Axotomy induces damage to glial cells remote from the transection site in the peripheral nervous system
2018-05-05AnatolyB.Uzdensky
Traumatic cerebral or spinal cord injury induced by military, traffic,and sports accidents, falls or environmental and anthropogenic catastrophes are among main causes of people mortality and disability, especially in young and middle age men (Kobeissy, 2015). Axon transection, or axotomy, occurs in wounds and during surgery.Central neurons do not regenerate and die, but in the peripheral nervous system 25–30% of axotomized motor or sensory neurons survive and can regenerate and restore lost connections to their target cells. In order to treat the consequences of nerve injury, the balance between neurodegeneration and neuroprotection processes should be rapidly shifted to neuron survival. Unfortunately, reliable neuroprotective medications with proven efficiency are not found yet. So, comprehensive and deep study of molecular processes that occur after axon transection is required.
Intercellular neuroglial interactions provide the integrity of the nervous tissue and its resistance to harmful impacts. Glia injury or malfunction contribute to pathophysiology of stroke, neurotrauma, and various neurodegenerative diseases such as Alzheimer’s,Huntington’s and Parkinson’s diseases, amyotrophic lateral sclerosis. In the adult mammals neurotrauma induces activation of astrocytes and microgliocytes that participate in neuronal survival and functional recovery. On the contrary, oligodendrocytes are more vulnerable and die (Verkhratsky and Butt, 2013). Glia damage can suppress neuronal functions and lead to death of neurons. On the other hand, neuron injury can induce death of surrounding glial cells. For example, localized soma destruction in the crayfish mechanoreceptor neuron by a focused laser beam increased photoinduced apoptosis of surrounding glial cells. This suggests the anti-apoptotic influence of the neuronal body on glial cells(Kolosov and Uzdensky, 2006). In the course of axotomy glial cells located within the damaged region are mechanically injured and die. However, glial cells remote for a long distance away from the nerve transection site and undamaged mechanically can also die.For example, severe injury of the rat spinal cord induced apoptosis of oligodendrocytes located far away from the damaged site (Li et al., 1999). In the abdominal crayfish stretch receptor (CSR) the axon transection caused necrosis or apoptosis of satellite glial cells at a distance of several millimeters from the cut site (Khaitin et al.,2015). The molecular mechanisms of axotomy-induced death of remote glial cells have not been studied in detail yet.
Neuroglial interactions during brain injury are difficult to study in mammals because of numerous neuron-neuron, neuron-glia,glia-glia interconnections and interactions. In the mammalian brain astrocytes form the large astroglial network and interact with many neurons (Verkhratsky and Butt, 2013). Much easier to study the simpler nervous system of invertebrates where neuroglial interactions are more apparent. CSR is a simple but informative model object for studying of neuronal and glial responses to axotomy.It contains the single bipolar mechanoreceptor neuron (MRN)mounted on the receptor muscle, which is stretched between two adjacent abdominal segments. The information on the receptor muscle length is encoded by the frequency of MRN spikes, which are transferred along the axon to the ventral ganglion. MRN body and axon are surrounded by the multilayer glial envelope (Figure 1). The advantage of CSR is that both mechanoreceptor neuron and satellite glial cells, which envelope namely this neuron but do not form myelin, are well identified at the optical and electron-microscopic levels (Fedorenko et al., 2015). In the course of CSR isolation, MRN axon is usually transected, but the neuron survive and fire up to 8–12 hours with a frequency almost proportional to receptor muscle extension. Recently we developed the novel technique of CSR isolation without axon transection, which preserves the MRN connection to the nerve cord ganglion (Khaitin et al.,2015). Such undamaged (intact) CSR preparation was used as control (Khaitin et al., 2015, 2017).
Prolonged incubation of intact and axotomized CSRs in physiological solution induced apoptosis or necrosis of some remote glial cells surrounding the 2-mm proximal segment of the MRN axon at a distance of 5–8 mm from the cut end (Figure 1). Apoptotic fragmentation of the glial nuclei became noticeable since 8 hours after CSR isolation, whereas necrotic nuclei (revealed by red fluorescence of propidium iodide, which penetrates through the compromised plasma membrane of necrotic cells) were observed since 15 hours. These effects were significantly greater in the axotomized preparations. The glial cells surrounding the MRN body (at a longer distance from the cut site) also died but to the lesser extent than the glia around the proximal axon part (Khaitin et al., 2015). In rodents the sciatic nerve axotomy is known to induce death of sensory neurons and Schwann cells in the dorsal root ganglia. Therefore, axon integrity is necessary for survival of surrounding glial cells. This raises two important problems: what molecular signals transfer the information on axon injury to remote glial cells, and what intracellular signaling pathways regulate survival and death of remote glial cells? The possible intercellular molecular signals in the nervous system include some neuromediators, metabolites, neurotrophic factors, Ca2+and nitric oxide (NO).
Ca2+is the key element of various signaling pathways that control physiological functions and death of the cell. Calcium ions play the important role in neurodegeneration, in particular, in neuronal responses to axotomy (Rishal and Fainzilber, 2014). High levels of cytosolic Ca2+(> 10–4–10–3M) trigger necrosis or apoptosis.We showed recently the involvement of Ca2+in the regulation of axotomy-induced death of remote glial cells in the isolated CSR(Khaitin et al., 2017). Axotomy-induced apoptosis of remote glial cells enhanced in the presence of diverse modulators, which increase the cytosolic Ca2+level: triple CaCl2concentration in the saline, calcium ionophore ionomycin, or thapsigargin, inhibitor of Ca2+-ATPase, which pumps out cytosolic Ca2+into the endoplasmic reticulum (ER). Ionomycin and thapsigargin also increased axotomy-induced necrosis of remote glial cells. On the other hand, cadmium ions, which block different calcium channels in the plasma membrane and prevent Ca2+influx, and ryanodine, which blocks Ca2+release from ER into cytosol, reduced necrosis of glial cells.These data show the involvement of Ca2+, which can penetrate into the cytosol through different pathways, in the axotomy-induced apoptosis and necrosis of glial cells in the isolated CSR.
Gradual Ca2+accumulation in the glial envelope of the axotomized MRN axon at a distance of several millimeters from the transection site was demonstrated using Ca2+-sensitive fluorescence probe fluo-4 (Khaitin et al., 2017). One can suggest that Ca2+may serve as a messenger that transfers the information on axon injury to remote glial cells. Ca2+ions can propagate along the glial syncytium formed by gap junctions (Scemes, 2000) or small perforations in the paired glia-glial membranes (Fedorenko et al., 2015).Alternatively, the secondary Ca2+release from internal strores (ER,mitochondria) may be stimulated by Ca2+itself, or by neurotrophic factors and intracellular signaling proteins.
In the injured neurons various signaling pathways control survival and axon regeneration (Rishal and Fainzilber, 2014). Some signaling pathways regulate survival and death of remote glial cells in the axotomized crayfish stretch receptor. Prolonged 8-hour incubation of the axotomized CSR in the presence of inhibitors of MEK1/2 (FR18020), MAP kinase p38 (SB202190), protein kinase B/Akt (AktI), glycogen synthase kinase 3 beta (GSK-3β) (TDZD-8) and mammalian target of rapamycin (mTOR) (KU-0063784)increased axotomy-induced apoptosis of remote glial cells. On the other hand, inhibition of ERK1/2 and GSK-3β enhanced glial necrosis. This suggests the involvement of these signaling proteins in protective, antiapoptotic and antinecrotic processes in the remote glial cells surrounding the axotomized mechanoreceptor neuron(Berezhnaya et al., 2017). These proteins are the components of different signaling pathways: receptor tyrosine kinase/Ras/Raf/MEK/MAPK (ERK, p38, or JNK), and/or receptor tyrosine kinase/PI-3kinase/Akt/GSK-3β or mTOR, which may be initiated, for example,by neurotrophic factors released from the damaged neuron or from glial cellsviaparacrine or autocrine regulatory pathways.
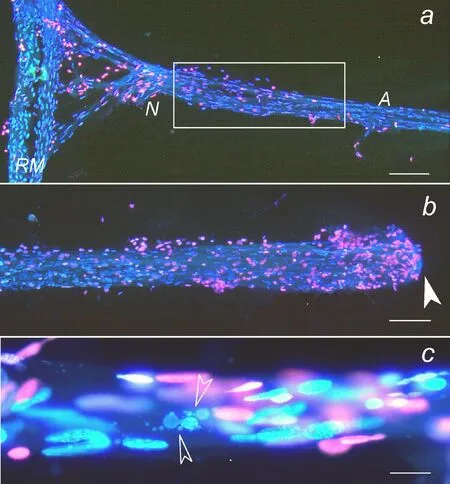
Figure 1 Fluorescent image of the crayfish stretch receptor isolated together with its ganglion.
The role of some of neurotrophic factors in protection of crayfish glial cells from oxidative damage has been characterized earlier. The application of recombinant human nerve growth factor (NGF) or glial-derived neurotrophic factor (GDNF) protected crayfish glial cells from necrosis and apoptosis induced by photodynamic impact(Uzdensky et al., 2015). One can suggest that in CSR some NGF- or GDNF-like neurotrophic factors are also involved in the protection of remote glial cells from axotomy-induced death.
NO effects were rather complicated. As shown earlier, NO generation by exogenous NONOate increased apoptosis of remote glial cells in the axotomized and photosensitized CSRs, whereas glial necrosis, oppositely, decreased (Uzdensky et al., 2015). In the photosensitized CSR, NO was generated mainly by glial cells (Kovaleva and Uzdensky, 2016). Thus, glia-produced NO exerted antinecrotic effect, but was proapoptotic for glial cells. So, NO may be also a potential messenger that signal to remote glial cells on axon injury.
Thus, axon integrity is necessary for survival of surrounding glial cells. Axon transection not only damages glial cells at the place of injury, but also induces necrosis or apoptosis of some remote glial cells in the isolated crayfish stretch receptor. The study of the role of different signaling processes in the axotomy-induced death of remote glial cells showed the involvement of Ca2+in propagation of proapoptotic signals from the axon transection site to remote glial cells. NGF-like and/or GDNF-like neurotrophic factors could serve as antiapoptotic and antinecrotic signals. Their effects could be mediated by various intracellular signaling cascades involving protein kinases MEK1/2, p38, protein kinase B/Akt, GSK-3β and mTOR. On the other hand, protein kinases ERK1/2 and GSK-3β were involved in protection of remote glial cells from axotomy-induced necrosis. One can also hypothesize that glia-produced NO exerts the proapoptotic and antinecrotic effects on glial cells in the axotomized CSR.
There are many unresolved problems remained to elucidate. The pathways of propagation of calcium signals from the injury site to remote glia cells should be investigated in details. How different Ca2+-dependent signaling proteins such as protein kinase C, calmodulin, calmodulin-dependent protein kinases, and other signaling proteins, which control functional and metabolic processes in the cell, regulate survival and death of remote glia after axotomy remains unstudied. The possible role of NO and neurotrophic factors in the intercellular neuron-to-glia or autocrine signaling should be also studied in future.
Supported by the Ministry of Education and Science of Russia grants 6.4951.2017/6.7 and 6.6З24.2017/8.9.
Anatoly B. Uzdensky*
Laboratory of Molecular Neurobiology, Academy of Biology and Biotechnology, Southern Federal University, Rostov-on-Don, Russia
orcid:0000-0002-0344-434X (Anatoly B. Uzdensky)
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Berezhnaya EV, Bibov MY, Komandirov MA, Neginskaya MA, Rudkovskii MV, Uzdensky AB (2017) Involvement of MAPK, Akt/GSK-3beta and AMPK/mTOR signaling pathways in protection of remote glial cells from axotomy-induced necrosis and apoptosis in the isolated crayfish stretch receptor. Mol Cell Neurosci 83:1-5.
Fedorenko G, Neginskaya M, Fedorenko A, Uzdensky A (2015) The paired neuroglial and interglial membranes in the crayfish stretch receptor and their local disorganization. J Neurosci Res 93:707-713.
Khaitin A, Rudkovskii M, Uzdensky A (2017) Ca2+mediates axotomy-induced necrosis and apoptosis of satellite glial cells remote from the transection site in the isolated crayfish mechanoreceptor. Mol Cell Neurosci 88:7-15.
Khaitin AM, Rudkovskii MV, Uzdensky AB (2015) The method of isolation of the crayfish abdominal stretch receptor maintaining a connection of the sensory neuron to the ventral nerve cord ganglion. Invert Neurosci 15:176.
Kobeissy FH (2015) Brain neurotrauma: molecular, neuropsychological,and rehabilitation aspects. Boca Raton, FL, USA: CRC Press/Taylor &Francis.
Kolosov M, Uzdensky A (2006) Crayfish mechanoreceptor neuron prevents photoinduced apoptosis of satellite glial cells. Brain Res Bull 69:495-500.
Kovaleva VD, Uzdensky AB (2016) Photodynamic therapy-induced nitric oxide production in neuronal and glial cells. J Biomed Opt 21:105005.
Li GL, Farooque M, Holtz A, Olsson Y (1999) Apoptosis of oligodendrocytes occurs for long distances away from the primary injury after compression trauma to rat spinal cord. Acta Neuropathol 98:473-480.
Rishal I, Fainzilber M (2014) Axon-soma communication in neuronal injury. Nat Rev Neurosci 15:32-42.
Scemes E (2000) Components of astrocytic intercellular calcium signaling.Mol Neurobiol 22:167-179.
Uzdensky A, Berezhnaya E, Khaitin A, Kovaleva V, Komandirov M,Neginskaya M, Rudkovskii M, Sharifulina S (2015) Protection of the Crayfish mechanoreceptor neuron and glial cells from photooxidative injury by modulators of diverse signal transduction pathways. Mol Neurobiol 52:811-825.
Verkhratsky A, Butt A (2013) Glial Physiology and Pathophysiology. Chichester, UK: Wiley-Blackwell.
杂志排行
中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration
