黏土负载纳米零价铁复合材料去除污染物研究进展
2018-05-02李益民
吕 瑛 李益民
(绍兴文理学院 化学化工学院,浙江 绍兴312000)
0 引言
近年来随着工业化和城镇化进程的加快,一些有毒有害、难降解的污染物由于未经处理或未达标排放,导致水体乃至土壤受到了严重污染,威胁生态安全,影响人体健康.因此,如何有效去除环境中有毒有害污染物,是环境领域中的主要研究热点.
零价铁(Zero Valent Iron, ZVI)原料廉价易得,还原能力较强(E0=-0.44V),20世纪90年代Gillham等[1]将零价铁用于氯代有机物的还原脱氯,开创了零价铁处理污染物的新途径,将其用作可渗透反应墙(Permeable Reactive Barrier,PRB)的活性材料[2-3],在欧美等国已有200多处实地应用.但是,零价铁与污染物反应时,溶液pH会升高,生成铁的氧化物、氢氧化物等腐蚀产物,与地下水中的无机组分反应得到的碳酸盐、硫酸盐等难溶物会覆盖在铁表面,减少铁表面反应活性位点,使反应活性降低[4-5].
1997年Zhang等[6]首先采用纳米铁(Nano-scale Zero-Valent Iron, NZVI)处理三氯乙烯和多氯联苯,取得了良好的还原脱氯效果.与微米铁相比,纳米铁比表面积大、表面能高,因而能更有效去除污染物,它与大多数氯代有机物的还原反应速率比微米铁要高1~3个数量级[7-8].然而,纳米铁粒子的胶体性质和它固有的铁磁性,使得它与污染物反应时易团聚、易氧化、稳定性差,影响了它的还原能力和使用寿命.此外,纳米铁表面疏水性差、选择性也较低,因而降低它对目标污染物的去除.
为了克服上述问题,人们尝试利用固体多孔材料(碳、树脂、沸石、黏土等)负载NZVI去除不同的污染物.Ponder等[9]用PolyFlo树脂制得负载纳米铁,处理废水中的Cr(Ⅵ)、Pb(Ⅱ),结果表明负载纳米铁具有较高的稳定性,反应活性与未负载的纳米铁相当.Jin等[10]则用片状石墨负载纳米铁用于还原三氯乙烯.采用上述方法得到的负载纳米铁较好地克服了纳米铁的团聚问题.Liu等[11]则用聚丙烯酸/聚偏乙烯膜负载纳米Pd/Fe用于三氯乙酸的降解,Wang等[12]用还原的氧化石墨烯负载纳米铁(NZVI/RGO)去除U(VI).这些负载纳米铁均较好地克服了纳米铁的团聚问题,在改善粒子表面疏水性、还原效率与重复使用性上都取得了理想的效果,但制备负载纳米铁的过程较复杂、成本较高.与以上负载材料相比较,黏土则具有价廉易得、环境相容性好、制备简单、易根据污染物的性能进行改性等优点,作为理想的载体材料,用于负载纳米铁处理各类污染物显示了独特的优点.本文主要就各类黏土负载纳米铁在污染物处理中的应用及作用机理进行综述,并对其实际应用进行展望.
1 黏土负载纳米零价铁的发展及其对污染物的去除作用
1.1 黏土
黏土矿物(Clay)是由硅氧四面体和铝氧八面体结构单元构成的一类层状硅酸盐矿物,在自然中储量丰富.其中,蒙脱石、伊利石、蛭石等由两层硅氧四面体中夹一层铝氧八面体(图1a)结构单元组合而成;坡缕石、海泡石则属于另一类2∶1型层链状硅酸盐,而高岭土则由硅氧四面体层和铝氧八面体交替叠加而成(图1b).由于黏土矿物在形成过程中发生同晶替代,导致黏土矿物表面带负电,可吸附Ca2+、Mg2+、Na+、K+等阳离子,在一定条件下,这些离子能被有机阳离子或多核金属离子(如keggin离子)等所取代,形成亲疏水性不同、荷电量各异的改性黏土,从而提高对相关物质的吸附性能.由于蒙脱土、膨润土具有较高阳离子交换容量(CEC=80~120 cmol/kg),较大比表面积(600~800 m2/g)[13],而且层间域高度大,这更容易使阳离子表面活性剂等进入膨润土或蒙脱土层间[14].因此,常选择蒙脱土、膨润土进行改性.
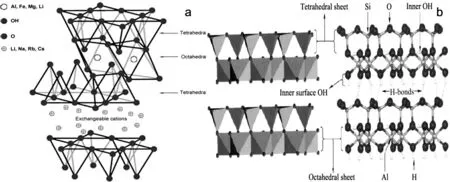
(a) 2∶1型硅酸盐结构[15] (b) 1∶1型硅酸盐结构[16] 图1 硅酸盐结构
1.2 黏土负载纳米零价铁的发展
黏土与零价铁(ZVI)复合去除污染物的报道始于2005年,Cho等[17]将十六烷基三甲基溴化铵(CTMAB)改性后的有机膨润土与ZVI均匀混合,用作模拟PRB柱试验中的活性物质处理三氯乙烯,去除速率是单一ZVI体系的7倍.此后,一些学者陆续开展了黏土负载纳米铁的制备及去除污染物的研究.Üzüm等[18]和Zhang等[19]采用高岭土、Gu等[20]、Fan等[21]和Shahwan等[22]采用蒙脱(石)土、Frost等[23]采用坡缕石分别制备了负载纳米铁,黏土负载纳米铁的主要制备方法是液相还原法:先将黏土在铁(Fe2+或Fe3+)盐溶液中浸渍数小时,达到吸附平衡后在N2保护下用NaBH4还原.反应式可表述为:
2Fe2++BH4-+3H2O→2Fe0↓+H2BO3-+4H++2H2↑
(1)
4Fe3++3BH4-+9H2O→4Fe0↓+3H2BO3-+12H++6H2↑
(2)
实际环境pH下(pH 4~6),带负电的天然黏土对阴离子污染物的吸附性能较弱,此外,由于黏土表面众多的亲水基团,使它们对疏水性有机污染物的吸附也不理想[14].为此,根据待处理污染物的性质对膨润土进行合适的改性[24-27],制得亲疏水性和表面Zeta电位各异的三种膨润土:有机膨润土(CTMAB-bent)、羟基铝柱撑膨润土(Al-bent)、钠基膨润土(Na-bent),并将其作载体制得相应的负载纳米铁,用于处理不同污染物:疏水性强的有机膨润土负载纳米铁(NZVI/CTMAB-bent)处理有机污染物;荷正电的羟基铝柱撑膨润土负载纳米铁NZVI/Al-bent,处理阴离子污染物CrO42-和NO3-;荷负电的钠基膨润土负载纳米铁NZVI/Na-bent,处理阳离子污染物UO22+、Ni2+.实验结果均显示改性膨润土对纳米铁处理相应污染物有明显的协同作用.
未负载的NZVI为直径约100 nm的链状团聚结构见图2,而在钠基膨润土、铝基膨润土和有机膨润土上固定的纳米铁均分散良好.Fan等[21]研究表明,纳米铁粒径与黏土中的铁盐加入量呈正相关.当加入的铁离子量低于黏土的阳离子交换量(CEC)时,交换到层间的铁离子与NaBH4发生层间限域还原反应,可以生成亚纳米粒度、高反应活性的零价铁[20,28-29].
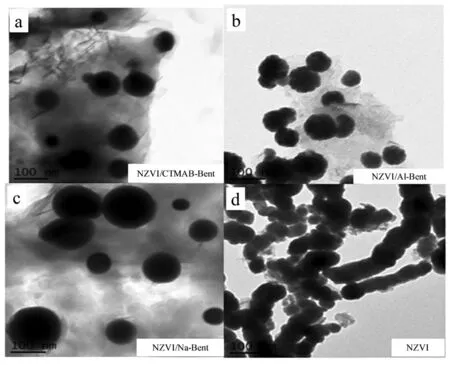
图2 各种纳米铁的TEM图谱[24,27]
1.3 黏土负载纳米零价铁对污染物的去除作用
已有文献报道,用于负载纳米铁的黏土载体有蒙脱(石)土[20,21,29-32]、膨润土[25,28,33-36]、高岭石[18-19]、海泡石[37-38]、坡缕石[39-40]、累托石[41]等,可处理的污染物包括氯代有机污染物、多溴联苯醚、硝基苯、染料、重金属和准金属离子、硝酸根等.黏土负载纳米零价铁复合物去除不同类型污染物代表性的研究见表1.
1.3.1 重金属或准金属离子
利用黏土负载纳米零价铁可有效去除水体中各种重金属离子[42],如Cr(VI)[26],Ni2+[43],As3+[30],Pb2+[19],Zn2+[44],Cd2+[45],Cu2+,Co2+[46],UO22+[33]和Se(VI)[47],其中,对Cr(VI)的研究报道最多[31,37].
六价铬是一般由电镀、冶金和制革等工业过程排放在地下水和地表水中[48],有毒性、致畸性和高溶解性,此外其高流动性严重污染环境[26].因此,修复这种毒性重金属具有重要环保意义.黏土负载纳米零价铁已被证明对Cr(VI)有高去除率.在零价铁修复体系中,Cr(VI)的去除机制基于零价铁对Cr(VI)的吸附和还原.其还原产物主要为三价铬的氢氧化物或与三价铬铁共沉淀的CrxFe(1-x)(OH)3[49].Zhang等[26]用制备的NZVI/Al-bent去除水中的六价铬,Cr(VI)的去除率随溶液pH降低和柱撑膨润土量增加而增加(见图3),Al-bent显示出明显的协同效应.

(a)不同的OH-Al-bent量对Cr(Ⅵ)去除的影响 (b)不同初始pH对Cr(Ⅵ)去除的影响图3[26] PH值与柱撑膨润土的影响
表1 不同黏土负载纳米铁对污染物的去除效率
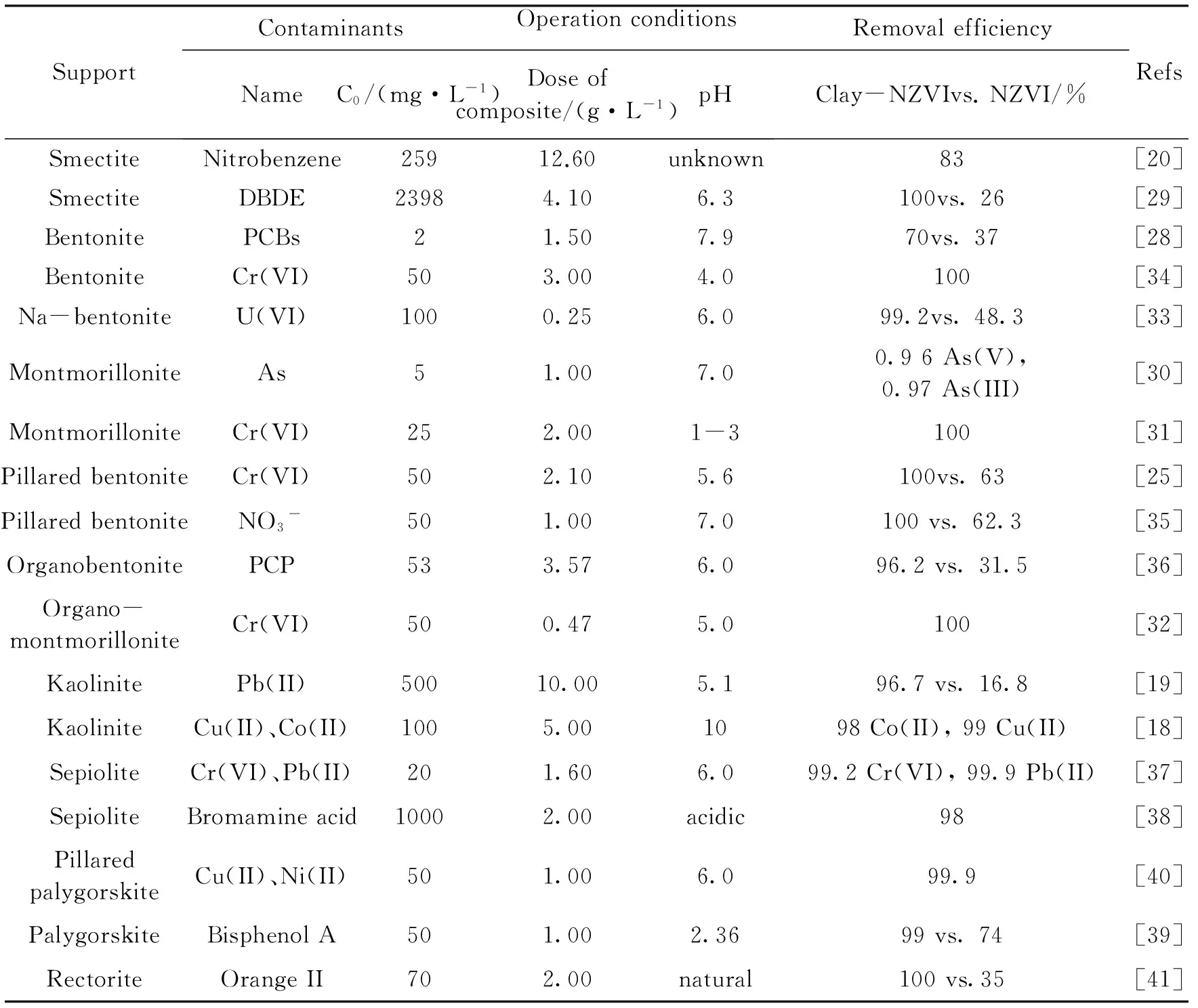
SupportContaminantsOperationconditionsNameC0/(mg·L-1)Doseofcomposite/(g·L-1)pHRemovalefficiencyClay-NZVIvs.NZVI/%RefsSmectiteNitrobenzene25912.60unknown83[20]SmectiteDBDE23984.106.3100vs.26[29]BentonitePCBs21.507.970vs.37[28]BentoniteCr(VI)503.004.0100[34]Na-bentoniteU(VI)1000.256.099.2vs.48.3[33]MontmorilloniteAs51.007.00.96As(V),0.97As(III)[30]MontmorilloniteCr(VI)252.001-3100[31]PillaredbentoniteCr(VI)502.105.6100vs.63[25]PillaredbentoniteNO3-501.007.0100vs.62.3[35]OrganobentonitePCP533.576.096.2vs.31.5[36]Organo-montmorilloniteCr(VI)500.475.0100[32]KaolinitePb(II)50010.005.196.7vs.16.8[19]KaoliniteCu(II)、Co(II)1005.001098Co(II),99Cu(II)[18]SepioliteCr(VI)、Pb(II)201.606.099.2Cr(VI),99.9Pb(II)[37]SepioliteBromamineacid10002.00acidic98[38]PillaredpalygorskiteCu(II)、Ni(II)501.006.099.9[40]PalygorskiteBisphenolA501.002.3699vs.74[39]RectoriteOrangeII702.00natural100vs.35[41]
Notes:C0, Initial concentration of contaminant; DBDE, decabromodiphenyl ether; PCBs, polychlorinated biphenyls; PCP, pentachlorophenol
结合表1中黏土负载纳米零价铁去除Cr(VI)的相关数据我们可以看出,黏土矿是纳米零价铁去除Cr(VI)的有效载体.影响黏土负载纳米零价铁复合物去除Cr(VI)的因素主要有:Cr(VI)初始浓度、黏土类型、复合物剂量、pH以及反应温度.Cr(VI)去除随初始pH降低和Cr(VI)初始浓度的升高而升高,随着复合材料用量减少而减少.
环境中硒的污染通常来源于农业、工业,采矿和石化活动[47].硒污染可能会引起皮肤和神经系统病变等健康问题.Se(VI)难以被矿物所吸附,在地下水中的流动性远远超过其他类型污染物.Dong等[47]用Al-bent-ZVI复合材料去除地下水中的Se(VI).结果表明:Al-bent的存在,促进了ZVI对Se(VI)的去除,还能使Se(VI)更多地还原为毒性和流动性更小的Se(0)和Se(-II).
黏土负载纳米零价铁对各种重金属离子的去除方式与重金属的电极电势大小及性质相关.当零价铁与电极电势远大于零价铁的重金属离子(Cr6+、Se6+和UO22+)反应时,则还原生成流动性小、低价态的物质;当重金属离子的电极电势比零价铁大时,则可通过还原与吸附方式去除污染物,如Pb2+和Ni2+;而当重金属离子的电极电势与零价铁相近时,则主要通过吸附作用方式去除,如Co2+.
1.3.2 氧化性酸根离子
NO3-、BrO3-、ClO4-等是水中常见的含氧酸盐[50],其中硝酸盐则作为水体的主要污染物质[51].硝酸盐主要来自动物饲养过程、农药、化肥和石油产品[52],它能对人体健康造成严重损害,如诱发肿瘤、畸形和突变缺陷[53].研究表明,黏土负载纳米零价铁可有效去除水中硝酸盐.
零价铁对硝酸盐的去除过程主要是将硝酸根还原为低价态的NH4+、亚硝酸根和氮气.Li等[52]用柱撑膨润土(Al-bent)负载纳米零价铁去除NO3-,考察了柱撑膨润土的类型、剂量、混合方式以及初始pH对NO3-去除的影响.Al-bent能有效促进NO3-在零价铁表面的吸附富集,使混合体系对NO3-去除率和反应寿命远高于未加Al-bent的体系,去除率远高于零价铁对NO3-的还原和柱撑膨润土对NO3-的吸附的加和.Zhang等[35]用Al-bent负载纳米零价铁去除NO3-,结果表明复合材料在pH 7时对NO3-去除率远高于相同条件下NZVI(62.3%)和柱撑膨润土(9.2%)的加和.特别有意义的是Al-bent负载纳米零价铁与NO3-反应时,可明显减少有毒中间体NO2-的生成,并能还原成更多的无毒产物N2.
1.3.3 有机污染物
染料[54]、氯代有机污染物和硝基芳香类污染物是地下水中常见的有机污染物.Fan等[55]采用膨润土负载纳米零价铁处理Orange II(OII),通过偶氮键的断裂,破坏了偶氮染料的发色团和共轭体系.Luo等[41]在中性pH下用0.2 g累托石负载的纳米零价铁去除70 mg/L的OII,10min内去除率达到100%,而相同条件下的NZVI对OII的去除率仅为35%.复合材料对OII的反应主要通过:OII先吸附于复合材料上,然后OII被黏土上的NZVI快速降解,由于黏土矿增强了对OII的吸附,减少了NZVI的团聚,增强了复合体系对OII的去除效率.
除OII外,不同类型的黏土负载NZVI还用于甲基橙、溴氨酸等染料的去除.例如,Chen等[56]在pH 6.5下用膨润土负载NZVI去除100 mg/L的甲基橙,10 min内去除率达到74.6%,远高于零价铁的去除率(40.0%).Yuan等[57]发现在pH 2~9条件下,累托石负载纳米零价铁均对甲基橙有良好的去除效果.海泡石、高岭土和膨润土的纳米复合体也被用于有效去除溴氨酸[38].
氯代有机物如多氯联苯、三氯乙烯、四氯乙烯和阿特拉津等对人类健康和水环境有严重危害.用有机膨润土负载纳米铁(NZVI/CTMAB-Bent)[58],在pH 5.0下能去除63.5%的阿特拉津,远大于NZVI还原量(26.6%)和有机金属膨润土吸附量(5.5%)的加和.这主要是因为有机膨润土负载纳米铁提高对了阿特拉津吸附能力,提高对疏水性氯代有机物的去除.此外,还用NZVI/CTMAB-Bent处理五氯酚,实验结果显示了非常高效的脱氯性能,复合材料能使五氯酚苯环上的5个氯完全脱去[36],而在其它关于纳米铁处理五氯酚的文献中还未见能完全脱去5个氯的报道(图4).
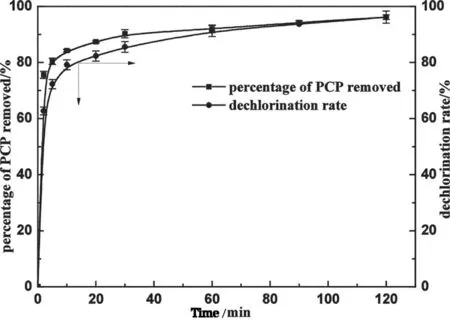
图4[36] NZVI/CTMAB-Bent对五氯酚(PCP)去除率和脱氯率
在有机膨润土负载纳米铁去除四种疏水性不同的氯代酚[59]的研究表明:有机膨润土负载纳米铁和纳米铁对四种氯代酚的去除率、拟一级速率常数(五氯酚>2,4,6-三氯酚>2,4-二氯酚>2-氯酚)与氯代酚的疏水性呈正相关;而且有机膨润土负载纳米铁与纳米铁体系的速率常数之比与氯代酚的疏水性呈正相关,即氯代酚的Kow越大,有机膨润土在纳米铁还原氯代酚过程中的强化作用越明显(见表2).
芳香族化合物因其高毒性和致畸性被列为优先控制污染物.黏土负载纳米铁对其也具有很强的去除效果.Gu等[20]研究了蒙脱石负载纳米
零价铁对硝基苯的去除,结果显示反应2 h后,有98%的硝基苯被转化为苯胺,硝基苯剩余量不足1%.
2 黏土在零价铁去除污染物中的协同作用原理
2.1 黏土的富集作用强化污染物与零价铁间的电子转移反应
黏土对污染物的吸附性能是影响零价铁对目标污染物去除率的重要因素.水体中污染物与零价铁之间的复相反应中,污染物穿过液膜扩散(富集)到零价铁外铁氧化物壳层的传质过程被认为是决速步骤.利用黏土对目标污染物的吸附作用,其负载纳米铁更有助于污染物在铁表面的富集,进而强化纳米铁对污染物的去除,且强化作用的大小与所用黏土对污染物的吸附富集能力成正相关.图5是我们课题组利用三种亲疏水性、表面zeta电位各异(图5a)的改性膨润土分别处理相关污染物的去除情况:用NZVI/CTMAB-bent处理硝基苯[60]和氯代有机污染物(图5b)[34],荷负电的NZVI/Na-bent处理UO22+(图5c)[33]、Ni2+等无机阳离子污染物,荷正电的NZVI/Al-bent处理Cr(Ⅵ)(图5d)[32]、Se(Ⅵ)[61]等无机阴离子污染物.结果表明,利用上述与污染物性质相匹配的改性黏土负载纳米铁,不仅显著提高了零价铁对目标污染物的去除率,同时将污染物还原为毒性更低的物质,提高了零价铁对目标污染物的反应选择性.
表2 NZVI/CTMAB-Bent和NZVI去除不同氯代酚的反应速率之比
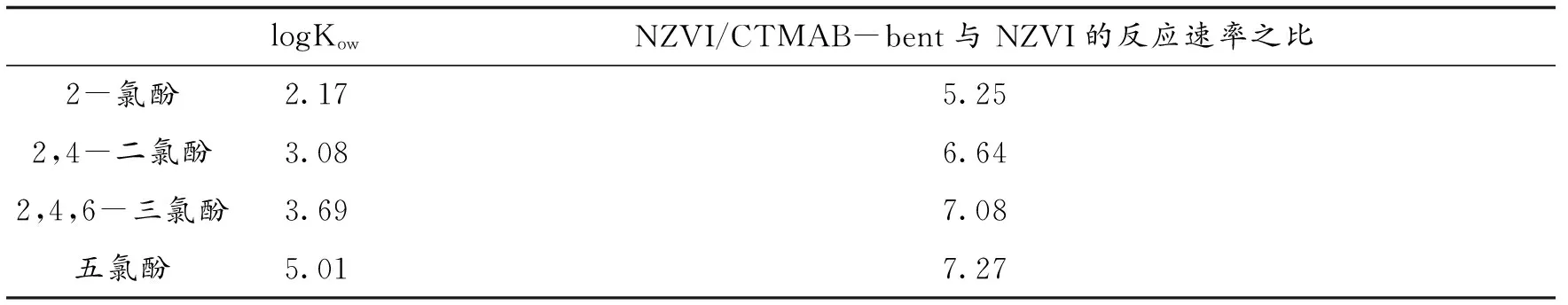
logKowNZVI/CTMAB-bent与NZVI的反应速率之比2-氯酚2.175.252,4-二氯酚3.086.642,4,6-三氯酚3.697.08五氯酚5.017.27
2.2 黏土缓冲pH和减缓零价铁表面钝化产物的生成
黏土特有的pH缓冲和转化钝化产物的能力可显著减少零价铁表面的钝化产物、延长其使用寿命.黏土表面丰富的Si-OH和Al-OH会随着零价铁与污染物反应体系pH的升高而离解出H+,从而降低反应介质pH值(可降低0.5~1.0个单位)[31,33],减少铁表面钝化产物生成.此外,黏土良好的阳离子交换性能可使反应产生的铁离子或其它还原产物吸附到黏土上.EAXFS分析证实了NZVI/Al-bent与Cr(Ⅵ)[32]、Se(Ⅵ)[61]反应后产生的沉淀或钝化产物能部分转移到黏土载体上.pH降低和钝化产物转移均有助于保持零价铁表面活性、延长其反应寿命和重复使用性[61].此外,由于黏土对环境中共存物质具有较好的吸附性能,可以提高零价铁对腐殖酸及常见阴离子(Cl-、NO3-、HCO3-、SO42-)的抗干扰能力[47].
2.3 黏土提高纳米铁去除容量
黏土可以提高负载纳米铁单位铁量对污染物的去除容量.研究表明,黏土中存在三种结合形态的Fe(II),即矿物结构Fe(Ⅱ)、表面端羟基结合Fe(Ⅱ)和层间结合Fe(Ⅱ).后两种结合形态的变化与介质pH有关,当pH<7.5时,主要以还原性较弱的层间结合Fe(Ⅱ)存在,当pH>7.5时,则以还原活性最强的端羟基结合Fe(Ⅱ)为主(还原能力远高于游离态Fe(Ⅱ)).中性介质中零价铁与大多数污染物反应导致pH≥8,此时,作为纳米铁载体的黏土中的端羟基结合Fe(Ⅱ)能还原具有一定氧化性的氯代有机物、硝基苯、U(VI)、Se(VI)等.图6为不同pH缓冲介质中,过量Se(VI)分别与相同铁量NZVI/Al-bent或NZVI的反应结果.在pH=8.0时,NZVI只去除了0.42 mmol Se(VI)、NZVI/Al-bent则能去除0.6 mmol Se(VI),但在pH=6.0和7.0时,负载与未负载纳米铁对Se(VI)的去除量相近[61],表明在合适pH下黏土提高了单位铁量对污染物的去除容量.
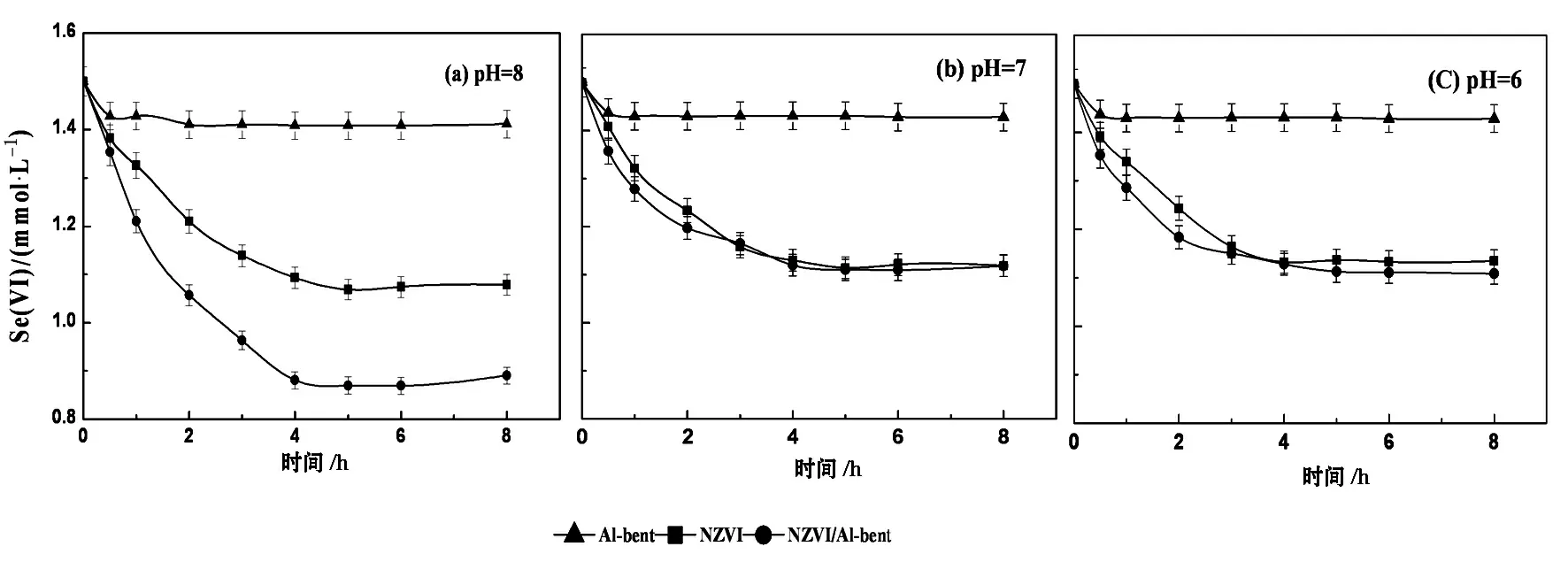
图6[61] 不同pH下负载纳米铁(NZVI/Al-bent)、NZVI(铁量均为2.0 mmol/L)分别与过量Se(VI)反应结果的比较
3 黏土零价铁复合材料在污染物修复中的现场应用
近年来已有学者开展了黏土零价铁复合材料在现场污染物修复的研究,如Olson等[62]利用黏土零价铁复合材料(混合材料中,零价铁和钠基膨润土分别为2%和3%,平均注入深度为7.6 m)现场修复位于美国海军陆战队基地中含有氯代有机物的污染场地.结果表明,膨润土对污染物的富集固定作用有利于零价铁与污染物之间的充分接触,促进了污染物的有效降解和去除,一年后,土壤和地下水中各监测点氯代有机物的平均去除率均分别达到99%和81%.Fjordb等[63]利用膨润土和零价铁复合材料现场修复位于丹麦斯库勒莱乌2号的四氯乙烯污染场地,零价铁的平均加入比例为3.1%,一年后土壤和地下水中四氯乙烯的去除率分别达到99%和76%,主要降解产物为乙烯,及少量的氯乙烯等.与挖掘修复方法相比,该零价铁修复技术的成本更低.例如,修复超过3 000 m3的污染场地,该技术的平均修复费用仅为260 USD/m3.
4 结论与展望
黏土负载纳米零价铁在去除地下水中有机和无机污染物的研究受到越来越多的关注.黏土负载纳米零价铁复合材料不仅无毒且能高效去除有机、无机、重金属污染物.黏土负载纳米零价铁通过调控改性黏土的组成及微界面的物理、化学性质:
1)改性黏土的存在,促进了污染物在零价铁表面的吸附与富集,有利于零价铁与污染物之间的电子转移,从而提高零价铁对污染物的还原去除效率.
2)改性黏土中大量可交换的Si-OH、Al-OH,在反应体系中起pH缓冲作用,减少纳米零价铁的团聚和铁表面腐蚀产物,提高其重复使用性,延长反应寿命.
3)反应过程中吸附在黏土上Fe(Ⅱ)的还原能力远高于游离态的Fe(Ⅱ),前者还能进一步还原污染物,从而提高单位铁的利用率;共同发挥改性黏土在零价铁去除各种污染物中的强化效应.
黏土负载纳米零价铁对污染物的去除效率主要受到反应物初始浓度、反应初始pH、复合材料用量以及实际污染体系共存离子的影响.文献中的研究结果表明改性黏土的存在,使反应体系显示出较强的抗共存组分干扰能力.
大多数对黏土负载纳米零价铁技术的研究还处于实验室规模.但最近已有课题组将零价铁、黏土、生物质材料复合,用于污染场地的实际修复,取得了良好的结果,说明黏土在实际环境应用具有巨大潜力.这为开发新型、高效、经济修复污染场地的活性反应材料提供了理论依据.
参考文献:
[1]JEEN S,GILLHAM R W,PRZEPIORA A. Predictions of Long-Term Performance of Granular Iron Permeable Reactive Barriers: Field-Scale Evaluation[j]. J Contam Hydrol, 2011,123(1-2): 50-64.
[2]HENDERSON A D, DEMOND A H. Long-term performance of zero-valent iron permeable reactive barriers: a critical review[J]. Environmental Engineering Science, 2007,24 (4): 401-423.
[3]DONG J, ZHAO Y S, ZHANG W , et al. Laboratory study on sequenced permeable reactive barrier remediation for landfill leachate-contaminated groundwater[J].Journal of Hazardous Materials, 2009,161 (1): 224-230.
[4]OH Y J, SONG H, SHIN W S, et al. Effect of amorphous silica and silica sand on removal of chromium(VI) by zero-valent iron[J]. Chemosphere, 2007 ,66(5): 858-865.
[5]KOHN T, LIVI K J T, ROBERTS A L, et al. Longevity of granular iron in groundwater treatment processes: corrosion product development[J]. Environmental Science & Technology, 2005,39 (8): 2867-2879.
[6]WANG C B, ZHANG W X. Synthesizing nanoscale iron particles for rapid and complete dechlorination of tce and pcbs[J]. Environmental Science and Technology, 1997,31 (7): 2154-2156.
[7]ZHANG W X. Nanoscale iron particles for environmental remediation: an overview[J]. Journal of Nanoparticle Research, 2003,5 (3): 323-332.
[8]LIU Y, MAJETICH S A, TILTON R D, et al. Tce dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties[J]. Environmental Science & Technology, 2005 39(5): 1338-1345.
[9]PONDER S M, DARAB J G, MALLOUK T E. Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron[J]. Environmental Science and Technology, 2000,34 (12): 2564-2569.
[10]ZHANG H, JIN Z, HANL, et al. Dechlorination of trichloroethylene in solution over supported nano zero valent Fe and Cu/Fe bimetal on exfoliated graphite[J]. Chinese Journal of Geochemistry, 2006 (S1): 132-133.
[11]WANG X, CHEN C, LIU H, et al. Preparation and characterization of PAA/PVDF membrane-immobilized Pd/Fe nanoparticles for dechlorination of trichloroacetic acid[J]. Water Research, 2008,42 (18): 4656-4664.
[12]LI J, CHEN C L, ZHANG R, et al. Nanoscale zero-valent iron particles supported on reduced graphene oxides by using a plasma technique and their application for removal of heavy-metal ions[J]. Chemistry-An Asian Journal, 2015,10 (6): 1410-1417.
[13]朱利中, 陈宝梁. 有机膨润土及其在污染控制中的应用[M].北京:科学出版社, 2006.
[14]朱利中,陈宝梁.膨润土吸附材料在有机污染控制中的应用[J].化学进展,2009,21(Z1):420-429.
[15]PAVLIDOU S, PAPASPYRIDES C D. A review on polymer-layered silicate nanocomposites[J]. Progress in Polymer Science, 2008,33 (12): 1119-1198.
[16]LV P, LIU C, RAO Z. Review on clay mineral-based form-stable phase change materials: preparation, characterization and applications[J]. Renewable and Sustainable Energy Reviews, 2017 (68): 707-726.
[17]CHO H H, LEE T, HUANG S J, et al. Iron and organo-bentonite for the reduction and sorption of trichloroethylene[J]. Chemosphere, 2005,58 (1): 103-108.
[18]ÜZüMÇ, SHAHWAN T, EROGLU A E, et al. Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+and Co2+ions[J]. Applied Clay Science, 2009 ,43(2): 172-181.
[19]ZHANG X, LIN S, CHEN Z, et al. Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+from aqueous solution: reactivity, characterization and mechanism[J]. Water Research, 2011,45 (11): 3481-3488.
[20]GU C, JIA H, LI H, et al. Synthesis of highly reactive subnano-sized zero-valent iron using smectite clay templates[J]. Environmental Science & Technology, 2010,44 (11): 4258-4263.
[21]FAN M, YUAN P, CHEN T, et al. Synthesis, characterization and size control of zerovalent iron nanoparticles anchored on montmorillonite[J]. Chinese Science Bulletin, 2010,55 (11): 1092-1099.
[22]SHAHWAN T,ÜZüMÇ, EROGLU A E, et al. Synthesis and characterization of bentonite/iron nanoparticles and their application as adsorbent of cobalt ions[J]. Applied Clay Science, 2010,47 (3): 257-262.
[23]FROST R L, XI Y, HE H. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption[J]. Journal of Colloid and Interface Science, 2010,341 (1): 153-161.
[24]REN C X, LI Y M, LI J, et al. Immobilization of nanoscale zero valent iron on organobentonite for accelerated reduction of nitrobenzene[J]. Journal of Chemical Technology & Biotechnology, 2014,89 (12): 1961-1966.
[25]LI Y M, LI J F, ZHANG Y. Mechanism insights into enhanced Cr(VI) removal using nanoscale zerovalent iron supported on the pillared bentonite by macroscopic and spectroscopic studies[J]. Journal of Hazardous Materials, 2012 (227): 211-218.
[26]ZHANG Y L, LI Y M, LI J F, et al. Enhanced Cr(VI) removal by using the mixture of pillared bentonite and zero-valent iron[J]. Chemical Engineering Journal, 2012 (185): 243-249.
[27]LI Z F, DONG H P, ZHANG Y L, et al. Enhanced removal of Ni(II) by nanoscale zero valent iron supported on Na-saturated bentonite[J]. Journal of Colloid and Interface Science, 2017 (497): 43-49.
[28]YU K, SHENG G D, MCCALL W. Cosolvent effects on dechlorination of soil-sorbed polychlorinated biphenyls using bentonite clay-templated nanoscale zero valent iron[J] Environmental Science & Technology, 2016 (50): 12949-12956.
[29]YU K, GU C, BOYD S A, et al. Rapid and extensive debromination of decabromodiphenyl ether by smectite clay-templated subnanoscale zero-valent iron[J]. Environmental Science and Technology, 2012 ,46(16): 8969-8975.
[30]BHOWMICK S, CHAKRABORTY S, MONDAL P, et al. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: kinetics and mechanism[J]. Chemical Engineering Journal, 2014 (243): 14-23.
[31]KADU B S, SATHE Y D, INGLE A B, et al. Efficiency and recycling capability of montmorillonite supported Fe-Ni bimetallic nanocomposites towards hexavalent chromium remediation[J]. Applied Catalysis B: Environmental, 2011,104 (4): 407-414.
[32]WU P, LI S, JU L, et al. Mechanism of the reduction of hexavalent chromium by organo-montmorillonite supported iron nanoparticles[J]. Journal of Hazardous Materials, 2012 (219): 283-288.
[33]SHENG G, SHAO X, LI Y M, et al. Enhanced removal of uranium(VI) by nanoscale zerovalent iron supported on Na-bentonite and an investigation of mechanism[J]. The Journal of Physical Chemistry A, 2014 (118): 2952-2958.
[34]SHI L N, ZHANG X, CHEN Z L. Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron[J]. Water Research, 2011,45 (2): 886-892.
[35]ZHANG Y, LI Y, LI J, et al. Enhanced removal of nitrate by a novel composite: Nanoscale zero valent iron supported on pillared clay[J]. Chemical Engineering Journal, 2011 (171): 526-531.
[36]LI Y, ZHANG Y, LI J, et al. Enhanced removal of pentachlorophenol by a novel composite: nanoscale zero valent iron immobilized on organobentonite[J]. Environmental Pollution, 2011 159(12): 3744-3749.
[37]FU R, YANG Y, XU Z, et al. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI)[J]. Chemosphere, 2015 (138): 726-734.
[38]FEI X, CAO L, ZHOU L, et al. Degradation of bromamine acid by nanoscale zero-valent iron (nZVI) supported on sepiolite[J]. Water Science and Technology, 2012,66 (12): 2539-2545.
[39]CHANG Y, HE Y Y, LIU T, et al. Aluminum pillared palygorskite-supported nanoscale zero-valent iron for removal of Cu(II), Ni(II) from aqueous solution[J]. Arabian Journal for Science and Engineering, 2014 (39): 6727-6736.
[40]LUO S, QIN P, SHAO J, et al. Synthesis of reactive nanoscale zero valent iron using rectorite supports and its application for orange II removal[J]. Chemical Engineering Journal, 2013 (223): 1-7.
[41]XI Y F, SUN Z M, HREID T, et al. Bisphenol a degradation enhanced by air bubbles via advanced oxidation using in situ generated ferrous ions from nano zero-valent iron/palygorskite composite materials[J]. Chemical Engineering Journal, 2014 (247): 66-74.
[42]EZZATAHMADI N, AYOKO G A, MILLAR G J, et al. Clay-supported nanoscale zero-valent iron composite materials for the remediation of contaminated aqueous solutions: a review[J]. Chemical Engineering Journal, 2017 (312): 336-350.
[43]WANG J, LIU G J, ZHOU C C, et al. Synthesis, characterization and aging study of kaolinite-supported zero-valent iron nanoparticles and its application for Ni(II) adsorption[J]. Materials Research Bulletin, 2014 (60): 421-432.
[44]SHI L N, ZHOU Y, CHEN Z L, et al. Simultaneous adsorption and degradation of Zn2+and Cu2+from wastewaters using nanoscale zero-valent iron impregnated with clays[J]. Environmental Science and Pollution Research, 2013,20 (6): 3639-3648.
[45]PANG Z H, LIU Y, LUO J, et al. Influence factors on removal of cadmium by montmorillonite supported nano zero-valent iron[J]. Advanced Materials Research, 2013: 539-542.
[46]UZUM C, SHAHWAN T, EROGLU A E, et al. Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+and Co2+ions[J]. Applied Clay Science, 2009 ,43(2): 172-181.
[47]DONG H P, CHEN Y, SHENG G D, et al. The roles of a pillared bentonite on enhancing Se(VI) removal by ZVI and the influence of co-existing solutes in groundwater[J]. Journal of Hazardous Materials, 2016 (304): 306-312.
[48]LIU T Y, WANG Z L, YAN X X, et al. Removal of mercury (II) and chromium (VI) from wastewater using a new and effective composite: pumice-supported nanoscale zero-valent iron[J]. Chemical Engineering Journal, 2014 (245): 34-40.
[49]LO I M C, LAM C S C, LAI K C K. Hardness and carbonate effects on the reactivity of zero-valent iron for Cr(VI) removal[J]. Water Research, 2006 ,40(3): 595-605.
[50]肖爱红. 活性炭负载纳米零价铁去除水中含氧酸盐的研究[D]. 赣州: 江西理工大学, 2015.
[51]童桂华. 去除地下水硝酸盐PRB介质试验研究[D]. 青岛: 中国海洋大学, 2008.
[52]LI J F, LI Y M, MENG Q L. Removal of nitrate by zero-valent iron and pillared bentonite[J]. Journal of Hazardous Materials, 2010 ,174(1): 188-193.
[53]DELLA R C, BELGIORNO V, MERIC S. Heterotrophic/autotrophic denitrification (HAD) of drinking water: prospective use for permeable reactive barrier[J]. Desalination, 2006,210 (1): 194-204.
[54]FU F L, DIONYSIOU D D, LIU H. The use of zero-valent iron for groundwater remediation and wastewater treatment: a review[J]. Journal of Hazardous Materials, 2014 (267): 194-205.
[55]FAN J, GUO Y H, WANG J J, et al. Rapid decolorization of azo dye methyl orange in aqueous solution by nanoscale zerovalent iron particles[J]. Journal of Hazardous Materials, 2009,166 (2): 904-910.
[56]CHEN Z X, JIN X Y, CHEN Z L, et al. Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron[J]. Journal of Colloid and Interface Science, 2011,363 (2): 601-607.
[57]YUAN N, ZHANG G K, GUO S, et al. Enhanced ultrasound-assisted degradation of methyl orange and metronidazole by rectorite-supported nanoscale zero-valent iron[J] Ultrasonics Sonochemistry, 2016 (28): 62-68.
[58]ZHANG Y, LI Y, ZHENG X. Removal of atrazine by nanoscale zero valent iron supported on organobentonite[J]. Science of The Total Environment, 2011,409 (3): 625-630.
[59]LI Y M, ZHANG Y, LI J F, et al. Enhanced reduction of chlorophenols by nanoscale zerovalent iron supported on organobentonite[J]. Chemosphere, 2013 ,92(4): 368-374.
[60]SU J, MINEGISHI T, KATAYAMA M, et al. Photoelectrochemical hydrogen evolution from water on a surface modified CdTe thin film electrode under simulated sunlight[J] Journal of Materials Chemistry A, 2017 (5): 4486-4492.
[61]LI Y M, CHENG W, SHENG G D, et al. Synergetic effect of a pillared bentonite support on Se(VI) removal by nanoscale zero valent iron[J]. Applied Catalysis B: Environmental, 2015 (174): 329-335.
[62]OLSON M R, SALE T C, SHACKELFORD C D, et al. Chlorinated solvent source-zone remediation via ZVI-clay soil mixing: 1-year results[J]. Ground Water Monitoring and Remediation, 2012 .32(3): 63-74.
[63]FJORDB A S, RIIS C, CHRISTENSEN A G, et al. ZVI-clay remediation of a chlorinated solvent source zone, skuldelev, denmark: 1. site description and contaminant source mass reduction[J]. Journal of Contaminant Hydrology, 2012 (140): 56-66.
