Fluorescence detection of Europiumdoped very small superparamagnetic iron oxide nanoparticles in murine hippocampal slice cultures
2018-05-05MartinPohland,YuskeKobayashi,JanaGlumm
In the late 1980s, superparamagnetic iron oxide nanoparticles(SPIO) moved into focus as contrast agents in magnetic resonance imaging (MRI), due to their strong relaxivity and resulting higher resolution of images. At the time, no one anticipated their high potential in basic research or for medical diagnostic and treatment. Since then, SPIO have been evaluated not only as specific markers for MRI, but also for cell labeling and tracking (Li et al., 2013). In addition, SPIO are being investigated as carriers for targeted administration of therapeutics,e.g., drugs or gene sequences , in cancer treatmentviamagnetic hyperthermia and in neuronal regeneration.
Even though U.S. Food and Drug Administration approved nanoparticles are already used in clinical settings, not enough is known about their adverse events when administered repeatedly. For example, Ferumoxytol is authorized exclusively for the treatment of iron-deficient anemia patients with chronic kidney disease. However, it is used off-label as a contrast agent with single bolus doses of approximately 0.16 mmol Fe/kg body weight to visualize vascular cerebral malformations or brain tumors in children, resulting in flooding of the central nervous system(CNS) with a high SPIO load and nanoparticle accumulation(Dosa et al., 2011). Consequently, possible side effects of SPIO and their cellular interactions as well as adverse effects within the CNS need to be investigated thoroughly.
First studies on this topic have been published showing an alteration in neuronal/microglial morphology and viability,depending on SPIO size, concentration and coating material.On the other hand, SPIO have been found to promote neurite outgrowth (Neubert et al., 2015) or to direct axonal elongation toward a desired destination using an external magnetic field.
So far, microscopic analysis of cellular SPIO interactions have been a major challenge, as their diameter at nano-scale makes it impracticable to verify without histologic sample processing.Even the most common technique using Prussian blue (PB)iron staining merely facilitates the visualization of agglomerated SPIO. This method is prone to interference from physiological or pathologic iron background, such as blood artifacts caused by hemorrhages in the region of interest, which hampers corresponding immune stainings (Kobayashi et al., 2017).
One solution for overcoming these problems is to dope SPIO with lanthanide ions (LI). LI do not exist physiologically and can be integrated easily into the SPIO core during their synthesis (de Schellenberger et al., 2017; Kobayashi et al., 2017). Very small superparamagnetic iron oxide particles (VSOP), doped with LI and electrostatically stabilized by a monomeric coating with citrate, are in this context a promising SPIO subcategory. Generally, VSOP are considerably smaller than sterically solidified SPIO and provide a strong T1-shortening effect in MRI (Rumenapp et al., 2012). We have recently published firstin vitroexperiments using the peritoneal macrophage cell line RAW264.7 derived from mice, showing that Europium-doped VSOP (Eu-VSOP)enable an improved detection using fluorescence spectrometry and microscopy which is more sensitive compared to bright field analysis of PB stainings (Kobayashi et al., 2017). In addition, doping of VSOP with Europium is accompanied by neither changes in their cellular uptake characteristics, physical properties, MRI signal or impact on cell biological functions (de Schellenberger et al., 2017; Kobayashi et al., 2017). The aim of our latest proof-of-principle study was to investigate the accumulation and visualization of Eu-VSOP using murine organotypic hippocampal slice cultures (OHSC) to evaluate their potential for further CNS tissue analyses.
We already showed that OHSC are a suitable model for evaluating the absorption, biocompatibility and safety of VSOPex vivo(Pohland et al., 2017). Synthesized Eu-VSOP provided by the Charité Institute of Radiology where applied. The synthesis and physicochemical classification have been previously described in detail (de Schellenberger et al., 2017). In summary, the Eu-VSOP we used (RH030812Eu) had a Z-average of 20.46 nm measured by dynamic light scattering, a polydispersity index of 0.167, a hydrodynamic diameter within 8.72 nm to 13.54 nm, a total iron(Fe2+/3+) concentration of 103.6 mM, a Eu3+concentration of 1.03 mM, a weight ratio m(Eu3+) to m(Fe2+/3+) of 0.0272 as well as a relaxivity (0.94 Tesla) of R1= 20.72 and R2= 63.06.
OHSC with a thickness of 200 μm were prepared from P0 C57 B6/J mice as stated previously (Pohland et al., 2017). Subsequently, slices were incubated until 2 daysin vitro(DIV) (44 hours) using Eu-VSOP at a concentration of 0.5 mM. In order to enhance Europium fluorescence emission, OHSC were methanol/acetone fixed and processed with modified antenna enhancement solution (MES), as described by Kobayashi et al.(2017). Microscopy was implemented using an Axio Observer.Z1, equipped with an Axio Vision Software ZEN 2012 and a Bandpass Filter 350/50. Background correction and adjustment of brightness and contrast were performed using Axio Vision Software ZEN 2012 and ImageJ. Identical settings were applied to every bright- field recording as well as every fluorescent image.Accordingly, we evaluated the accumulation of Eu-VSOP in the formations (Figure 1A) cornu ammonis (CA) and dentate gyrus (DG), which are involved in spatial and episodic memory in hippocampal neuronal ensembles (Leutgeb et al., 2005). OHSC without previous MES treatment (Figure 1C) served as control and showed no fluorescent signal. In comparison, MES-processed slices had a significant emission within the DG as well as in the CA1 and CA3 regions of the hippocampus (Figure 1E),indicating an increased Eu-VSOP uptake.
This result is in line with our previous data showing the distribution of un-doped VSOP within OHSC (Pohland et al., 2017)and is probably related to the types of cell populations and their density within the DG and CA. So far, it is unclear which kinds of cells are involved in Eu-VSOP accumulation. However, as our previous results show microglia seem to play a substantial role.For that reason, it would be interesting to compare the Eu-VSOP uptake in the absence of internal microglia.
In addition, the labeling of cells and tissue with Eu-VSOP does provide a fundamental groundwork for further histological clarification. Corresponding stainings will help us to answer the question if neurons, astrocytes or interneurons are involved.
We are aware of the comparatively diffuse fluorescent signal depicted inFigure 1. In our point of view this is an evidence for deep tissue penetration of Eu-VSOP and also related to the use of a fluorescence microscope as well as a tissue thickness of 200 μm. Adjustment of Eu-VSOP concentration, incubation time and slice diameter is necessary to improve the image quality in future. In addition, we are sure that an increase of resolution is possible if a confocal microscope is used. Furthermore, tests need to be done to compare the impact of Eu-doped and -undoped VSOP on slice viability or cytokine secretion.
In our point of view our results help to fill the gap between primary cell culture and animal experiment applying Eu-VSOP.Currently, our data can only just be rated as an initial experiment.
In our experimental setup, Eu-VSOP allowed an advanced and highly specified microscopic analysis of SPIO-exposed OHSC, and help to preclude any problems that are related to PB iron stainings.de Schellenberger et al. (2017) recently published promising data showing a fluorescence assessment of Eu-VSOP in dissolved organs, biological fluids and spleen explants afterin vivoapplication in mice. These results enabled an improved, unambiguous characterization of VSOP toxicology and bio-distribution.
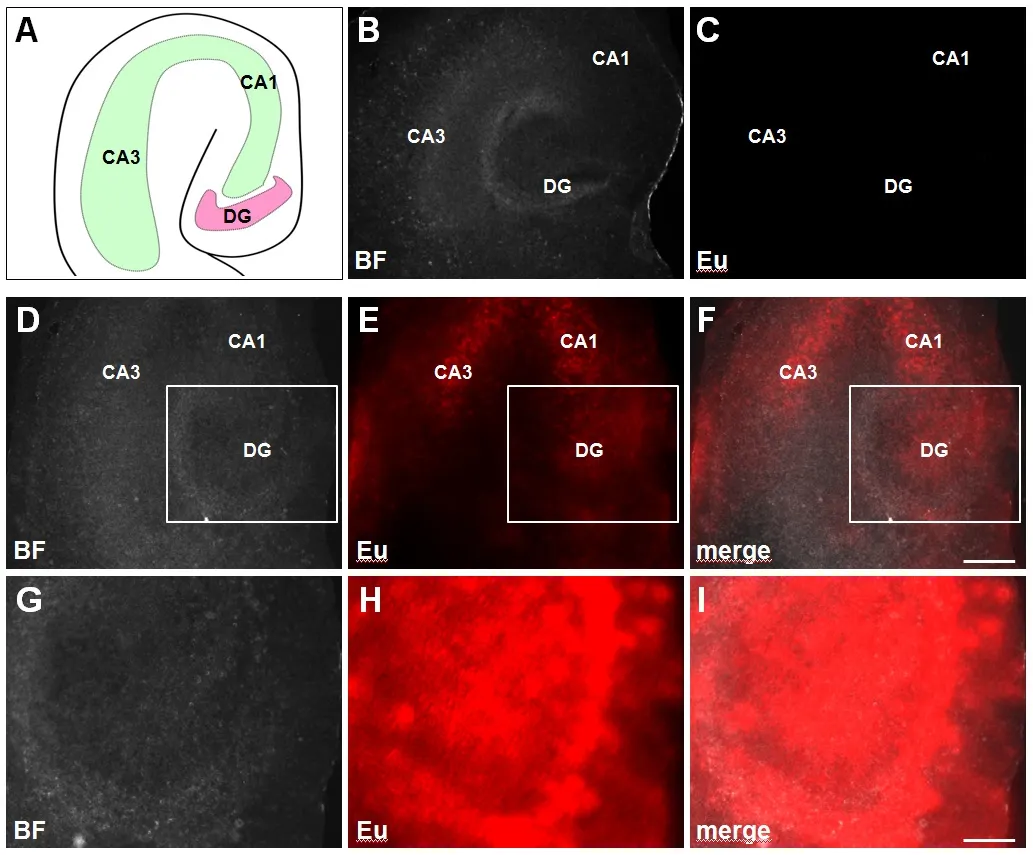
Figure 1 Europium-doped very small superparamagnetic iron oxide particles (Eu-VSOP) uptake within the cornu ammonis and dentate gyrus of organotypic hippocampal slice cultures.
Since SPIO will be used more frequently for MRI in humans,potential side effects of particle interaction need to be investigated in depth before one can start applying them broadly as contrast agents for cancer treatment or in neuronal regeneration.
Crucial for SPIO cytotoxicity is the release of free iron once the particles are degraded, affecting neurons and microglia (Xue et al., 2012). Increased neuronal cell death induced by SPIO application (Pisanic et al., 2007) lead to microglial activation promoting neurotoxicity through pro-inflammatory responses (Pais et al., 2008). On the other hand, we have shown that certain SPIO can enhance neuronal differentiation and neurite outgrowth (Neubert et al., 2015) in line with other authors who have demonstrated that SPIO can improve the survival of PC12 neurons in a dose-dependent manner (Kim et al., 2011).
More research is required to understand the role of various SPIO in regeneration, neurite differentiation and their subtle differences. Likewise, it is essential to reliably track themin vivo.
In this context our presented data might contribute to improve assessments of SPIO in CNS tissue.
This study was supported by deutsche Forschungsgemeinschaft Grant Klinische Forschungsgruppe 213 to JG.
Martin Pohland, Yuske Kobayashi, Jana Glumm*
Institute of Cell Biology and Neurobiology, Center for Anatomy,Charité -Universitätsmedizin Berlin, Berlin, Germany (Pohland M,Glumm J)Department of Interventional and Diagnostic Radiology and Nuclear Medicine, Universitätsklinikum Hamburg-Eppendorf,Hamburg, Germany (Kobayashi Y)Department of Neurosurgery, HELIOS Klinikum Berlin Buch,Berlin, Germany (Glumm J)
orcid:0000-0001-5756-3718 (Jana Glumm)
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review report:
Reviewer:Saritha Krishna, Baylor College of Medicine, USA.
Comments to authors:The current study describes the deposition and visualization of Europium-doped very small superparamagnetic iron oxide particle(s) in murine organotypic hippocampal slice cultures. This work is an extension of the author’s prior publication where they studied the MR signal enhancing effects of Eu-VSOP and VSOP in the RAW264.7 cell line. The recent work published by Pohland M et al. showed murine organotypic hippocampal slice culture as a good tool to screen nanoparticles and further evaluate their potential cytotoxic effects. The findings of the current work shows that doping of VSOP with Europium enables their visualization and deposition in hippocampal slice culture. Furthermore, authors assert that their findings will help to detect SPIO-exposed regions and further analyze the interaction of SPIO within CNS tissue and examine for any cytotoxic effects.
de Schellenberger AA, Hauptmann R, Millward JM, Schellenberger E,Kobayashi Y, Taupitz M, Infante-Duarte C, Schnorr J, Wagner S (2017)Synthesis of europium-doped VSOP, customized enhancer solution and improved microscopy fluorescence methodology for unambiguous histological detection. J Nanobiotechnology 15:71.
Dosa E, Tuladhar S, Muldoon LL, Hamilton BE, Rooney WD, Neuwelt EA(2011) MRI using ferumoxytol improves the visualization of central nervous system vascular malformations. Stroke 42:1581-1588.
Kim JA, Lee N, Kim BH, Rhee WJ, Yoon S, Hyeon T, Park TH (2011) Enhancement of neurite outgrowth in PC12 cells by iron oxide nanoparticles. Biomaterials 32:2871-2877.
Kobayashi Y, Hauptmann R, Kratz H, Ebert M, Wagner S, Taupitz M (2017)Europium doping of superparamagnetic iron oxide nanoparticles enables their detection by fl uorescence microscopy and for quantitative analytics.Technol Health Care 25:457-470.
Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB(2005) Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 309:619-623.
Li L, Jiang W, Luo K, Song H, Lan F, Wu Y, Gu Z (2013) Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 3:595-615.
Neubert J, Wagner S, Kiwit J, Brauer AU, Glumm J (2015) New findings about iron oxide nanoparticles and their different effects on murine primary brain cells. Int J Nanomedicine 10:2033-2049.
Pais TF, Figueiredo C, Peixoto R, Braz MH, Chatterjee S (2008) Necrotic neurons enhance microglial neurotoxicity through induction of glutaminase by a MyD88-dependent pathway. J Neuroinflammation 5:43.
Pisanic TR, 2nd, Blackwell JD, Shubayev VI, Finones RR, Jin S (2007)Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 28:2572-2581.
Pohland M, Glumm R, Wiekhorst F, Kiwit J, Glumm J (2017) Biocompatibility of very small superparamagnetic iron oxide nanoparticles in murine organotypic hippocampal slice cultures and the role of microglia.Int J Nanomedicine 12:1577-1591.
Rumenapp C, Gleich B, Haase A (2012) Magnetic nanoparticles in magnetic resonance imaging and diagnostics. Pharm Res 29:1165-1179.
Xue Y, Wu J, Sun J (2012) Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol Lett 214:91-98.
杂志排行
中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration
