Cell cycle and complement inhibitors may be speci fic for treatment of spinal cord injury in aged and young mice: transcriptomic analyses
2018-04-04MingHaoXinranJiHuaChenWeiZhangLichengZhangLihaiZhangPeifuTangNingLu
Ming Hao, Xin-ran Ji, Hua Chen, Wei Zhang, Li-cheng Zhang, Li-hai Zhang, Pei-fu Tang, Ning Lu
Department of Orthopedic Surgery, General Hospital of People’s Liberation Army (301 Hospital), Beijing, China
Introduction
Spinal cord injury (SCI) is a common traumatic event in orthopedic clinics due to rapid industrial and economic development in China, with an estimated incidence of 23.7 per million cases in Tianjin, 25 in Shanghai, and 60 in Beijing (Hua et al., 2013). SCI results in severe or permanent motor, sensory and autonomic dysfunction, which affects a patient’s quality of life and imposes a huge economic burden on family and society (Krueger et al., 2013; Ravensbergen et al., 2016; Zhang et al., 2016b; Rabchevsky et al., 2017). More importantly, recent studies have suggested that the pathological and behavioral outcomes after SCI may be age-dependent, with elderly patients exhibiting markedly less remyelination compared with younger patients, which consequently leads to worsened functional recovery and a higher mortality rate (Siegenthaler et al., 2008; Wilson et al., 2014). Thus, distinguishing the cellular and molecular response mechanisms in aged and young people is necessary to develop targeted treatments.
Recently, the role of aging following SCI was investigated(Geoffroy et al., 2016). Accordingly, the number of M1 macrophages at the injury epicenter was increased by 50% in aged compared with young rats (Hooshmand et al., 2014), while M2 macrophages were reduced (Zhang et al., 2015), thereby inducing apoptotic cell death and greater locomotor deficits.Similarly, a lower number of in filtrating neutrophils and secreted pro-inflammatory cytokines/chemokines (e.g., interleukin 6; tumor necrosis factor α; and C-X-C motif chemokine ligand 1) were detected in microglia from young compared with adult mice (Kumamaru et al., 2012). Further studies suggest that inflammatory activation may be NADPH oxidase (NOX)- (Zhang et al., 2016) or adipokine-mediated (Bigford et al., 2012) in chronic SCI and advanced age, with high expression of NOX2 and the leptin signaling inhibitor, suppressor of cytokine signaling 3 (SOCS3), as well as lower long-form leptin receptor(LepRb) and Janus kinase 2/signal transducer and activator of transcription 3 Jak2/Stat3 signaling. High throughput analysis of gene expression pro files between aged and young rats following SCI has also been performed. Cortical transcriptome analysis of the left hemisphere suggests that genes enriched in biological processes such as apoptosis (1 day post-operation), activation of immune responses (7 days post-operation), and cell cycle and cell adhesion (35 days post-operation) may be speci fic to aged animals (Jaerve et al., 2012). However, specific treatments for aged and young SCI patients are not fully understood.
In this study, we aimed to further investigate gene expression differences in the injured spinal cord between aged and young mice using microarray data downloaded from the Gene Expression Omnibus (GEO) database (Takano et al., 2017). A total of 364 differential genes between aged and young mice were identified in the study by Takano et al. (2017), among which 169 down-regulated genes were involved in regulation of synapse-, ion transport-, or axon-related functions,while 195 up-regulated genes were involved in the cell cycle,cell stress responses, or maintenance of extracellular matrix(Takano et al., 2017). Shared or unique differentially expressed genes (DEGs) for aged and young mice were not identified.Thus, our study focused on screening crucial genes and pathways for aged and young mice, and as a result, is able to suggest targeted treatments.
Materials and Methods
Animals
Ten female C57BL/6J mice (young, 2—3 months old, n = 6;aged, 15—18-months old, n = 4) were housed in groups under 12-hour light/dark cycles with free access to food and water.All protocols were approved by the Institutional Animal Care and Use Committee of Keio University School of Medicine,Japan, and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Keio University School of Medicine, Japan.
Young and aged mice were randomly assigned to undergo SCI or control treatment: young injured mice (n = 3), young normal mice (n = 3), aged injured mice (n = 3), and aged normal mice (n= 3). SCI model was induced using a commercially available SCI device (In finite Horizon Impactor, 70-kdyn; Precision Systems& Instrumentation, Fairfax Station, VA, USA) at the thoracic level, Th9. Spinal cord samples were collected nine days after injury (Takano et al., 2017). Injured mice exhibiting low Basso Mouse Scale scores indicate successful model establishment(Takano et al., 2017). Normal mice underwent no treatment.
Microarray data
SCI microarray data were extracted from the GEO database(http://www.ncbi.nlm.nih.gov/geo/) using the accession number, GSE93561 (Takano et al., 2017). This contains spinal cord samples from three young injured mice (GSM2454721_AG1408, GSM2454722_AG1409, and GSM2454723_AG1410), three young normal mice (GSM2454718_AG1405,GSM2454719_AG1406, and GSM2454720_AG1407), three aged injured mice (GSM2454727_AG1414, GSM2454728_AG1415, and GSM2454729_AG1416), and three aged normal mice (GSM2454724_AG1411, GSM2454725_AG1412, and GSM2454726_AG1413). Because of its expression, sample GSM2454729_AG1416 was deemed “not available”. Consequently, this aged injured sample and the corresponding aged normal sample (GSM2454726_AG1413) were removed from the study. As a result, the study ultimately included: young injured mice (n = 3), young normal mice (n = 3), aged injured mice (n = 2), and aged normal mice (n = 2).
Data normalization and DEG identi fication
Raw CEL files were preprocessed and normalized using the Robust Multichip Average algorithm (Irizarry et al., 2003) as implemented in the Bioconductor R package (http://www.bioconductor.org/packages/release/bioc/html/affy.html).DEGs between injured and control samples were screened using the Linear Models for Microarray data method (Ritchie et al., 2015), also in the Bioconductor R package (http://www.bioconductor.org/packages/release/bioc/html/limma.html).After performing t-tests, P-values were adjusted by the Benjamini-Hochberg algorithm (Thissen, 2002). Adjusted P < 0.05 and |logFC(fold change)| > 1.5 were set as threshold values.A Venn diagram was constructed to show unique or shared genes in aged and young injured mice using an online tool(http://bioinformatics.psb.ugent.be/webtools/Venn/).
Protein–protein interaction (PPI) network construction
To screen crucial genes associated with SCI (aged or young),DEGs were mapped onto PPI data collected from the SearchTool for the Retrieval of Interacting Genes (STRING) 10.0 database (http://string db.org/) (Szklarczyk et al., 2015). Combined scores > 800 were set as cut-off values for identifying signi ficant protein pairs for constructing PPI networks. These were then visualized using Cytoscape software 2.8 (www.cytoscape.org/) (Kohl, 2011). To identify functionally related and highly interconnected clusters from PPI networks, module analysis was performed using the Molecular Complex Detection plugin of Cytoscape software, with a degree cutoff of 5,node score cutoff of 0.5, k-core of 5, and maximum depth of 100 (ftp://ftp.mshri.on.ca/pub/BIND/Tools/MCODE) (Bader and Hogue, 2003). Significant modules were identified with Molecular Complex Detection scores ≥ 4 and nodes ≥ 6.
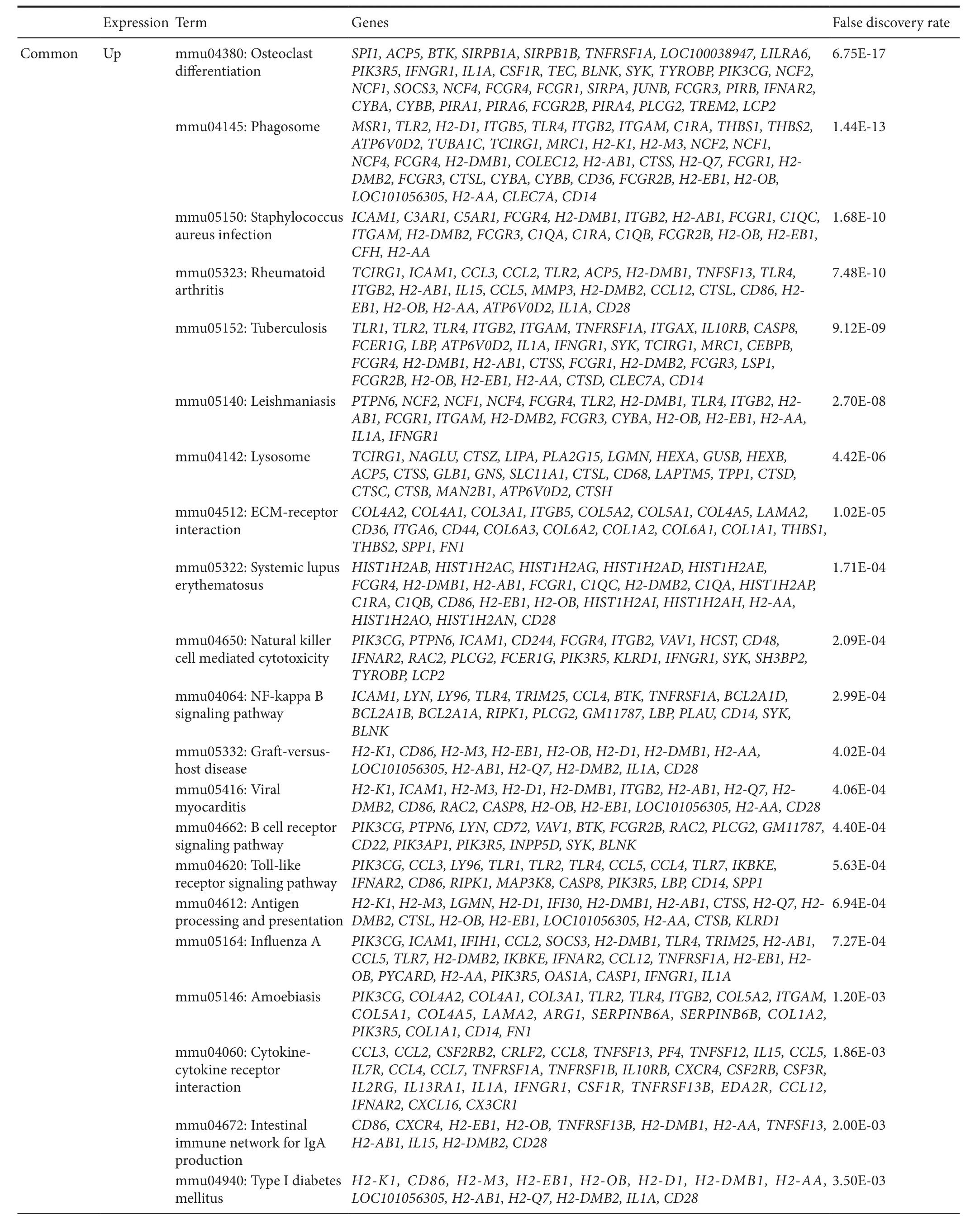
Table 1 KEGG pathway enrichment for differentially expressed genes in spinal cord tissue of aged and young injured mice

Table 1 Continued

Figure 1 Heat map of differentially expressed genes between young/aged spinal cord injury and normal control mice.

Figure 2 Venn diagram of differentially expressed genes between young/aged spinal cord injury and normal control mice.
Functional enrichment analysis
Kyoto encyclopedia of genes and genomes (KEGG) pathway and Gene Ontology (GO) enrichment analyses were performed to investigate the potential function of all DEGs (shared or unique DEGs), or genes in modules using The Database for Annotation,Visualization and Integrated Discovery (DAVID) 6.8 online tool(http://david.abcc.ncifcrf.gov). False discovery rate < 0.05 was chosen as the cut-off point for GO and KEGG analyses.
Results
Identi fication of DEGs in aged and young SCI mice
Based on a threshold of adjusted P < 0.05 and |logFC| > 1.5,a relatively higher number of DEGs were identi fied after SCI in aged mice (1,604: 952 up-regulated and 652 down-regulated) compared with young mice (1,153: 721 up-regulated and 432 down-regulated). These genes clearly differentiated the samples (Figure 1). Further, Venn diagram showed 960 shared DEGs between young and aged injured groups (640 up-regulated and 320 down-regulated), suggesting these genes are important for development of SCI. Additionally, 644 (312 up-regulated and 332 down-regulated) and 193 (81 up-regulated and 112 down-regulated) DEGs were unique for the aged and young injured groups, respectively, suggesting these genes are age-dependent (Figure 2).

Figure 3 Modules obtained from protein–protein interaction networks of shared differentially expressed genes between young/aged spinal cord injury and normal control mice.
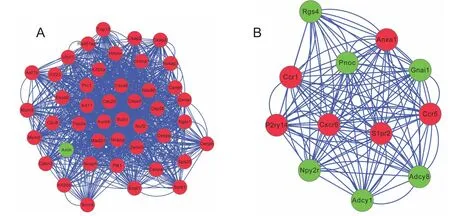
Figure 4 Modules obtained from protein–protein interaction networks of unique differentially expressed genes for aged spinal cord injury mice.
Functional enrichment analysis of shared and unique DEGs
Shared and unique DEGs were subjected to functional enrichment analysis using the online tool DAVID, with the mouse genome as background and false discovery rate < 0.05 as the cut-off point. For shared up-regulated DEGs, 33 KEGG pathways were enriched including osteoclast differentiation,phagosome, extracellular matrix (ECM)—receptor interaction,nuclear factor-kappa B (NF-κB) signaling pathway, cytokine-cytokine receptor interaction, and focal adhesion. Further, nine pathways were identi fied for shared down-regulated DEGs, including synaptic vesicle cycle and glutamatergic syn-apse (Table 1). Furthermore, three pathways showed enrichment in unique genes of the aged injured group: up-regulated(cell cycle and lysosome) and down-regulated (cholinergic synapse). While four pathways were enriched in unique genes of the young injured group: up-regulated (pertussis, and complement and coagulation cascades) and down-regulated (nicotine addiction and retrograde endocannabinoid signaling).

Table 2 Signi ficant functional modules from protein–protein interaction networks constructed by shared or unique differentially expressed genes in spinal cord tissue of aged and young injured mice
PPI network construction and module analysis for shared and unique DEGs
PPI networks were constructed after mapping shared or unique DEGs onto PPI data. For shared DEGs, four signi ficant modules were screened from the PPI network (Figure 3andTable 2).Module 1 was involved in neuroactive ligand—receptor interaction, module 2 in ECM-receptor interaction, focal adhesion,and phosphoinositide 3-kinase (PI3K)-Akt signaling pathway-related, and module 4 in osteoclast differentiation and NF-κB signaling pathway-associated (Table 3). In the aged injured group, two significant modules were screened from the PPI network for unique DEGs (Figure 4), with module 1 involved in the cell cycle and module 2 in the chemokine signaling pathway. No pathways or significant pathways were enriched in module 3 of shared and unique DEGs from the young injured group (Figure 5). Moreover, GO analysis indicated that unique DEGs in the young injured group exert effects on SCI via inflammatory processes (Table 2).
Discussion
By integrating functional analyses of all DEGs and module genes,our present study preliminarily demonstrates that cell cycle (including polo like kinase 1 [PLK1], cell division cycle 6 [CDC6];cell division cycle 20 [CDC20], and BUB1 mitotic checkpoint serine/threonine kinase [BUB1]) and complement-related genes(including complement C3 [C3]) may be specifically altered in spinal cord of aged and young injured mice, respectively. All DEGs were up-regulated, consequently use of cell cycle and complement pathway inhibitors may be potential treatment measures for aged and young SCI patients. Indeed, our hypothesis has been indirectly demonstrated by previous studies.
Increasing evidence, including gene expression profiles in spinal cord (Di, 2003), indicate that cell cycle activation plays an important role in the pathophysiology of SCI (Wu et al.,2011). First, cell cycle activation contributes to neuronal and oligodendroglial apoptosis after SCI (postmitotic cells) (Byrnes et al., 2007). Further, it also promotes microglial proliferation(mitotic cells), which produce pro-inflammatory cytokines and cause functional deficits (Tian et al., 2007a, b). Cell cycle-related proteins, such as cyclin D1, cyclin dependent kinase 4 (CDK4), and proliferating cell nuclear antigen are all signi ficantly up-regulated following SCI (Wu et al., 2012,2014). Moreover, systemic administration of CDK inhibitors,such as olomoucine, flavopiridol, or CR8, suppresses these processes and improves neurodegeneration and neuropathic pain (Ren et al., 2014; Wu et al., 2016). However, whether cell cycle activation is specific for aged SCI (Jaerve et al., 2012),and whether there are treatment differences in CDK inhibitors for aged and young mice is unclear and needs further confirmation. Human PLK1 is an evolutionarily conserved serine/threonine kinase that regulates cell division at the M phase. PLK1 can phosphatase CDC6 (Yim and Erikson, 2010),Cdc25C (Toyoshimamorimoto et al., 2002), CDC20 (Jia et al.,2016), CCD14B (Bassermann et al., 2008), BUB1 (Qi et al.,2006), BubR1 (BUB1-related) (Elowe et al., 2007), and CDK5 regulatory subunit associated protein 2 (CDK5RAP2) (Hanafusa et al., 2015) to promote spindle checkpoint signaling.Use of PLK1 inhibitors, such as RO3280 (Wang et al., 2015),GSK461364 (Chou et al., 2016; Pajtler et al., 2017), and BI2536(Frost et al., 2012; Kumar et al., 2015), induces cell cycle arrest and growth inhibition, enabling treatment of various diseases.In our PPI network, we found that PLK1 interacts with 44 DEGs, including CDC20, CDC6, and BUB1. These findings suggest a possible crucial role of PLK1 in SCI and an underlying therapeutic effect for PLK1 inhibitors. Unfortunately,there are no experimental studies that con firm our conclusion,but this may be a new direction for our future studies.
Classical (C1q and C4), alternative (Factor B), and terminal(C5b-9) complement pathways in neurons and oligodendrocytes are suggested to initiate an inflammatory cascade and induce secondary injury and functional de ficits following traumatic SCI (Anderson et al., 2004). Mice with a de ficiency in thecomplement component C1q (Galvan et al., 2008), C3 (Qiao et al., 2006), complement receptor 2, and complement receptor C5aR (Li et al., 2014; Brennan et al., 2015), or treated with complement antagonists (Qiao et al., 2006; Li et al., 2009; Brennan et al., 2016; Biggins et al., 2017) exhibit improved functional outcomes. As expected, complement activation was detected in SCI mice in our study. More importantly, our study reveals that this pathway may be speci fic to young injured mice, although this is not consistent with a previous study (Jaerve et al., 2012).We believe this may be due to the following reasons: (1) Jaerve et al., (2012) investigated cortical samples from the left hemisphere, which was the resulting site induced by SCI. Thus, a delayed effect may be present; (2) our sample size is small; and(3) C1qb, C3, and C4 are early complement proteins after SCI,while C5, C6, C7, and C9 are terminal complement proteins after SCI (Nguyen et al., 2008). Therefore, we speculate that different gene expression pro files may explain the different phenomena. As anticipated, C3 (as a complement activation pathway gene) was signi ficantly up-regulated in young SCI mice in our study. Accordingly, the C3 inhibitor, CR2-Crry (Qiao et al., 2006), may be an effective treatment for young SCI patients.Nevertheless, further con firmation is still needed.

Table 3 KEGG pathway enrichment for functional modules screened from protein–protein interaction networks constructed by shared or unique differentially expressed genes in spinal cord tissue of aged and young injured mice
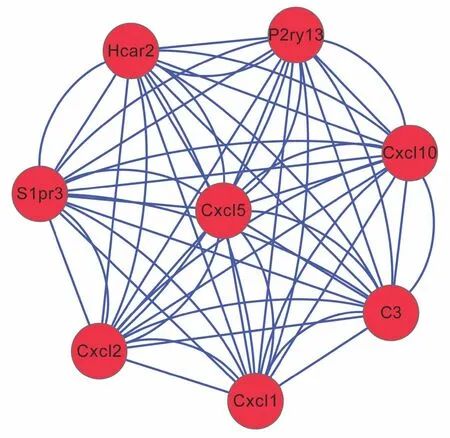
Figure 5 Module obtained from protein–protein interaction network of unique differentially expressed genes for young spinal cord injury mice.
In addition to unique DEGs, we also found several shared between aged and young injured mice. These DEGs are involved in the NF-κB signaling pathway, indicating that these genes and pathway may be important for SCI, regardless of age. These findings are in accordance with previous studies. For example, NF-κB and related in flammatory cytokines were up-regulated in the injured rat spinal cord (Ni et al., 2015; Yarar-Fisher et al., 2016).Treatment with hyperbaric oxygen (Yang et al., 2013; Kang et al.,2015), curcumin (Ni et al., 2015), and butein (Ming et al., 2013)ameliorated SCI-induced hindlimb locomotion de ficits, spinal cord edema, and apoptosis by down-regulating the toll-like receptor 4 (TLR4)/NF-κB in flammatory signaling pathway.
In conclusion, our present study reveals preliminarily findings showing differences in speci fic genes in aged and young injured mice. Cell cycle- (PLK1) and complement (C3)-related gene inhibitors may be more effective for treatment of SCI in aged and young mice, respectively. However, further in vivo experimental studies are needed to con firm our findings due to small sample size, which is a limitation of our study.
Author contributions:PFT and NL designed this study. XRJ, HC, LHZ and WZ performed experiments. LCZ analyzed data. XRJ and MH wrote the paper. All authors approved the final version of the paper.
Con flicts of interest:None declared.
Financial support:TThis study was supported by the National Science Fund for Distinguished Young Scientists of China, No. 81601052. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Research ethics:The study protocol was approved by the Institutional Animal Care and Use Committee of Keio University School of Medicine, Japan.The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Additional file:
Additional file 1: Abbreviations in Tables 1–3 and Figures 3, 4.
Anderson AJ, Robert S, Huang W, Young W, Cotman CW (2004) Activation of complement pathways after contusion-induced spinal cord injury. J Neurotrauma 21:1831-1846.
Bader GD, Hogue CW (2003) An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics 4:2.
Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M (2008) The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 134:256-267.
Bigford GE, Bracchiricard VC, Nash MS, Bethea JR (2012) Alterations in mouse hypothalamic adipokine gene expression and leptin signaling following chronic spinal cord injury and with advanced age. PLoS One 7:e41073.
Biggins P, Brennan F, Taylor S, Woodruff T, Ruitenberg M (2017) The alternative receptor for complement component 5a, C5aR2, conveys neuroprotection in traumatic spinal cord injury. J Neurotrauma 34:2075-2085.
Brennan FH, Gordon R, Lao HW, Biggins PJ, Taylor SM, Franklin RJ,Woodruff TM, Ruitenberg MJ (2015) The complement receptor C5aR controls acute in flammation and astrogliosis following spinal cord injury. J Neurosci 35:6517-6531.
Brennan FH, Kurniawan ND, Jana V, Bartlett PF, Fabian K, Arumugam TV,Milan B, Ruitenberg MJ (2016) IVIg attenuates complement and improves spinal cord injury outcomes in mice. Ann Clin Transl Neurol 3:495-511.
Byrnes KR, Stoica BA, Fricke S, Di GS, Faden AI (2007) Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain 130:2977-2992.
Chou YS, Yen CC, Chen WM, Lin YC, Wen YS, Ke WT, Wang JY, Liu CY,Yang MH, Chen TH, Liu CL (2016) Cytotoxic mechanism of PLK1 inhibitor GSK461364 against osteosarcoma: Mitotic arrest, apoptosis, cellular senescence, and synergistic effect with paclitaxel. Int J Oncol 48:1187-1194.
Di Giovanni S1, Knoblach SM, Brandoli C, Aden SA, Hoffman EP, Faden AI (2003) Gene pro filing in spinal cord injury shows role of cell cycle in neuronal death. Ann Neurol 53:454-468.
Elowe S, Hümmer S, Uldschmid A, Li X, Nigg EA (2007) Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore—microtubule interactions. Genes Dev 21:2205-2219.
Frost A, Mross K, Steinbild S, Hedbom S, Unger C, Kaiser R, Trommeshauser D, Munzert G (2012) Phase I study of the Plk1 inhibitor BI 2536 administered intravenously on three consecutive days in advanced solid tumours. Curr Oncol 19:28-35.
Galvan MD, Luchetti S, Burgos AM, Nguyen HX, Hooshmand MJ, Hamers FP, Anderson AJ (2008) De ficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury. J Neurosci 28:13876-13888.
Geoffroy CG, Meves JM, Zheng B (2016) The age factor in axonal repair after spinal cord injury: a focus on neuron-intrinsic mechanisms. Neurosci Lett 652:41-49.
Hanafusa H, Kedashiro S, Tezuka M, Funatsu M, Usami S, Toyoshima F, Matsumoto K (2015) PLK1-dependent activation of LRRK1 regulates spindle orientation by phosphorylating CDK5RAP2. Nat Cell Biol 17:1024-1035.
Hooshmand MJ, Galvan MD, Partida E, Anderson AJ (2014) Characterization of recovery, repair, and in flammatory processes following contusion spinal cord injury in old female rats: is age a limitation? Immun Ageing 11:15.
Hua R, Shi J, Wang X, Yang J, Zheng P, Cheng H, Li M, Dai G, An Y (2013)Analysis of the causes and types of traumatic spinal cord injury based on 561 cases in China from 2001 to 2010. Spinal Cord 51:218-221.
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U,Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249-264.
Jaerve A, Kruse F, Malik K, Hartung HP, Müller HW (2012) Age-dependent modulation of cortical transcriptomes in spinal cord injury and repair.PLoS One 7:e49812.
Jia L, Li B, Yu H (2016) The Bub1-Plk1 kinase complex promotes spindle checkpoint signalling through Cdc20 phosphorylation. Nat Commun 7:10818.
Kang N, Hai Y, Yang J, Liang F, Gao CJ (2015) Hyperbaric oxygen intervention reduces secondary spinal cord injury in rats via regulation of HMGB1/TLR4/NF-κB signaling pathway. Int J Clin Exp Patho 8:1141-1153.
Kohl MW, S. Warscheid, B. (2011) Cytoscape: software for visualization and analysis of biological networks. Methods Mol Biol 696:291-303.
Krueger H, Noonan VK, Trenaman LM, Joshi P, Rivers CS (2013) The economic burden of traumatic spinal cord injury in Canada. Chronic Dis Inj Can 33:113-122.
Kumamaru H, Saiwai H, Ohkawa Y, Yamada H, Iwamoto Y, Okada S (2012)Age-related differences in cellular and molecular pro files of in flammatory responses after spinal cord injury. J Cell Physiol 227:1335-1346.
Kumar BNP, Rajput S, Bharti R, Parida S, Mandal M (2015) BI2536—A PLK inhibitor augments paclitaxel efficacy in suppressing tamoxifen induced senescence and resistance in breast cancer cells. Biomed Pharmacother 74:124-132.
Li L, Li J, Zhu Y, Fan G (2009) Ephedra sinica inhibits complement activation and improves the motor functions after spinal cord injury in rats.Brain Res Bull 78:261-266.
Li L, Xiong ZY, Qian ZM, Zhao TZ, Feng H, Hu S, Hu R, Ke Y, Lin J (2014)Complement C5a is detrimental to histological and functional locomotor recovery after spinal cord injury in mice. Neurobiol Dis 66:74-82.
Ming L, Shouyu W, Xin H, Decheng L (2013) Butein inhibits NF-κB activation and reduces in filtration of in flammatory cells and apoptosis after spinal cord injury in rats. Neurosci Lett 542:87-91.
Nguyen HX, Galvan MD, Anderson AJ (2008) Characterization of early and terminal complement proteins associated with polymorphonuclear leukocytes in vitro and in vivo after spinal cord injury. J Neuroin flammation 5:26.
Ni H, Jin W, Zhu T, Wang J, Yuan B, Jiang J, Liang W, Ma Z (2015) Curcumin modulates TLR4/NF-κB in flammatory signaling pathway following traumatic spinal cord injury in rats. J Spinal Cord Med 38:199-206.
Pajtler KW, Sadowski N, Ackermann S, Althoff K, Schönbeck K, Batzke K, Schäfers S, Odersky A, Heukamp L, Astrahantseff K, Künkele A DH,Schramm A, Sprüssel A, Thor T, Lindner S, Eggert A, Fischer M, Schulte JH. (2017) The GSK461364 PLK1 inhibitor exhibits strong antitumoral activity in preclinical neuroblastoma models. Oncotarget 8:6730-6741.
Qi W, Tang Z, Yu H (2006) Phosphorylation- and Polo-Box—dependent binding of Plk1 to Bub1 is required for the kinetochore localization of Plk1. Mol Biol Cell 17:3705-3716.
Qiao F, Atkinson C, Song H, Pannu R, Singh I, Tomlinson S (2006) Complement plays an important role in spinal cord injury and represents a therapeutic target for improving recovery following trauma. Am J Pathol 169:1039-1047.
Rabchevsky AG, Patel SP, Sullivan PG (2017) Targeting mitoNEET with pioglitazone for therapeutic neuroprotection after spinal cord injury. Neural Regen Res 12:1807-1808.
Ravensbergen HJ, De GS, Post MW, Bongers-Janssen HM, Lh VDW, Claydon VE (2016) Is there an association between markers of cardiovascular autonomic dysfunction at discharge from rehabilitation and participation one and five years later in individuals with spinal cord injury? Arch Phys Med Rehabil 97:1431-1439.
Ren H, Han M, Zhou J, Zheng ZF, Lu P, Wang JJ, Wang JQ, Mao QJ, Gao JQ, Ouyang HW (2014) Repair of spinal cord injury by inhibition of astrocyte growth and in flammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials 35:6585-6594.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015)limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47.
Siegenthaler MM, Ammon DL, Keirstead HS (2008) Myelin pathogenesis and functional deficits following SCI are age-associated. Exp Neurol 213:363-371.
Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C (2015) STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43:D447-452.
Takano M, Kawabata S, Shibata S, Yasuda A, Nori S, Tsuji O, Nagoshi N,Iwanami A, Ebise H, Horiuchi K, Okano H, Nakamura M (2017) Enhanced functional recovery from spinal cord injury in aged mice after stem cell transplantation through hgf induction. Stem Cell Reports 8:509-518.
Thissen DS, L. Kuang, D. (2002) A Modi fied benjamini-hochberg multiple comparisons procedure for controlling the false discovery rate. J Educ Behav Stat 27:77-83.
Tian DS, Dong Q, Pan DJ, He Y, Yu ZY, Xie MJ, Wang W (2007a) Attenuation of astrogliosis by suppressing of microglial proliferation with the cell cycle inhibitor olomoucine in rat spinal cord injury model. Brain Res 1154:206-214.
Tian DS, Xie MJ, Yu ZY, Zhang Q, Wang YH, Chen B, Chen C, Wang W(2007b) Cell cycle inhibition attenuates microglia induced in flammatory response and alleviates neuronal cell death after spinal cord injury in rats. Brain Res 1135:177-185.
Toyoshimamorimoto F, Taniguchi E, Nishida E (2002) Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep 3:341-348.
Wang NN et al. (2015) Molecular targeting of the oncoprotein PLK1 in pediatric acute myeloid leukemia: RO3280, a novel PLK1 inhibitor, induces apoptosis in leukemia cells. Int J Mol Sci 16:1266-1292.
Wilson JR, Davis AM, Kulkarni AV, Kiss A, Frankowski RF, Grossman RG,Fehlings MG (2014) Defining age-related differences in outcome after traumatic spinal cord injury: analysis of a combined, multicenter dataset.Spine J 14:1192-1198.
Wu J, Stoica BA, Faden AI (2011) Cell cycle activation and spinal cord injury. Neurotherapeutics 8:221-228.
Wu J, Stoica BA, Dinizo M, Pajooheshganji A, Piao C, Faden AI (2012) Delayed cell cycle pathway modulation facilitates recovery after spinal cord injury. Cell Cycle 11:1782-1795.
Wu J, Zhao Z, Zhu X, Renn CL, Dorsey SG, Faden AI (2016) Cell cycle inhibition limits development and maintenance of neuropathic pain following spinal cord injury. Pain 157:488-503.
Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, Skovira J, Faden AI (2014) Spinal cord injury causes brain in flammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci 34:10989-11006.
Yang J, Liu X, Zhou Y, Wang G, Gao C, Su Q (2013) Hyperbaric oxygen alleviates experimental (spinal cord) injury by downregulating HMGB1/NF-κB expression. Spine (Phila Pa 1976) 38:E1641-1648.
Yarar-Fisher C, Bickel CS, Kelly NA, Stec MJ, Windham ST, Mclain AB, Oster RA, Bamman MM (2016) Heightened TWEAK-NF-kB signaling and in flammation-associated fibrosis in paralyzed muscles of men with chronic spinal cord injury. Am J Physiol Endocrinol Metab 310:E754-761.
Yim H, Erikson RL (2010) Cell division cycle 6, a mitotic substrate of polo-like kinase 1, regulates chromosomal segregation mediated by cyclin-dependent kinase 1 and separase. Proc Natl Acad Sci U S A 107:19742-19747.
Zhang B, Bailey WM, Braun KJ, Gensel JC (2015) Age decreases macrophage IL-10 expression: implications for functional recovery and tissue repair in spinal cord injury. Exp Neurol 273:83-91.
Zhang B, Bailey WM, Mcvicar AL, Gensel JC (2016a) Age increases reactive oxygen species production in macrophages and potentiates oxidative damage after spinal cord injury. Neurobiol Aging 47:157-167.
Zhang W, Zhu XQ, Zhang DC (2016b) Transplantation of bone marrow mesenchymal stem cells overexpressing Shootin1 for treatment of spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu 20:7507-7517.
杂志排行
中国神经再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Cerebral ischemia and neuroregeneration
- SNARE complex in axonal guidance and neuroregeneration
- Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease have impaired microglial function and defective repair of myelin damage
- The relaxin peptide family – potential future hope for neuroprotective therapy? A short review
- Roles of neural regeneration in memory pharmacology
