine and nerve growth factor are required to induce cytoplasmic dynein expression in rat dorsal root ganglion cultures. Brain Res Dev Brain Res 96:109-119.
Barakat-Walter I, Duc C, Puymirat J (1993) Changes in nuclear 3,5,3’-triiodothyronine receptor expression in rat dorsal root ganglia and sciatic nerve during development: comparison with regeneration. Eur J Neurosci 5:319-326.
Barakat-Walter I, Kraftsik R, Schenker M, Kuntzer T (2007) Thyroid hormone in biodegradable nerve guides stimulates sciatic nerve regeneration:a potential therapeutic approach for human peripheral nerve injuries. J Neurotrauma 24:567-577.
Barakat-Walter I, Duc C, Sarlieve LL, Puymirat J, Dussault JH, Droz B (1992)The expression of nuclear 3,5,3’ triiodothyronine receptors is induced in Schwann cells by nerve transection. Exp Neurol 116:189-197.
Barde YA (1989) Trophic factors and neuronal survival. Neuron 2:1525-1534.
Bardou P, Merle JC, Woillard JB, Nathan-Denizot N, Beaulieu P (2013)Electrical impedance to detect accidental nerve puncture during ultrasound-guided peripheral nerve blocks. Can J Anaesth 60:253-258.
Benbrook D, Pfahl M (1987) A novel thyroid hormone receptor encoded by a cDNA clone from a human testis library. Science 238:788-791.
Berenberg RA, Forman DS, Wood DK, DeSilva A, Demaree J (1977) Recovery of peripheral nerve function after axotomy: effect of triiodothyronine.Exp Neurol 57:349-363.
Bernal J (2002) Action of thyroid hormone in brain. J Endocrinol Invest 25:268-288.
Bernal J, Nunez J (1995) Thyroid hormones and brain development. Eur J Endocrinol 133:390-398.
Bessede T, Alsaid B, Ferretti L, Pierre M, Bernabe J, Giuliano F, Karam I,Benoit G, Droupy S (2010) Effect of a local delivery of triiodothyronine(T3) within neuroregenerative guide on recovery of erectile function in a rat-model of cavernous nerve injury. J Sex Med 7:1798-1806.
Bhumika S, Darras VM (2014) Role of thyroid hormones in different aspects of nervous system regeneration in vertebrates. Gen Comp Endocrinol 203:86-94.
Bianco AC, Kim BW (2006) Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 116:2571-2579.
Bini TB, Gao S, Xu X, Wang S, Ramakrishna S, Leong KW (2004) Peripheral nerve regeneration by microbraided poly(L-lactide-co-glycolide) biodegradable polymer fibers. J Biomed Mater Res A 68:286-295.
Brunelli GA, Vigasio A, Brunelli GR (1994) Different conduits in peripheral nerve surgery. Microsurgery 15:176-178.
Bryan DJ, Wang KK, Chakalis-Haley DP (1996) Effect of Schwann cells in the enhancement of peripheral-nerve regeneration. J Reconstr Microsurg 12:439-436.
Buti M, Verdu E, Labrador RO, Vilches JJ, Fores J, Navarro X (1996) Influence of physical parameters of nerve chambers on peripheral nerve regeneration and reinnervation. Exp Neurol 137:26-33.
Camboni D, Roskoden T, Schwegler H (2003) Effect of early thyroxine treatment on brain-derived neurotrophic factor mRNA expression and protein amount in the rat medial septum/diagonal band of Broca. Neurosci Lett 350:141-144.
Chakraborty G, Magagna-Poveda A, Parratt C, Umans JG, MacLusky NJ,Scharfman HE (2012) Reduced hippocampal brain-derived neurotrophic factor (BDNF) in neonatal rats after prenatal exposure to propylthiouracil(PTU). Endocrinology 153:1311-1316.
Chamberlain LJ, Yannas IV, Hsu HP, Strichartz G, Spector M (1998) Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp Neurol 154:315-329.
Chen YS, Wang-Bennett LT, Coker NJ (1989) Facial nerve regeneration in the silicone chamber: the influence of nerve growth factor. Exp Neurol 103:52-60.
Chen YS, Hsieh CL, Tsai CC, Chen TH, Cheng WC, Hu CL, Yao CH (2000)Peripheral nerve regeneration using silicone rubber chambers filled with collagen, laminin and fibronectin. Biomaterials 21:1541-1547.
Cheng SY, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocr Rev 31:139-170.
Ciardelli G, Chiono V (2006) Materials for peripheral nerve regeneration.Macromol Biosci 6:13-26.
Clos J, Legrand J (1970) Effects of thyroid deficiency and underfeeding on growth and myelination of the sciatic nerve fibers in the young white rat.An electron microscopic study. Brain Res 22:285-297.
Cockett SA, Kiernan JA (1973) Acceleration of peripheral nervous regeneration in the rat by exogenous triiodothyronine. Exp Neurol 39:389-394.
Cotrufo R, Dattola R, Deodato M, Pisani F, Messina C (1979) Experimental hyperthyroidism fails to expedite reinnervation of muscles denervated by crushing sciatic nerves in rabbits. Exp Neurol 65:271-277.
Courtin F, Zrouri H, Lamirand A, Li WW, Mercier G, Schumacher M,Goascogne CL, Pierre M (2005) Thyroid hormone deiodinases in the central and peripheral nervous system. Thyroid 15:931-942.
Crupi R, Paterniti I, Campolo M, Di Paola R, Cuzzocrea S, Esposito E (2013)Exogenous T3 administration provides neuroprotection in a murine model of traumatic brain injury. Pharmacol Res 70:80-89.
Cuppini R, Sartini S, Ambrogini P, Gallo G (1996) Muscle reinnervation in hypothyroid rats. J Peripher Nerv Syst 1:223-229.
D’Intino G, Lorenzini L, Fernandez M, Taglioni A, Perretta G, Del Vecchio G, Villoslada P, Giardino L, Calza L (2011) Triiodothyronine administration ameliorates the demyelination/remyelination ratio in a non-human primate model of multiple sclerosis by correcting tissue hypothyroidism.J Neuroendocrinol 23:778-790.
Danielsen N, Dahlin LB, Ericson LE, Crenshaw A, Lundborg G (1986)Experimental hyperthyroidism stimulates axonal growth in mesothelial chambers. Exp Neurol 94:54-65.
Danielsen N, Williams LR, Dahlin LB, Varon S, Lundborg G (1988) Peripheral nerve regeneration in Gore-tex chambers. Scand J Plast Reconstr Surg Hand Surg 22:207-210.
De A, Chaudhury S, Sarkar PK (1991) Thyroidal stimulation of tubulin and actin in primary cultures of neuronal and glial cells of rat brain. Int J Dev Neurosci 9:381-390.
Delaloye S, Kraftsik R, Kuntzer T, Barakat-Walter I (2009) Does the physical disector method provide an accurate estimation of sensory neuron number in rat dorsal root ganglia? J Neurosci Methods 176:290-297.
den Dunnen WF, van der LB, Robinson PH, Holwerda A, Pennings AJ,Schakenraad JM (1995) Biological performance of a degradable poly(lactic acid-epsilon-caprolactone) nerve guide: influence of tube dimensions.J Biomed Mater Res 29:757-766.
Den Dunnen WF, Van der Lei B, Schakenraad JM, Blaauw EH, Stokroos I,Pennings AJ, Robinson PH (1993) Long-term evaluation of nerve regeneration in a biodegradable nerve guide. Microsurgery 14:508-515.
Diffee GM, Haddad F, Herrick RE, Baldwin KM (1991) Control of myosin heavy chain expression: interaction of hypothyroidism and hindlimb suspension. Am J Physiol 261:C1099-1106.
Doolabh VB, Hertl MC, Mackinnon SE (1996) The role of conduits in nerve repair: a review. Rev Neurosci 7:47-84.
Dubuisson AS, Beuermann RW, Kline DG (1993) Sciatic nerve regeneration across gaps within collagen chambers: the influence of epidermal growth factor. J Reconstr Microsurg 9:341-346.
Dugas JC, Ibrahim A, Barres BA (2012) The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol Cell Neurosci 50:45-57.
Fields RD, Le Beau JM, Longo FM, Ellisman MH (1989) Nerve regeneration through artificial tubular implants. Prog Neurobiol 33:87-134.
Finkelstein DI, Dooley PC, Luff AR (1993) Recovery of muscle after different periods of denervation and treatments. Muscle Nerve 16:769-777.
Forman DS, Padjen AL, Siggins GR (1977) Axonal transport of organelles visualized by light microscopy: cinemicrographic and computer analysis.Brain Res 136:197-213.
Franklin RJ, Gilson JM, Franceschini IA, Barnett SC (1996) Schwann celllike myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia 17:217-224.
Frizell M, McLean WG (1979) The effect of triiodothyronine on axonal transport in regenerating peripheral nerves. Exp Neurol 64:225-230.
Gambke B, Lyons GE, Haselgrove J, Kelly AM, Rubinstein NA (1983) Thyroidal and neural control of myosin transitions during development of rat fast and slow muscles. FEBS Lett 156:335-339.
Garcia MD, Escobar del Rey F, Morreale de Escobar G (1976) Thyrotropin-releasing hormone and thyroid hormone interactions on thyrotropin secretion in the rat: lack of inhibiting effects of small doses of triiodo-L-thyronine in the hypothyroid rat. Endocrinology 98:203-213.
Ghosh S, Rahaman SO, Sarkar PK (1999) Regulation of neurofilament gene expression by thyroid hormone in the developing rat brain. Neuroreport 10:2361-2365.
Gilbert ME, Lasley SM (2013) Developmental thyroid hormone insufficiency and brain development: a role for brain-derived neurotrophic factor(BDNF)? Neuroscience 239:253-270.
Glauser L, Barakat Walter I (1997) Differential distribution of thyroid hormone receptor isoform in rat dorsal root ganglia and sciatic nerve in vivo and in vitro. J Neuroendocrinol 9:217-227.
Glinoer D (2007) Clinical and biological consequences of iodine deficiency during pregnancy. Endocr Dev 10:62-85.
Gonzalez SL, Labombarda F, Gonzalez Deniselle MC, Mougel A, Guennoun R,Schumacher M, De Nicola AF (2005) Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J Steroid Biochem Mol Biol 94:143-149.
Gordon T, Sulaiman O, Boyd JG (2003) Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst 8:236-250.
Grenningloh G, Soehrman S, Bondallaz P, Ruchti E, Cadas H (2004) Role of the microtubule destabilizing proteins SCG10 and stathmin in neuronal growth.J Neurobiol 58:60-69.
Hayashi M, Patel AJ (1987) An interaction between thyroid hormone and nerve growth factor in the regulation of choline acetyltransferase activity in neuronal cultures, derived from the septal-diagonal band region of the embryonic rat brain. Brain Res 433:109-120.
Himes BT, Tessler A (1989) Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adult and neonatal rats. J Comp Neurol 284:215-230.
Hulbert AJ (2000) Thyroid hormones and their effects: a new perspective. Biol Rev Camb Philos Soc 75:519-631.
Ibarrola N, Rodriguez-Pena A (1997) Hypothyroidism coordinately and transiently affects myelin protein gene expression in most rat brain regions during postnatal development. Brain Res 752:285-293.
Ijkema-Paassen J, Jansen K, Gramsbergen A, Meek MF (2004) Transection of peripheral nerves, bridging strategies and effect evaluation. Biomaterials 25:1583-1592.
Isenschmid R (1932) Ueber den einfluss von thymus und schilddrüse auf die nerven-regeneration. Versuche mit thymokreszin und thyroxin. Schweizerische medizinische Wochenschrift 62.
Kalyanaraman H, Schwappacher R, Joshua J, Zhuang S, Scott BT, Klos M,Casteel DE, Frangos JA, Dillmann W, Boss GR, Pilz RB (2014) Nongenomic thyroid hormone signaling occurs through a plasma membrane-localized receptor. Sci Signal 7:ra48.
Kaselis A, Treinys R, Vosyliute R, Satkauskas S (2014) DRG axon elongation and growth cone collapse rate induced by Sema3A are differently dependent on NGF concentration. Cell Mol Neurobiol 34:289-296.
Kazakov VM (1992) Terminal intramuscular motor innervation and motor endplates in thyrotoxic myopathy. Neuromuscul Disord 2:343-349.
Keeley R, Atagi T, Sabelman E, Padilla J, Kadlcik S, Keeley A, Nguyen K, Rosen J (1993) Peripheral nerve regeneration across 14-mm gaps: a comparison of autograft and entubulation repair methods in the rat. J Reconstr Microsurg 9:349-358.
Keynes RJ (1987) Schwann-cells during neural development and regeneration -leaders or followers. Trends Neurosci 10:137-141.
Kilby MD (2003) Thyroid hormones and fetal brain development. Clin Endocrinol (Oxf) 59:280-281.
Kirschbaum BJ, Kucher HB, Termin A, Kelly AM, Pette D (1990) Antagonistic effects of chronic low frequency stimulation and thyroid hormone on myosin expression in rat fast-twitch muscle. J Biol Chem 265:13974-13980.
Koenig RJ, Lazar MA, Hodin RA, Brent GA, Larsen PR, Chin WW, Moore DD(1989) Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature 337:659-661.
Laird JM, Mason GS, Thomas KA, Hargreaves RJ, Hill RG (1995) Acidic fibroblast growth factor stimulates motor and sensory axon regeneration after sciatic nerve crush in the rat. Neuroscience 65:209-216.
Lechan RM, Qi Y, Berrodin TJ, Davis KD, Schwartz HL, Strait KA, Oppenheimer JH, Lazar MA (1993) Immunocytochemical delineation of thyroid hormone receptor beta 2-like immunoreactivity in the rat central nervous system. Endocrinology 132:2461-2469.
Legrand C, Clos J (1991) Biochemical, immunocytochemical and morphological evidence for an interaction between thyroid hormone and nerve growth factor in the developing cerebellum of normal and hypothyroid rats. Dev Neurosci 13:382-396.
Li S, Xue C, Yuan Y, Zhang R, Wang Y, Wang Y, Yu B, Liu J, Ding F, Yang Y, Gu X (2015) The transcriptional landscape of dorsal root ganglia after sciatic nerve transection. Sci Rep 5:16888.
Li WW, Le Goascogne C, Ramauge M, Schumacher M, Pierre M, Courtin FO(2001) Induction of type 3 iodothyronine deiodinase by nerve injury in the rat peripheral nervous system. Endocrinology 142:5190-5197.
Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR (2001) BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci 14:135-144.
Liuzzi FJ, Tedeschi B (1991) Peripheral nerve regeneration. Neurosurg Clin N Am 2:31-42.
Lundborg G (1987) Nerve regeneration and repair. A review. Acta orthopaedica Scandinavica 58:145-169.
Lundborg G (2004) Alternatives to autologous nerve grafts. Handchir Mikrochir Plast Chir 36:1-7.
Lundborg G, Dahlin L, Dohi D, Kanje M, Terada N (1997) A new type of “bioartificial” nerve graft for bridging extended defects in nerves. J Hand Surg Br 22:299-303.
Luo M, Faure R, Ruel J, Dussault JH (1988) A monoclonal antibody to the rat nuclear triiodothyronine receptor: production and characterization. Endocrinology 123:180-186.
Mackinnon SE, Dellon AL (1988) A comparison of nerve regeneration across a sural nerve graft and a vascularized pseudosheath. J Hand Surg Am 13:935-942.
Marinesco G, Minea J (1910) Nouvelles recherches sur l’influence qu’exerce l’ablation du corps thyroide sur la dégénérescence et et la régénérescence des nerfs.Compte rendude la Société de biologie 68:188.
Martini R (1994) Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J Neurocytol 23:1-28.
Mason MR, Lieberman AR, Grenningloh G, Anderson PN (2002) Transcriptional upregulation of SCG10 and CAP-23 is correlated with regeneration of the axons of peripheral and central neurons in vivo. Mol Cell Neurosci 20:595-615.
Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H (2012) Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain.Endocrinology 153:1528-1537.
McIsaac G, Kiernan JA (1975) Acceleration of neuromuscular re-innervation by triiodothyronine. J Anat 120:551-560.
McQuarrie IG (1975) Nerve regeneration and thyroid hormone treatment. J Neurol Sci 26:499-502.
Meek MF, Van Der Werff JF, Nicolai JP, Gramsbergen A (2001) Biodegradable p(DLLA-epsilon-CL) nerve guides versus autologous nerve grafts: electromyographic and video analysis. Muscle Nerve 24:753-759.
Melville S, Sherburn TE, Coggeshall RE (1989) Preservation of sensory cells by placing stumps of transected nerve in an impermeable tube. Exp Neurol 105:311-315.
Merle M, Dellon AL, Campbell JN, Chang PS (1989) Complications from silicon-polymer intubulation of nerves. Microsurgery 10:130-133.
Mitsuhashi T, Tennyson G, Nikodem V (1988) Nucleotide sequence of novel cDNAs generated by alternative splicing of a rat thyroid hormone receptor gene transcript. Nucleic Acids Res 16:5697.
Mohammadi R, Amini K, Yousefi A, Abdollahi-Pirbazari M, Belbasi A, Abedi F(2013) Functional effects of local administration of thyroid hormone combined with chitosan conduit after sciatic nerve transection in rats. J Oral Maxillofac Surg 71:1763-1776.
Moir MS, Wang MZ, To M, Lum J, Terris DJ (2000) Delayed repair of transected nerves: effect of brain-derived neurotrophic factor. Arch Otolaryngol Head Neck Surg 126:501-505.
Moleti M, Sturniolo G, Trimarchi F, Vermiglio F (2016) The changing phenotype of iodine deficiency disorders: a review of thirty- five years of research in north-eastern Sicily. Ann Ist Super Sanita 52:550-557.
Munoz A, Rodriguez-Pena A, Perez-Castillo A, Ferreiro B, Sutcliffe JG, Bernal J(1991) Effects of neonatal hypothyroidism on rat brain gene expression. Mol Endocrinol 5:273-280.
Nakai A, Sakurai A, Macchia E, Fang V, DeGroot LJ (1990) The roles of three forms of human thyroid hormone receptor in gene regulation. Mol Cell Endocrinol 72:143-148.
Nakamura T, Inada Y, Fukuda S, Yoshitani M, Nakada A, Itoi S, Kanemaru S,Endo K, Shimizu Y (2004) Experimental study on the regeneration of peripheral nerve gaps through a polyglycolic acid-collagen (PGA-collagen) tube. Brain Res 1027:18-29.
Newman JP, Verity AN, Hawatmeh S, Fee WE, Jr., Terris DJ (1996) Ciliary neurotrophic factors enhances peripheral nerve regeneration. Arch Otolaryngol Head Neck Surg 122:399-403.
Nixon AB, Casey PJ (2004) Analysis of the regulation of microtubule dynamics by interaction of RGSZ1 (RGS20) with the neuronal stathmin, SCG10. Methods Enzymol 390:53-64.
Noble J, Munro CA, Prasad VS, Midha R (1998) Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma 45:116-122.
Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K(2004) Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur J Neurosci 20:2881-2895.
Oble DA, Burton L, Maxwell K, Hassard T, Nathaniel EJ (2004) A comparison of thyroxine- and polyamine-mediated enhancement of rat facial nerve regeneration. Exp Neurol 189:105-111.
Oppenheimer JH, Koerner D, Schwartz HL, Surks MI (1972) Specific nuclear triiodothyronine binding sites in rat liver and kidney. J Clin Endocrinol Metab 35:330-333.
Panaite PA, Barakat-Walter I (2010) Thyroid hormone enhances transected axonal regeneration and muscle reinnervation following rat sciatic nerve injury. J Neurosci Res 88:1751-1763.
Papakostas I, Mourouzis I, Mourouzis K, Macheras G, Boviatsis E, Pantos C (2009)Functional effects of local thyroid hormone administration after sciatic nerve injury in rats. Microsurgery 29:35-41.
Patel AJ, Hayashi M, Hunt A (1988) Role of thyroid hormone and nerve growth factor in the development of choline acetyltransferase and other cell-specific marker enzymes in the basal forebrain of the rat. J Neurochem 50:803-811.
Peters A, Muir AR (1959) The relationship between axons and Schwann cells during development of peripheral nerves in the rat. Q J Exp Physiol Cogn Med Sci 44:117-130.
Petersen J, Russell L, Andrus K, MacKinnon M, Silver J, Kliot M (1996) Reduction of extraneural scarring by ADCON-T/N after surgical intervention. Neurosurgery 38:976-983.
Politis MJ, Ederle K, Spencer PS (1982) Tropism in nerve regeneration in vivo. Attraction of regenerating axons by diffusible factors derived from cells in distal nerve stumps of transected peripheral nerves. Brain Res 253:1-12.
Popken GJ, Farel PB (1997) Sensory neuron number in neonatal and adult rats estimated by means of stereologic and profile-based methods. J Comp Neurol 386:8-15.
Porter field SP, Hendrich CE (1993) The role of thyroid hormones in prenatal and neonatal neurological development--current perspectives. Endocr Rev 14:94-106.
Pregelj P, Crne-Finderle N, Sketelj J (2003) Effect of thyroid hormones on acetylcholinesterase mRNA levels in the slow soleus and fast extensor digitorum longus muscles of the rat. Neuroscience 116:657-667.
Rami A, Krieglstein J (1992) Thyroxine attenuates hippocampal neuronal damage caused by ischemia in the rat. Life Sci 50:645-650.
Reier PJ, Hughes AF (1972) An effect of neonatal radiothyroidectomy upon nonmyelinated axons and associated Schwann cells during maturation of the mouse sciatic nerve. Brain Res 41:263-282.
Robinson PH, van der LB, Knol KE, Pennings AJ (1989) Patency and long-term biological fate of a two-ply biodegradable microarterial prosthesis in the rat. Br J Plast Surg 42:544-549.
Ruel J, Faure R, Dussault JH (1985) Regional distribution of nuclear T3 receptors in rat brain and evidence for preferential localization in neurons. J Endocrinol Invest 8:343-348.
Schenker M, Riederer BM, Kuntzer T, Barakat-Walter I (2002) Thyroid hormones stimulate expression and modification of cytoskeletal protein during rat sciatic nerve regeneration. Brain Res 957:259-270.
Schenker M, Kraftsik R, Glauser L, Kuntzer T, Bogousslavsky J, Barakat-Walter I (2003) Thyroid hormone reduces the loss of axotomized sensory neurons in dorsal root ganglia after sciatic nerve transection in adult rat. Exp Neurol 184:225-236.
Schmalbruch H (1987) Loss of sensory neurons after sciatic nerve section in the rat. Anat Rec 219:323-329.
Seckel BR, Jones D, Hekimian KJ, Wang KK, Chakalis DP, Costas PD (1995) Hyaluronic acid through a new injectable nerve guide delivery system enhances peripheral nerve regeneration in the rat. J Neurosci Res 40:318-324.
Sendtner M, Kreutzberg GW, Thoenen H (1990) Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature 345:440-441.
Shulga A, Blaesse A, Kysenius K, Huttunen HJ, Tanhuanpaa K, Saarma M, Rivera C (2009) Thyroxin regulates BDNF expression to promote survival of injured neurons. Mol Cell Neurosci 42:408-418.
Snider WD, Thanedar S (1989) Target dependence of hypoglossal motor neurons during development in maturity. J Comp Neurol 279:489-498.
Speidel CC (1964) In vivo studies of myelinated nerve fibers. Int Rev Cytol 16:173-231.
Stelmack BM, Kiernan JA (1977) Effects of triiodothyronine on the normal and regenerating facial nerve of the rat. Acta Neuropathol 40:151-155.
Stettner M, Zenker J, Klingler F, Szepanowski F, Hartung HP, Mausberg AK,Kleinschnitz C, Chrast R, Kieseier BC (2018) The role of peripheral myelin protein 2 in remyelination. Cell Mol Neurobiol 38:487-496.
Strait KA, Carlson DJ, Schwartz HL, Oppenheimer JH (1997) Transient stimulation of myelin basic protein gene expression in differentiating cultured oligodendrocytes: a model for 3,5,3’-triiodothyronine-induced brain development.Endocrinology 138:635-641.
Swoap SJ, Haddad F, Caiozzo VJ, Herrick RE, McCue SA, Baldwin KM (1994)Interaction of thyroid hormone and functional overload on skeletal muscle isomyosin expression. J Appl Physiol (1985) 77:621-629.
Tagami T, Nakamura H, Sasaki S, Mori T, Yoshioka H, Yoshida H, Imura H (1990)Immunohistochemical localization of nuclear 3,5,3’-triiodothyronine receptor proteins in rat tissues studied with antiserum against C-ERB A/T3 receptor.Endocrinology 127:1727-1734.
Tentes I, Asimakopoulos B, Hellman U, Nikolettos N, Kortsaris A, Kontoleon-Vakalopoulou E (2003) Subcellular fractionation and electrophoretic analysis of proteins from the regenerating nerve in rabbits following treatment with triiodothyronine (T3). In Vivo 17:601-608.
Thi AD, Jung-Testas I, Baulieu EE (1998) Neuronal signals are required for estrogen-mediated induction of progesterone receptor in cultured rat Schwann cells.J Steroid Biochem Mol Biol 67:201-211.
Thompson CC, Weinberger C, Lebo R, Evans RM (1987) Identification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science 237:1610-1614.
Tosic M, Torch S, Comte V, Dolivo M, Honegger P, Matthieu JM (1992) Triiodothyronine has diverse and multiple stimulating effects on expression of the major myelin protein genes. J Neurochem 59:1770-1777.
Utley DS, Lewin SL, Cheng ET, Verity AN, Sierra D, Terris DJ (1996) Brain-derived neurotrophic factor and collagen tubulization enhance functional recovery after peripheral nerve transection and repair. Arch Otolaryngol Head Neck Surg 122:407-413.
van Gucht AL, Meima ME, Zwaveling-Soonawala N, Visser WE, Fliers E, Wennink JM, Henny C, Visser TJ, Peeters RP, van Trotsenburg AS (2016) Resistance to thyroid hormone alpha in an 18-month-old girl: clinical, therapeutic,and molecular characteristics. Thyroid 26:338-346.
Varejao AS, Cabrita AM, Geuna S, Patricio JA, Azevedo HR, Ferreira AJ, Meek MF (2003) Functional assessment of sciatic nerve recovery: biodegradable poly(DLLA-epsilon-CL) nerve guide filled with fresh skeletal muscle. Microsurgery 23:346-353.
Vestergaard S, Tandrup T, Jakobsen J (1997) Effect of permanent axotomy on number and volume of dorsal root ganglion cell bodies. J Comp Neurol 388:307-312.
Voinesco F, Glauser L, Kraftsik R, Barakat-Walter I (1998) Local administration of thyroid hormones in silicone chamber increases regeneration of rat transected sciatic nerve. Exp Neurol 150:69-81.
Voria I, Hauser J, Axis A, Schenker M, Bichet S, Kuntzer T, Grenningloh G,Barakat-Walter I (2006) Improved sciatic nerve regeneration by local thyroid hormone treatment in adult rat is accompanied by increased expression of SCG10. Exp Neurol 197:258-267.
Walter IB (1993) Nuclear triiodothyronine receptor expression is regulated by axon-Schwann cell contact. Neuroreport 5:137-140.
Walter MA, Toriumi DM, Patt BS, Bhattacharyya TK, O’Grady K, Caul field JB,Thompson JA (1996) Fibroblast growth factor-induced motor end plate regeneration in atrophic muscle. Arch Otolaryngol Head Neck Surg 122:425-430.
Wang-Bennett LT, Coker NJ (1990) Analysis of axonal regeneration through the silicone regeneration chamber: a retrograde tracing study in the rabbit facial nerve. Exp Neurol 107:222-229.
Williams LR, Danielsen N, Müller H, Varon S (1988) Influence of the acellular fibrin matrix on nerve regeneration success within the silicone chamber model.In: The Current Status of Peripheral Nerve Regeneration. Alan R ed. Liss Inc.
Xu X, Yee WC, Hwang PY, Yu H, Wan AC, Gao S, Boon KL, Mao HQ, Leong KW, Wang S (2003) Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials 24:2405-2412.
Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S (2004) Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials 25:1891-1900.
Yokota T, Nakamura H, Akamizu T, Mori T, Imura H (1986) Thyroid hormone receptors in neuronal and glial nuclei from mature rat brain. Endocrinology 118:1770-1776.
Yu F, Gothe S, Wikstrom L, Forrest D, Vennstrom B, Larsson L (2000) Effects of thyroid hormone receptor gene disruption on myosin isoform expression in mouse skeletal muscles. Am J Physiol Regul Integr Comp Physiol 278:R1545-1554.
Yu WH, Srinivasan R (1981) Effect of thyroid hormone on the regeneration of the hypoglossal nerve in rats. Exp Neurol 73:325-329.
杂志排行
中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Harnessing migraines for neural regeneration
- Synaptic dysfunction in Alzheimer’s disease: the effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention
