Targeted tissue engineering: hydrogels with linear capillary channels for axonal regeneration after spinal cord injury
2018-05-05ShengwenLiu,ArminBlesch
Spinal cord injury (SCI) frequently results in the permanent loss of function below the level of injury due to the failure of axonal regeneration in the adult mammalian central nervous system (CNS).The limited intrinsic growth capacity of adult neurons, a lack of growth-promoting factors and the multifactorial inhibitory microenvironment around the lesion site contribute to the lack of axonal regeneration. Strategies such as transplantation of cells, delivery of bioactive compounds and gene transfer have been investigated as a means to promote axonal regrowth through the lesion, to form new synaptic connections and to improve functional outcomes. Although growth of some axonal populations can be robustly enhanced by cellular implants alone or in combination with neurotrophic factors,axons usually extend in random orientation and even reverse growth direction in the lesion site (Figure 1A) (Gros et al., 2010; Günther et al., 2015). Thus, regenerating axons often fail to approach the distal edge of the lesion site, a pre-requisite for proper contact with spared host neurons. The lack of a 3-dimensional organization in the injury site is therefore an additional barrier for successful axonal bridging.Two approaches, physical guidance through structured scaffolds and chemical guidance by growth factor gradients, have emerged as potential means to provide directional cues for axonal growth through the lesion.
Scaffolds with linear channels:Advances in tissue engineering and biomaterials have provided promising leads for spinal cord repair.Biomaterials with high bio-compatibility and low toxicity can either be chemically synthesized or derived from natural polymers. After engraftment into sites of SCI, biomaterials can bridge the lesion cavity to restore continuity of the spinal cord. By adjusting the chemical and physical conditions during gelation, hydrogel scaffolds consisting of porous chambers, linear channels or aligned fibers can be fabricated. The internal structure can serve as a conduit to physically guide axon growth across the lesion, reduce contact of regenerating axons to the inhibitory microenvironment and act as a vehicle for cells and bioactive factors, which in turn create a permissive microenvironment for axonal growth (Figure 1B). Arrangement of cells in parallel channels may also contribute to axon orientation. Cells and blood vessels that are organized in distinct channels attract axons to sprout along the linear pores (Moore et al., 2006). For example, transplantation of freeze-dried agarose scaffolds or alginate hydrogels composed of uniaxial channels stimulate and guide axonal growth in a linear fashion after SCI (Stokols and Tuszynski, 2006;Günther et al., 2015).
Cell filling and growth factors enhance axon growth in hydrogel channels:In most studies, biological effects of biomaterials without additional manipulations are limited following transplantation to the injured spinal cord despite a delicately fabricated microarchitecture. Therefore, biomaterials have frequently been combined with cells to improve morphological and functional outcomes. Biomaterials can provide a matrix for cell adhesion, and enhance cell survival as well as migration. In addition, cells that are co-transplanted within a scaffold can interact with the surrounding host tissue and increase axon growth into and beyond the graft/host border, a major obstacle for long-distance axonal regeneration (Figure 1C). As biomaterials effectively fill portions of the lesion cavity, the number of cells required for transplantation also decreases. This is particularly important for clinical translation and more extended lesions when large cell numbers that might be difficult to obtain are needed. Several cell types used for transplantation in animal models of SCI have been examined in combination with different biomaterials. These include studies using poly(lactic-co-glycolic acid) (PLGA) scaffolds composed of multiple channels together with Schwann cells (SCs), templated agarose scaffolds with bone marrow stromal cells (BMSCs) and alginate hydrogels with BMSC-or SC- filled channels. In PLGA scaffolds, axons were found to grow throughout the full extent of channels containing SCs but not in channels without SCs (Moore et al., 2006). In templated agarose scaffolds, oriented axonal growth in a highly linear topography throughout the channels was demonstrated when channels were pre-loaded with BMSCs and overexpressing brain derived neurotrophic factor (BDNF) robustly increased axon density within the scaffold (Stokols et al., 2006). Similarly, channels in templated agarose scaffolds seeded with neurotrophin-3 expressing BMSCs facilitated axonal growth towards the distal aspect of the graft, in comparison to animals that were grafted with cell suspensions without agarose scaffolds (Gros et al., 2010). As in studies with agarose scaffolds, BMSCs expressing BDNF enhanced axonal regeneration into channels of alginate hydrogels. However, axonal regeneration generally decreases in the central portion of hydrogel implants and axonal extension into the distal host spinal cord tissue was not observed (Günther et al., 2015).
Influences of hydrogel channel diameter:The channel diameter of multi-channel biomaterials used in most studies ranged from 200 μm to 600 μm. In contrast to other techniques, alginate hydrogels with smaller anisotropic channels can be easily fabricated by diffusion of divalent cations through an alginate solution (Prang et al., 2006). Ranging from 10 μm to 100 μm, channel diameters depend on the cations utilized to cross-link alginate polymers(Pawar et al., 2015).In vitrocultures of neonatal cortex, spinal cord or dorsal root ganglia on alginate hydrogels have shown that the density of axon growth into the hydrogel positively correlates with the diameter of channels. In contrast, the linear orientation of axons diminishes with increasing channel diameter (Pawar et al.,2015). Other aspects that can be affected by microchannel diameter within the scaffold include the number and type of cells migrating into channels and newly formed blood vessels that occupy part of the channel lumen available for axonal regeneration. The influence of channel diameter in anisotropic alginate hydrogels seeded with BMSCs was recently evaluated in a cervical spinal cord hemisection lesion. Comparing channels with 41 μm (cross-linked by Sr2+) to 64 μm (cross-linked by Zn2+) diameter, axonal growth was supported throughout the lesion and the 50% difference in channel diameter did not influence axon density. While axon growth was overall oriented in a linear pattern irrespective of the diameter of hydrogel channels and similar to that observed in intact white matter of the spinal cord, even this small increase in channel diameter resulted in a measurable decrease in linear axonal orientation (Günther et al., 2015).
Tissue integration of hydrogels and axonal bridging:Similar to transplantation studies with other biomaterials (Gros et al., 2010),one major obstacle impeding axonal growth beyond an alginate hydrogel is the graft/host interface. Host cells including astrocytes and fibroblasts that generate a myriad of inhibitors respond to the lesion and impede axon extension (Günther et al., 2015; Liu et al., 2017).To overcome the inhibitory environment, neurotrophin gradients extending from the adjacent host spinal cord into the graft can shiftthe balance from inhibition to growth promotion and induce some longer-distance axonal regeneration (Taylor et al., 2006). Degradation of inhibitors by enzymes such as chondroitinase, or modification of the density of the barrier to allow axon penetration have also shown success in combination with biomaterial implants (reviewed in Günther et al., 2016). In addition, SCs, currently under investigation in an FDA-approved clinical trial in SCI can improve the host-graft continuity. SCs can modify the interface to become more permissive for axonal growth by intermingling with astrocytic pro-cesses of the glial scar (Williams et al., 2015). Recent studies using a combination of alginate hydrogels with SCs demonstrate that axons can extend throughout the full length of channels without a decline in the number of axons in the central portion of the scaffold. While axonal growth is enhanced by BDNF deliveredviaviral gene transfer into the caudal tissue, this chemotropic gradient beyond the lesion was not sufficient for axons to bridge the lesion site. Only when SCs are co-injected into the caudal spinal cord, regrowing axons penetrate the caudal interface and extend into the host parenchyma(Figure 1D) (Liu et al., 2017). These findings further highlight the importance of the biomaterial/host interface and the critical role of barriers immediately surrounding the scaffold for successful axonal regeneration.
Glial fibrillary acid protein (GFAP)-labeled astrocytes, which are commonly used to define the border of the spinal parenchyma after injury, are generally confined to areas around biomaterials rather than migrating into the channels. These astrocytes are considered to be beneficial by limiting secondary injury and possibly facilitating axonal growth (Anderson et al., 2016). Although implants provide for the structural continuity across the lesion, a“gap” composed of invading cells and small cysts frequently exists between hydrogels and the rostral and caudal astrocytic edges/host parenchyma.In vitrostudies using alginate hydrogels have shown that growth of fetal CNS axons into alginate channels is always accompanied by astrocytes, suggesting that glia might be required for axon elongation (Pawar et al., 2015). Incorporation of suitable astrocyte subtypes into channels that can mingle and interact with host astrocytes may therefore help to bridge this “gap” and further improve axonal growth into and out of the channels. Studies investigating this hypothesis are ongoing.
Conclusions:Taken together, anisotropic biomaterials composed of channels for axon orientation and guidance provide several advantages compared to isotropic biomaterials or transplantation of cell suspensions. Additional approaches such as a combination with cells and transient neurotrophin delivery can improve anatomical and behavioral effects (Figure1). Further modifications of biomaterials by electrostatic or covalent coating with bioactive molecules and alterations of the stiffness of biomaterials in particular at the host/graftinterface might lead to even better tissue integration, may enhance and accelerate vascularization and cell migration, and provide the support necessary for axons to bridge across larger lesions.
Supported by grants from the Deutsche Forschungsgemeinschaft(BL414/3-1), International Foundation for Research in Paraplegia, the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative, Indiana Spinal Cord and Brain Injury Research Fund and Morton Cure Paralysis Fund to AB and a Heinz Götze Memorial Fellowship to SL.
Shengwen Liu, Armin Blesch*
Department of Neurosurgery, Tongji Hospital, Tongji Medical College,Huazhong University of Science and Technology, Wuhan, Hubei
Province, China (Liu S)
Stark Neurosciences Research Institute, Indiana University School of Medicine, Department of Neurological Surgery and Goodman
Campbell Brain and Spine, Indianapolis, IN, USA (Blesch A)
orcid:0000-0003-1133-1174 (Armin Blesch)
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review reports:
Reviewer 1:Hedong Li, Pennsylvania State University, USA.
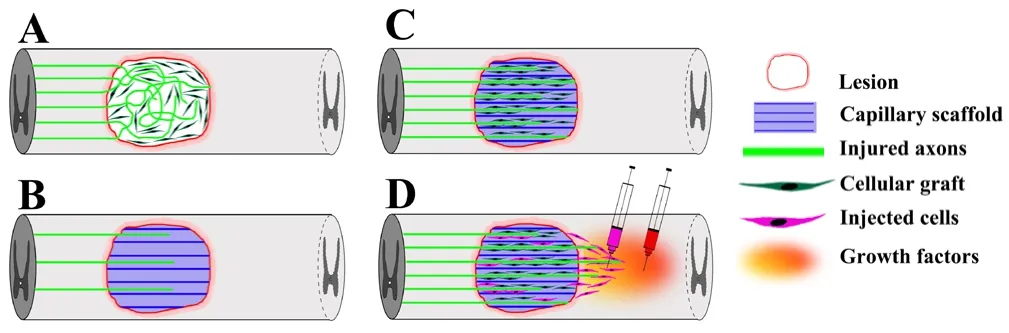
Figure 1 Schematic of linear guidance of axonal growth by biomaterials containing a capillary channel structure filled with supporting cells.
Comments to authors:This is a nice and concise review on a specific topic in spinal cord regeneration. The review touched on multiple aspects of the problem and provided helpful insights on potential strategies that one may consider to proceed and solve the problem. As complex as it is, spinal cord injury involves multiple steps in its pathogenesis, which would require combinatory strategies to circumvent. The authors nicely dissect out steps of axonal regeneration with the help of combination of biomaterials (hydrogels) and cells, and give readers a overview of the potential problems and their solutions in a step-wise manner.
Reviewer 2:Meng-Jen Lee, Chaoyang University of Technology, China.
Comments to authors:This paper is an interesting topic-focused review about the alginate hydrogel use for spinal cord injury.
Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G,Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195-200.
Günther MI, Weidner N, Müller R, Blesch A (2015) Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater 27:140-150.
Günther MI, Schackel T, Weidner N, Blesch A (2016) Hydrogel biomaterials in spinal cord repair and regeneration. In: AOSpine Masters Series. Vol. 7:Spinal Cord Injury and Regeneration (Vialle LR, Fehlings M, Weidner N,eds), pp 107-121. New York, USA: Thieme.
Gros T, Sakamoto JS, Blesch A, Havton LA, Tuszynski MH (2010) Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials 31:6719-6729.
Liu S, Sandner B, Schackel T, Nicholson L, Chtarto A, Tenenbaum L, Puttagunta R, Muller R, Weidner N, Blesch A (2017) Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater 60:167-180.
Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S, Knight AM, Lu L, Currier BL, Spinner RJ, Marsh RW, Windebank AJ,Yaszemski MJ (2006) Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials 27:419-429.
Pawar K, Prang P, Muller R, Caioni M, Bogdahn U, Kunz W, Weidner N (2015)Intrinsic and extrinsic determinants of central nervous system axon outgrowth into alginate-based anisotropic hydrogels. Acta Biomater 27:131-139.
Prang P, Muller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, Faber C,Vroemen M, Bogdahn U, Weidner N (2006) The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials 27:3560-3569.
Stokols S, Tuszynski MH (2006) Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 27:443-451.
Stokols S, Sakamoto J, Breckon C, Holt T, Weiss J, Tuszynski MH (2006)Templated agarose scaffolds support linear axonal regeneration. Tissue Eng 12:2777-2787.
Taylor L, Jones L, Tuszynski MH, Blesch A (2006) Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci 26:9713-9721.
Williams RR, Henao M, Pearse DD, Bunge MB (2015) Permissive Schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant 24:115-131.
杂志排行
中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration
