Dissecting the multifactorial nature of demyelinating disease
2018-05-05KarolinaKucharovaWilliamStallcup
Karolina Kucharova, William B. Stallcup
Sanford Burnham Prebys Medical Discovery Institute, Cancer Center; Tumor Microenvironment and Cancer Immunology Program,La Jolla, CA, USA
Chondroitin Sulfate Proteoglycan-4 (Cspg4)Proteoglycan as a Tool for Probing Myelin
Damage and Repair
Extensive research has identified multiple factors that contribute to the failure of remyelination in multiple sclerosis (Franklin, 2002). This pathological complexity is due at least in part to the fact that several different cell types participate in disease progression. In addition to oligodendrocytes and neurons (axons), immune cells and other elements of the microenvironment also play important roles in myelin damage and repair.Defining in more depth the respective contributions of these various cell types to demyelination and remyelination will be required for a better understanding of multiple sclerosis that can lead to improved treatment of patients. Toward this end, we have identified chondroitin sulfate proteoglycan-4 (CSPG4, also known as the NG2 proteoglycan) as a surface component of three cell types present in lysolecithin-induced demyelinated lesions in mouse spinal cord. Oligodendrocyte progenitor cells (OPCs), myeloid cells (macrophages and microglia), and microvascular pericytes in demyelinated lesions all exhibit enhanced CSPG4 expression one week after lysolecithin microinjection (Kucharova et al., 2011). CSPG4 thus not only serves as a marker for recognizing these cells, but also provides a means of manipulating the cells for experimental purposes.We have utilized two types of manipulations to study the respective roles of CSPG4-expressing cell types in myelin damage and repair.
Cell type-specific CSPG4 ablation
We have used Cre-Lox technology to ablate CSPG4 specifically in two cell types, OPCs and myeloid cells.By crossing CSPG4 floxed mice (Chang et al., 2012)withOlig2-Cremice (Schüller et al., 2008) and withLysM-Cremice (Stockmann et al., 2008), we have created OPC-specific CSPG4 null mice (CSPG4 fl/ fl;Olig2-Cre)and myeloid-specific NG2 null mice (CSPG4 fl/ fl;LysMCre), respectively. These have allowed us to define the functional role of CSPG4 in the biology of OPCs and myeloid cells during lysolecithin-induced spinal cord demyelination and remyelination. In addition, these mouse models allow us to study the effects of reduced participation of these two cell types on demyelination and remyelination (Kucharova and Stallcup, 2015).
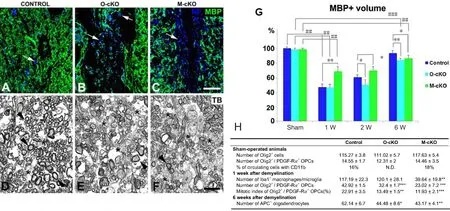
Figure 1 Myelin repair in NG2 null mice.
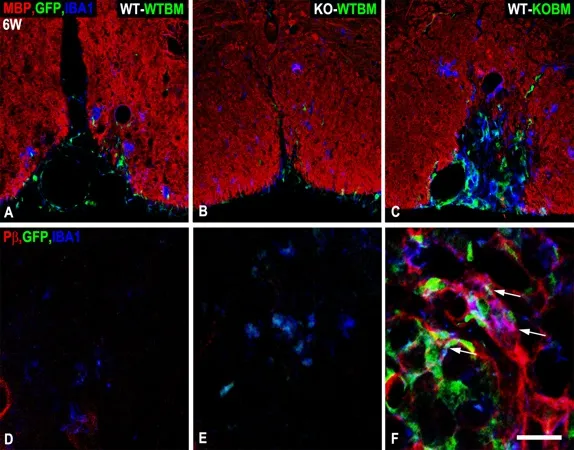
Figure 2 Persistence of atypical NG2 null macrophages in lesions in WT-KOBM mice.
Bone marrow transplantation
We have used bone marrow transplantation to discriminate between the actions of macrophages and microglia in myelin damage and repair. By transplanting bone marrow (BM) fromβ-actin EGFPgermline CSPG4 null (KO) donors into irradiated wild type(WT) recipients, we created chimeric WT-KOBM mice that lack CSPG4 in EGFP-labeled bone marrow-derived macrophages, but retain CSPG4 in host cells,including microglia and OPCs. Conversely, by transplanting bone marrow fromβ-actin EGFPwild type donors into irradiated germline CSPG4 null recipients,we created chimeric KO-WTBM mice that lack CSPG4 in OPCs and microglia, but retain CSPG4 in EGFP-labeled macrophages. WT-WTBM chimeric mice provide controls for lysolecithin-based experiments with WT-KOBM and KO-WTBM mice in which various aspects of spinal cord demyelination and repair are quantified (Kucharova and Stallcup, 2017).
Importance of CSPG4 in OPC Function
Because OPCs produce the mature oligodendrocytes needed for remyelination of demyelinated axons(Gensert and Goldman, 1997), we would expect deficits in OPC generation to have a negative impact on remyelination. Our finding that ablation of CSPG4 diminishes OPC proliferation (Kucharova and Stallcup, 2010; Kucharova et al., 2011) thus suggests that CSPG4 loss should also impair remyelination. Using the lysolecithin model, negative effects on OPC proliferation (40% decrease) and abundance (25% decrease)at 1-week post-injury, with resulting deficits in mature oligodendrocyte number (30% decrease) at 6-weeks post-injury, are clearly observed in spinal cord lesions inCSPG4 fl/ fl;Olig2-Cremice. These data are based on quantification of OPC and oligodendrocyte numbers(Figure 1H).
It is important to note in Figure 1H that numbers of OPCs and oligodendrocytes do not differ between sham-operated control, OPC-specific, and myeloid-specific CSPG4 null mice. Similarly, levels of myelination are the same in the three lines of mice at the starting point (Figure 1G, Sham). Differences seen in the three mouse lines therefore occur in response to the demyelinating event. Figure 1G shows that although the initial extent of demyelination at 1-week is not affected by OPC loss of CSPG4, CSPG4-dependent OPC deficits cause myelin repair inCSPG4fl/fl;Olig2-Cremice to lag behind that seen in controls at 2-weeks. Even at 6-weeks post-injury, when repair is virtually complete in control mice, myelin volume is not fully restored inCSPG4 fl/ fl;Olig2-Cremice. This is illustrated more dramatically at 6-weeks post-injury in Figure 1A, D and B,E by the deficit in well-myelinated axons inCSPG4 fl/fl;Olig2-Cremice, compared to controls. Significantly, the overall number of axons, regardless of size, is also reduced by 40% in lesions inCSPG4fl/fl;Olig2-Cremice, reflecting the importance of myelination for neuronal cell survival (Kucharova and Stallcup, 2015).The effects of CSPG4 loss on OPCs are also seen in the bone marrow transplantation studies. In the lysolecithin model, the absence of CSPG4 from OPCs in KO-WTBM chimeric mice results in a 30% decrease in OPC number at one-week post-injury. The resulting deficit in generation of mature oligodendrocytes translates into a significant decrease in the extent of myelin repair observed at 6-weeks after lysolecithin microinjection (Kucharova and Stallcup, 2017).
Importance of CSPG4 in Myeloid Cell Function
Previous findings from spinal cord demyelination experiments using germline CSPG4 null mice have suggested that loss of CSPG4 greatly reduces the number of myeloid cells associated with demyelinated lesions(Kucharova et al., 2011). This result is confirmed by our subsequent examination of lysolecithin-induced spinal cord lesions inCSPG4fl/fl;LysM-Cremice.Although numbers of circulating CD11b-positive monocytes/macrophages are similar in sham-operated control andCSPG4 fl/ fl;LysM-Cremice, lesions in these mice at 1-week post-injury contain 70% fewer myeloid cells than lesions in control mice (Figure 1H). This deficit in myeloid cell recruitment to lesions is associated with changes in both the initial extent of myelin damage and the subsequent extent of myelin repair. At one-week after lysolecithin microinjection, lesions inCSPG4 fl/ fl;LysM-Cremice are only about half the size of lesions in control mice (Figure 1G), likely due to the involvement of myeloid cells in causing myelin damage. In spite of this reduced extent of myelin damage,remyelination of axons in CSPG4fl/fl;LysM-Cre mice lags behind remyelination in control mice and is still incomplete at 2 and 6-weeks post-injury. Compared to controls (Figure 1A, D), numbers of well-myelinated axons inCSPG4 fl/ fl;LysM-Cremice (Figure 1C, F) are reduced to a similar extent to that seen inCSPG4fl/fl;Olig2-Cremice (Figure 1B, E). These results indicate an important role for myeloid cells in myelin repair, in addition to their contribution to myelin damage. One intriguing function of myeloid cells appears to be their ability to support expansion of the OPC population, as reflected by a 50% decrease in OPC proliferation and a 40% reduction in OPC abundance seen in lesions inCSPG4fl/fl;LysM-Cremice at 1-week post-injury(Figure 1H). These decreases are even greater than the OPC deficits observed inCSPG4fl/fl;Olig2-Cremice, indicating that loss of myeloid cells due to myeloid-specific ablation of NG2 is more deleterious to expansion of the OPC pool than loss of CSPG4 by the OPCs themselves. Myeloid cells may promote OPC proliferation by at least two mechanisms: (1) phagocytosis of myelin debris that is inhibitory to OPC proliferation and/or (2) production of cytokines/growth factors that stimulate OPC mitosis.
We have noted two drawbacks of the Cre/Lox approach for evaluating CSPG4 function in myeloid cells.First, LysM-Cre mediated ablation of CSPG4 does not allow discrimination between the effects of ablation on macrophages and microglia, since CSPG4 is ablated in both populations. Second, because CSPG4 expression is quite transient in myeloid cells, it has been difficult to determine the efficiency of LysM-Cre mediated CSPG4 ablation in these cells. The bone marrow transplantation approach offers improvement in both of these areas. Because CSPG4 is ablated in the germline in these experiments, we know that CSPG4 is completely lost in cells derived from recipient mice in KOWTBM chimeras and in cells derived from donor mice in WT-KOBM chimeras. In addition, ionized calcium binding adapter molecule 1 (Iba-1) positive bone marrow-derived macrophages are marked with the EGFP tracer, while Iba-1 positive resident microglia are not.Examination of WT-KOBM mice at 1-week after lysolecithin microinjection reveals that the absence of CSPG4 in macrophages results in a 5-fold decrease in macrophage number in spinal cord lesions, confirming our results withCSPG4fl/fl;LysM-Cremice. An additional finding is that the abundance of resident microglia is increased 6-fold in WT-KOBM lesions,possibly as compensation for decreased macrophage recruitment. Double labeling for Iba-1 and myelin basic protein in WT-WTBM mice demonstrates that macrophages and microglia are both active in phagocytosis of myelin debris, while evaluation of WTKOBM and KO-WTBM chimeras, respectively, shows that CSPG4 ablation diminishes phagocytic activity in both macrophages and microglia. Interestingly, the increased numbers of microglia in lesions in WT-KOBM mice are not able to replace the effects of macrophages in promoting expansion of the OPC pool, as evidenced by the greatly reduced abundance of lesional OPCs in these chimeras. This may suggest that phagocytosis of myelin debris is less important for OPC expansion than other microenvironmental macrophage contributions that affect OPC proliferation and/or recruitment.As previously shown in Figure 1A–C for cell type-specific CSPG4 ablation, double immunolabeling for myelin basic protein and neurofilament protein at 6-weeks after lysolecithin injection identifies myelinated axons.In the case of WT-KOBM chimeras, myelin repair is only 35% complete, compared to 88% complete in control WT-WTBM mice, confirming the effect of macrophage loss on myelin repair. The persistent myelin damage seen in WT-KOBM mice in Figure 2C occurs in conjunction with elevated numbers of bone marrow-derived macrophages in the lesion. In contrast,macrophage numbers have returned to baseline levels in control WT-WTBM (Figure 2A) and KO-WTBM(Figure 2B) mice at this time point. Although somewhat low in number at 6-weeks compared to 1-week,these persistent macrophages in WT-KOBM mice are characterized by their expression of platelet-derived growth factor receptor β (PDGFRβ) (Figure 2F), a marker that is absent in lesions in WT-WTBM (Figure 2D) and KO-WTBM (Figure 2E) mice. Activation of PDGFRβ has been previously linked to maintenance of macrophages in a less differentiated state (Reiterer and Yen, 2007), possibly contributing to aberrant functions that distinguish these immature cells from mature macrophages.
Conclusions
Our use of both cell type-specific CSPG4 ablation and germline CSPG4 ablation coupled with bone marrow transplantation has allowed us to accomplish two objectives in the context of myelin damage and repair.First, we have been able to demonstrate important roles for CSPG4 in establishing populations of both OPCs and myeloid cells in demyelinated lesions in mouse spinal cord. Second, we have generated mouse models that allow us to assess the consequences of reduced OPC and myeloid cell participation in the processes of demyelination and remyelination.
In the case of OPCs, our findings demonstrate that reduced numbers of OPCs lead to diminished capability for myelin repair. This is hardly surprising, given the fact that newly-generated oligodendrocytes are required for axon remyelination. However, both of our CSPG4 ablation paradigms also support an important role for CSPG4 in expansion of the OPC pool, and thus for generating a population of oligodendrocytes that is sufficient for carrying out myelin repair. While our data firmly support diminished proliferation of OPCs as a likely reason for insufficient generation of oligodendrocytes in lesions in both OPC-specific and myeloid-specific CSPG4 null mice, it is also possible that impaired OPC differentiation as a result of CSPG4 ablation may contribute to the oligodendrocyte deficiency (Franklin, 2002). This possibility remains to be investigated.
In the case of myeloid cells, both experimental approaches support a key role for CSPG4 in establishing substantial myeloid populations in demyelinated lesions. We have also observed dramatic reductions in myeloid cell abundance in brain tumors in CSPG4 null mice (Yotsumoto et al., 2015; Cejudo-Martin et al.,2016), suggesting a general requirement for CSPG4 in recruitment or maintenance of myeloid cells at sites of inflammation. Reduced myeloid cell numbers result in a decrease in the initial extent of axon demyelination,due to reduced myeloid cell participation in myelin damage. More interesting, however, is the diminished myelin repair observed in myeloid cell deficient mice.This appears to stem, at least in part, from the ability of myeloid cells to stimulate expansion of the OPC population,viaphagocytosis of inhibitory myelin debris and/or production of factors that promote OPC proliferation. An additional factor contributing to poor myelin repair may be the actions of atypical, incompletely-differentiated macrophages that persist in lesions in WT-KOBM mice. The identity and function of aberrant immune cells will be critical issues in improving therapy for chronic demyelinating pathologies.
Author contributions:KK and WBS were responsible for planning and executing experimental protocols, analyzing data, and writing and editing the manuscript.
Financial support:This study was supported by National Institutes of Health R01 CA095287 (WBS) and Sanford Burnham Prebys Medical Discovery Institute Lab Funding Initiative (WBS).
Conflicts of interest:None declared.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review reports:
Reviewer 1:Maximilian Michael Saller, Ludwig-Maximilians-Universitat Munchen, Germany.
Comments to authors:The manuscript provides an interesting summary of the proteoglycan CSPG4 function in the turnover and repair of myelin after lysolecithin treatment in mice.
Reviewer 2:Qun Li, Johns Hopkins Medicine, USA.
Comments to authors:Development of oligodendrocyte progenitor cells (OPCs) contributes to remyelination in multiple sclerosis (MS)and other demyelination events. In this article, the authors applytype-specific NG2 ablation models and show that genetically deleting NG2 in OPCs and myeloid cells diminishes capability for OPC proliferation and myelin repair after lesion. In addition, the authors investigate the distinct functions of macrophages and microglia for demyelination and remyelination in subacute (1 week) and chronic(6 weeks) injuries using germline NG2 ablation coupled with bone marrow transplantation. Action of atypical and incompletely-differentiated macrophages for myelination inability is also reported.The mechanisms that NG2 in OPCs and myeloid cells contribute to myelin repair are relevantly discussed. The manuscript is well organized, and authors clearly elucidate their viewpoints.
Cejudo-Martin P, Kucharova K, Stallcup WB (2016) Role of NG2 proteoglycan in macrophage recruitment to brain tumors and sites of CNS demyelination. Trends Cell Mol Biol 11:55-65.
Chang Y, She ZG, Sakimura K, Roberts A, Kucharova K, Rowitch DH, Stallcup WB (2012) Ablation of NG2 proteoglycan leads to deficits in brown fat function and to adult onset obesity. PLoS One 7:e30637.
Franklin RJ (2002) Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci 3:705-714.
Gensert JM, Goldman JE (1997) Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 19:197-203.
Kucharova K, Stallcup WB (2010) The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination. Neuroscience 166:185-194.
Kucharova K, Stallcup WB (2015) NG2-proteoglycan-dependent contributions of oligodendrocyte progenitors and myeloid cells to myelin damage and repair. J Neuroinflammation 12:161.
Kucharova K, Stallcup WB (2017) Distinct NG2 proteoglycan-dependent roles of resident microglia and bone marrow-derived macrophages during myelin damage and repair. PLoS One 12:e0187530.
Kucharova K, Chang Y, Boor A, Yong VW, Stallcup WB (2011)Reduced inflammation accompanies diminished myelin damage and repair in the NG2 null mouse spinal cord. J Neuroinflammation 8:158.
Reiterer G, Yen A (2007) Platelet-derived growth factor receptor regulates myeloid and monocytic differentiation of HL-60 cells.Cancer Res 67:7765-7772.
Schüller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG,Huillard E, Sun T, Ligon AH, Qian Y, Ma Q, Alvarez-Buylla A,McMahon AP, Rowitch DH, Ligon KL (2008) Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell 14:123-134.
Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N,Greenberg JI, Cheresh DA, Johnson RS (2008) Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456:814-818.
Yotsumoto F, You WK, Cejudo-Martin P, Kucharova K, Sakimura K, Stallcup WB (2015) NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. Oncoimmunology 4:e1001204.
杂志排行
中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration
