Young blood products: emerging treatment for Alzheimer’s disease?
2018-05-05RitaKhouryEliasGhossoub
Rita Khoury, Elias Ghossoub
Department of Psychiatry and Behavioral Neuroscience, Saint Louis University, Saint Louis, MO, USA
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, affecting 46.8 million persons worldwide with a projected prevalence of 131 million in the year 2050 (Alzheimer’s Association, 2017). Despite recent advances in the research of biomarkers, early diagnosis and pharmacotherapy of AD, no treatment that can halt the disease process or reverse the brain changes has been discovered. Only two classes of medications are currently Food and Drug administration (FDA)-approved for the symptomatic treatment of this disease:the cholinesterase inhibitors and the NMDA-receptor antagonist, memantine (Khoury et al., 2017). To date,advancing age remains the most important risk factor for developing AD (Dartigues and Feart, 2011). With population aging on the rise, finding a cure for this burdensome disease is urgently needed. Research has shown that neurogenesis continues to occur in the adult brain in a distinct region of the hippocampus, the dentate gyrus, and in the sub-ventricular zone along the walls of the lateral ventricles. The path of these adult neural stem cells (NSCs) also extends to the olfactory bulb (Urbán and Guillemot, 2014). However, neurogenesis has been shown to decline in these regions with age. Given the proximity of adult NSCs to brain vasculature, it is not surprising that they have been shown to be sensitive to exposure to blood-borne factors circulating in the systemic environment (Smith et al., 2018). Animal experiments using heterochronic parabiosis, a surgical procedure that joins the organs and circulatory systems of 2 animals of different ages (Conboy et al., 2013), have demonstrated the rejuvenating effects of the exposure to a youthful systemic milieu on aged tissues, including the brain. The reverse is also true, as research has shown that a young animal exposed to an old systemic environment develops decreased synaptic plasticity and cognitive functioning (Villeda et al., 2011; Rebo et al., 2016).Moreover, Rebo and colleagues have found that simple blood exchange between two animals of different ages may be a promising technique for neural regeneration,with the added benefits of it being less invasive and more applicable to human subjects compared to heterochronic parabiosis. However, repeated transfusions might be needed to achieve better results with respect to cognitive enhancement (Rebo et al., 2016). Plasma rather than whole blood infusions, have replicated the same promising pro-cognitive findings, pointing to the fact that one or more soluble plasma factors and not blood cells, are specifically implicated in promoting or inhibiting brain neurogenesis (Villeda et al., 2011).
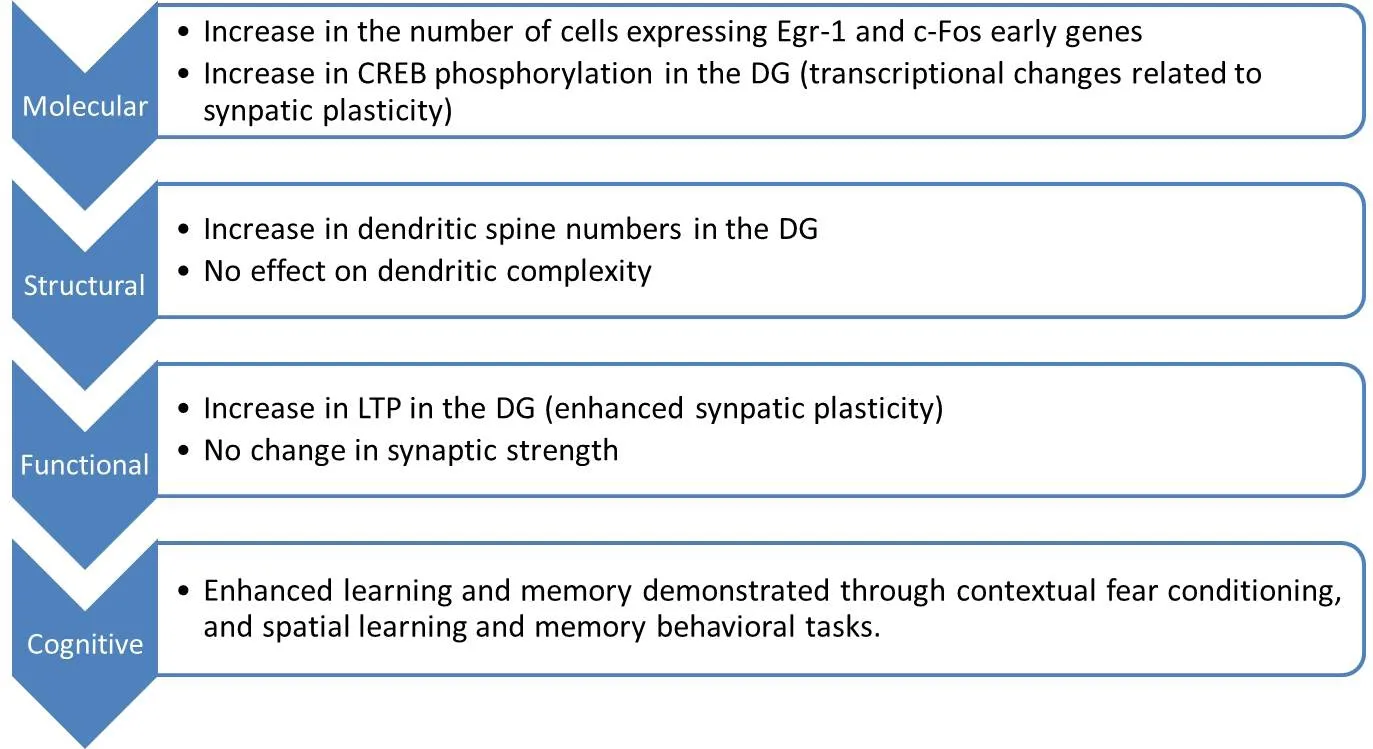
Figure 1 Changes induced by exposure of aged mice to young blood products (parabiosis or plasma infusions) (Villeda et al., 2014).
Rejuvenating Effects of Blood Factors in Aging Animal Models
In animal models, parabiosis studies have shown the impact of blood-factors on both neurogenesis and synaptic plasticity in the hippocampus (Villeda et al., 2011, 2014).Exposure to old blood has led to a significant reduction in neurogenesis in the dentate gyrus of the hippocampus,correlating with increasing levels of C-C motif chemokine 11 (CCL-11) also known as eotaxin-1. CCL-11 has thus been identified to be an age-related systemic factor associated with decreased neurogenesis in healthy aged mice.The systemic administration of CCL-11 alone to young adult miceviastereotaxic injections increased systemic CCL-11 levels, leading to neurogenesis inhibition and impaired learning and memory in young mice (Villeda et al.,2011). Additionally, blood-borne factors are thought to affect synaptic plasticity in the hippocampus: aged mice exposed to young blood products showed an increase in both dendritic spine number/density and long-term potentiation in their dentate gyrus. Those changes were correlated with increased cAMP response element-binding protein (Creb) signaling, which is implicated in synaptic plasticity signaling pathways. The molecular, structural,functional and cognitive changes induced in aged mice by exposure to “pro-youthful” factors contained in young plasma are presented in Figure 1 (Villeda et al., 2014).
In another five-week parabiosis study, neurogenesis in the sub-ventricular zone of young, two-month-old mice, was found to be significantly affected when those mice were joined to old (21-month-old) mice but not to middle-aged (15-month-old) ones. This finding shows an age-dependent accumulation of blood factors affecting neurogenesis. Young-blood circulating factors were shown to remodel the aging cerebral vasculature and to increase blood vessels’ volume and branching, thus enhancing neurogenesis. Moreover, administration of growth differentiation factor 11 (GDF-11) alone to old mice was shown to particularly improve brain blood vasculature and enhance neurogenesis (Katsimpardi et al., 2014). These findings confirm that, during the aging process, brain vasculature and neurogenesis can be modifiable through the administration of young circulating blood factors, despite the accumulation of deleterious systemic factors.
Where does the Evidence Stand in AD?
Promising findings about the rejuvenating effect of young plasma factors on the healthy brains of aging animal models by means of parabiosis, plasma transfusion or direct administration of GDF-11 have prompted researchers to experiment their effect in subjects with an established neurocognitive disorder, such as AD. The hypotheses stipulated that the modification of brain vasculature, reactivation of adult NSCs, and remodeling of their synaptic activity/plasticity may lead to cognitive enhancement and increased neurogenesis (Laviano, 2014).Aged mice with established AD, that carry a mutant amyloid precursor protein (APP) gene, were treated ei-ther surgically (parabiosis) for five weeks, or with repeated intravenous plasma infusions from young mice, twice a week, for four weeks. Both procedures reversed the depletion of certain synaptic and neuronal proteins such as synaptophysin, and the excessive phosphorylation of extracellular signal-regulated kinase (ERK), which is usually involved in the pathophysiology of AD. Clinically,repeated plasma transfusions were also associated with improvements in APP-mutant mice’s working and associative memory, even though beta-amyloid plaque accumulation and microglial activation were not significantly affected (Middeldorp et al., 2016).
The PLasma for Alzheimer Symptom Amelioration(PLASMA) Study is the first pilot study to test the feasibility, safety and tolerability of repeated intravenous plasma infusions in humans diagnosed with AD. It was conducted in Stanford University and sponsored by Alkahest. Plasma was drawn from 18 to 30 year-old donors, and was given to 18 patients suffering from mild to moderate AD according to the National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria, with a Mini-Mental Status Examination (MMSE) ranging between 12 and 24 (NCT02256306, 2017). Nine participants received an infusion of either saline placebo or young donor plasma, once weekly for four weeks, and were then crossed over to the other infusion type six weeks later.The remaining nine participants received only plasma infusions once weekly for 4 weeks. The primary outcome measures were the incidence of adverse events as a measure of safety and tolerability, and the compliance rate with the research protocol as a measure of feasibility.Secondary outcome measures included changes in cognitive function and functionality using the following scales:Alzheimer’s Disease Assessment Scale-Cog (ADAS-cog);Trail-Making test, Clinical Dementia Rating Scale Sum of Boxes (CDR-SB), Functional Activities Questionnaire(FAQ), and Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL). The trial’s results were encouraging: the technique was found to be feasible and safe among patients with mild to moderate AD. However, despite modest improvements in functioning and performing activities of daily living, no statistically significant cognitive benefit was found in this trial; potential explanations for this result might be that the sample size was small or that plasma derived from 30-year old adults may not contain blood factors that are beneficial for patients with AD (Scudellari, 2015). In the future, Alkahest is considering conducting larger clinical trials in different stages of AD, trying different administration protocols and using specific plasma products(pro-cognitive growth factors) rather than whole plasma infusions to enhance both safety and effectiveness of the trials (Kaiser, 2017).
Limitations and Future Challenges
Although promising, findings from animal research studies need to be interpreted with caution as they might not necessarily be extrapolated to humans. Therefore there is a need to conduct additional placebo-controlled human studies in larger samples. Moreover, researchers should expect a difference in outcome depending on whether they study healthy aging, minor neurocognitive disorders(mild cognitive impairment), or major neurocognitive disorders such as AD. Although it is tempting to view neurodegenerative diseases as a continuum of aging or the result of an accelerated aging process, research has shown that lesions and cognitive changes associated with aging are distinct from those associated with neurodegenerative diseases (Wyss-Coray, 2016). Some researchers believe that memory impairment occurring in the elderly with normal aging may be due to impairment in neuronal plasticity and/or connectivity, without neuronal loss,as seen in AD (Paul and Reddy, 2014). It is also crucial to further identify the mechanisms underlying the mode of action of blood/plasma transfusions and the settings in which activation of adult neurogenesis is still an achievable goal. Many researchers postulate that intervening at the AD stage, even early on, is rather too late, as neural regeneration at this stage is unlikely to happen. Alzheimer’s disease is thought to begin many decades before the onset of symptoms, and earlier interventions have now been the focus of clinical trials (Sperling et al., 2014).Thus, intervening at an early, pre-morbid stage may carry a higher chance to undo cognitive changes, when they are still reversible (Paul and Reddy, 2014). Hence, determining an “optimal age” at which an intervention in humans may yield significant cognitive enhancement needs to be explored in future studies.
Furthermore, there are no clear recommendations regarding whether to use transfusions of blood, plasma,or isolated plasma products. Although blood/plasma infusions do not incur the risk of tissue rejection, they are associated with developing allergic reactions ranging from mild reactions to anaphylaxis. It is well-known that whole blood products infusions are associated with more adverse allergic events than plasma products infusions(Conese et al., 2017). Other side effects include the risk of infections, hemolysis, volume overload and acute lung injury especially in older adults with heart failure (Aicardi,2017). Thus, there is a need to identify the specific factors implicated in stimulating adult neurogenesis, in order to rely on isolated plasma products infusions in the future.
To date, GDF-11 has been shown to increase angiogenesis and neurogenesis in the brain, whereas CCL-11/eotaxin-1 has been found to inhibit neurogenesis and impair memory and learning in animal models (Conese et al.,2017). Deficiencies in growth hormone (GH) and insulin-growth factor-1 (IGF-1) have been linked to memory deficits. Systemic administration of GH-releasing hormone (GHRH) increased circulating levels of both GH and IGF-1 and was associated with improved cognition in healthy elderly people and in people with mild cognitive impairment (Wyss-Coray, 2016). Furthermore, systemic treatment of aged mice with gonadotropin-releasing hormone I (GnRH I) was found to increase neurogenesis and to improve cognitive function (Wyss-Coray, 2016).Hence, isolating and infusing specific factors may play a key role in developing innovative disease-modifying therapies for AD (Aicardi, 2017). However, this approach is not without its risks: a long-term concern is the increase in the incidence of cancerization, as these factors are thought to activate systemic stem cells, resulting in excessive cell division (Conese et al., 2017).
In the meantime, scientists are urged to examine the ethical challenges these innovative blood-based therapies may carry. One challenge lies in reinforcing the conceptualization of aging as a disease that must be treated.“Treatment success” can lead to increased population growth and have major societal repercussions. The ethical principles of beneficence, non-malfeasance and justice may also be at stake: issues to address include the risk-benefit balance of treatment, the equal access to resources and the potential exploitation of youth (Hofmann,2018). Prospective, large, randomized-controlled trials are needed to evaluate the efficacy and safety of plasma factor infusions as a prophylactic or therapeutic option for neurodegenerative diseases: only then will we find out whether the benefits of this treatment justify its risks.
Author contributions:RK contributed to the conception/design and writing of the manuscript. EG contributed to the writing and critical revision of the manuscript. Both authors approved the final version of the manuscript to be published.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Shasha Li, La Escuela Superior de Medicina del Instituto Politécnico Nacional, Mexico.
Aicardi G (2017) Young blood plasma administration to fight Alzheimer’s disease? Rejuvenation Res doi: 10.1089/rej.2017.1940.
Alzheimer’s Association (2017) Alzheimer’s disease facts and figures. Alzheimers Dement 2017;13:325-373
Conboy MJ, Conboy IM, Rando TA (2013) Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 12:525-530.
Conese M, Carbone A, Beccia E, Angiolillo A (2017) The fountain of youth: a tale of parabiosis, stem cells, and rejuvenation.Open Med (Wars) 12:376-383.
Dartigues JF, Feart C (2011) Risk factors for Alzheimer disease:aging beyond age? Neurology 77:206-207.
Hofmann B (2018) Young blood rejuvenates old bodies: A call for reflection when moving from mice to men. Transfus Med Hemother 45:67-71.
Kaiser J (2017) Blood from young people does little to reverse Alzheimer’s in first test. http://www.sciencemag.org/news/2017/11/blood-young-people-does-little-reverse-alzheimer-s- first-test. Accessed January 10, 2018.
Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL (2014) Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344:630-634.
Khoury R, Patel K, Gold J, Hinds S, Grossberg GT (2017) Recent progress in the pharmacotherapy of Alzheimer’s disease.Drugs Aging 34:811-820.
Laviano A (2014) Young blood. N Engl J Med 371:573-575.
Middeldorp J, Lehallier B, Villeda SA, Miedema SS, Evans E,Czirr E, Zhang H, Luo J, Stan T, Mosher KI, Masliah E, Wyss-Coray T (2016) Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol 73:1325-1333.
NCT02256306 (2017) The plasma for Alzheimer symptom amelioration (PLASMA) study (PLASMA). https://clinicaltrials.gov/ct2/show/NCT02256306. Accessed January 17, 2018.
Paul SM, Reddy K (2014) Young blood rejuvenates old brains.Nat Med 20:582-583.
Rebo J, Mehdipour M, Gathwala R, Causey K, Liu Y, Conboy MJ, Conboy IM (2016) A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat Commun 7:13363.
Scudellari M (2015) Ageing research: Blood to blood. Nature 517:426-429.
Smith LK, White CW 3rd, Villeda SA (2018) The systemic environment: at the interface of aging and adult neurogenesis. Cell Tissue Res 371:105-113.
Sperling R, Mormino E, Johnson K (2014) The evolution of preclinical Alzheimer’s disease: Implications for prevention trials.Neuron 84:608-622.
Urbán N, Guillemot F (2014) Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci 8:396.
Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T (2014) Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 20:659-663.
Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA,Quinn JF, Galasko DR, et al. (2011) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477:90-94.
Wyss-Coray T (2016) Ageing, neurodegeneration and brain rejuvenation. Nature 539:180-186.
杂志排行
中国神经再生研究(英文版)的其它文章
- Acupuncture and neuroregeneration in ischemic stroke
- The adjustment of γ-aminobutyric acidA tonic subunits in Huntington’s disease: from transcription to translation to synaptic levels into the neostriatum
- Bridging the gap: axonal fusion drives rapid functional recovery of the nervous system
- Collagen for brain repair: therapeutic perspectives
- Stimulating effect of thyroid hormones in peripheral nerve regeneration: research history and future direction toward clinical therapy
- Harnessing migraines for neural regeneration
