Cell proliferation during hair cell regeneration induced by Math1 in vestibular epithelia in vitro
2018-04-04YiboHuangRuiMaJuanmeiYangZhaoHanNingCongZhenGaoDongdongRenJingWangFangluChi
Yi-bo Huang Rui Ma Juan-mei Yang Zhao Han Ning Cong Zhen Gao Dongdong Ren Jing WangFang-lu Chi
1 Department of Otology and Skull Base Surgery, EYE & ENT Hospital of Fudan University, Shanghai, China
2 Shanghai Clinical Medical Center of Hearing Medicine, Shanghai, China
3 Key Laboratory of Hearing Medicine, Ministry of Health, Shanghai, China
Introduction
Mammalian sensorineural hearing loss and some balance diseases are incurable. The most important reason for this is that cochlear hair cells cannot be regenerated, and only a few balance cells can be spontaneously regenerated. However,Math1over-expression-induced robust production of hair cells provides the potential to develop a strategy for functional hair cell regeneration (Zheng and Gao, 2000; Kawamoto et al., 2003; Staecker et al., 2007; Izumikawa et al., 2008; Han et al., 2010). Induced by Math1, stem cells, cochlear and vestibular cells have the capacity to differentiate into new hair cells(Zheng and Gao, 2000; Kawamoto et al., 2003; Staecker et al.,2007; Gubbels et al., 2008; Huang et al., 2009; Han et al., 2010;Jeon et al., 2011). However, new hair cells cannot be regener-ated using Math1gene transfer (Izumikawa et al., 2008). This then leads to the question: what types of cells have the potential to differentiate into new hair cells induced by Math1? No con firmed factors have been reported to date.
In birds, hair cells can be spontaneously regenerated after injury. Two means of regeneration have been reported (Stone and Cotanche, 2007): the direct transdifferentiation of supporting cells into hair cells and supporting cell proliferation with the subsequent conversion into hair cells. In the mammalian embryonic stage, hair cells are generated by transdifferentiation with a lack of cell division and Math1playing a role in cell fate determination (Chen et al., 2002; Woods et al., 2004);After birth, in vivo cochlear hair cells could not be regenerated spontaneously, while in vitro supporting cells divided and transdifferentiated into hair cells after dissociation (White et al., 2006). Spontaneous regeneration or repair of balance hair cells with proliferation was observed in vitro and in vivo (Forge et al., 1993; Warchol et al., 1993). Previous studies showed that cells successfully differentiated into new hair cells by Math1had a high proliferative ability (Zheng and Gao, 2000; Gubbels et al., 2008; Huang et al., 2009; Han et al., 2010). The current study explored the relationship between Math1induced hair cell regeneration and cell division in mammals.
Materials and Methods
Animals
All animal experiments were performed under the guideline of the Ethics Committee Protocols of the Eye and Ear,Nose and Throat Hospital of Fudan University of China, and approved by the Chinese Science Academy Committee on Care and Use of Animals. The day when male speci fic-pathogen-free C57BL/6 mice (provided by Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China; license No. SCXK(Hu) 2012-0002) were born was designated P0, the next day as P1, and P2, P3, and P4. P2—4 mice were used in this study.
Sample collection
A detailed protocol on dissecting vestibular end organs was previously reported (Huang et al., 2009). The dissection process was carried out in a sterile environment and samples were placed in chilled D-Hank’s solution. Two fine forceps (0.1 mm at the point end; Dumont Biology, La Sagne,Switzerland), pairs of Vannas scissors and iris scissors, and stainless steel needles were used.
The heads of postnatal mice were removed and bisected through the midline. The brain tissue was removed with forceps. Utricle and cristae were harvested together, and attached to cover-slips pretreated with poly-L-lysine (Sigma, St. Louis, MO, USA). With the forceps, the otolithic membrane and nerve fibers at the back of the epithelia were removed before attachment. The utricle and cristae were attached to cover-slips with the hair cell side upwards. To obtain damaged utricles (Meyers and Corwin, 2007), stainless steel needles were pressed into utricles to form lesions in the hair cell epithelium, and cells within the lesion were removed with a sharp needle and forceps.
Culture and transfection of vestibular epithelia
Vestibular epithelia were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum(Gibco) for the first 12—15 hours. DMEM/F12 medium supplemented with B27 was used in the following culture.Half of the medium was replaced with fresh culture medium every two days. The cultures were incubated in a 95% air,5% CO2-humidified environment at 37°C. Ad-Math1-enhanced green fluorescent protein (EGFP) vectors (AD5-E1/E3-defected-Math1/EGFP, PFU 1.0 × 1011, Ad0112d, Beijing Sinogenemax Co., Beijing, China) or Ad-EGFP vectors (as controls) (AD-EGFP, PFU 1 × 1011, Beijing Sinogenemax Co.) with a final concentration of 1 × 108/mL were added to the culture medium at 1 day in vitro (cultures were denoted as 0 day in vitro on the day of explantation) for 6—8 hours,and then the medium containing virus was replaced with fresh culture medium.
To track cell division during hair cell transformation,BrdU (Sigma) and Ad-Math1-EGFP were added to the culture media at different time points (Figure 1), at a concentration of 10—15 μg/mL.
Immunocytochemistry
All samples were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) for 2 hours, permeabilized with 0.3% Triton X-100 in PBS for 30 minutes at room temperature, and then blocked with 5% normal goat serum for 30 minutes. BrdU samples were pretreated in 2 N HCl for 30 minutes at 37°C. Immunocytochemistry was performed using primary antibodies incubated for 12—48 hours at 4°C: rabbit polyclonal anti-Myosin VIIa antibody (1:100; Proteus BioSciences Inc.,Ramona, CA, USA) followed by a secondary antibody, goat monoclonal anti-rabbit IgG conjugated to TRITC (1:400;Molecular Probes, Inc., Eugene, OR, USA) or Alexa Fluor 647 goat anti-rabbit IgG (A21245, diluted 1:150; Molecular Probes,Inc.) for 30 minutes at 37°C. Mouse anti-BrdU antibody (1:100;Sigma) followed by a secondary antibody, goat anti-mouse IgG conjugated to TRITC (1:400; Molecular Probes, Inc.) or Alexa Fluor 647 goat anti-mouse IgG (1:400; Molecular Probes,Inc.) for 30 minutes at 37°C. Hair bundles were stained with phalloidin-TRITC (1:400; Sigma) for 30 minutes at 37°C. The samples were examined and photographed using a Leica SP2 or SP5 confocal microscope (Wetzlar, German).
Results
Cells with different proliferative potentials had different capabilities to generate new hair cells induced by Math1 gene transfer
Cells in the non-sensory region and mechanically damaged region of sensory epithelia had high proliferative potential and were induced into hair cells by Math1 gene transferAs discussed in a previous study (Huang et al., 2009), when vestibular epithelia were attached to culture dishes, cubic shaped cells spread and moved from the sensory region to the periphery, forming a non-sensory region. All of these cells exhibited high cell proliferative potential as demonstrated by BrdU staining (Figure 2A) (BrdU was added asFigure 1A). Ad-Math1-EGFP vectors were used in the culture media, and some of these non-sensory cells were transfected. Very few cells in the sensory region were transfected and differentiated into new hair cells as we previously reported (Huang et al., 2009). Some transfected cells differentiated into new hair cells, which were labeled by a hair cell speci fic protein, Myosin VIIa (Figure 2B). The ratio of new hair cells to transfected cells at 10 days in vitro was 69.5%.In the control group, Ad-EGFP vectors were used under the same conditions, and no new hair cells were found as previously reported (Huang et al., 2009).
When a hole or damage is made mechanically in the cultured postnatal mouse vestibular utricle, supporting cells around and in the damaged region spread and move to the center of the hole, and these cells have high proliferative capability (Meyers and Corwin, 2007). Our experiment indicated that when these cells in the damaged region were infected by Ad-Math1-EGFP vectors, some became new hair cells at 3 and 10 days in vitro (Figure 3). The ratio of new hair cells to transfected cells at 10 days in vitro was 58.2%.In the control group, Ad-EGFP vectors were used under the same conditions, but no new hair cells were found.
Cells with low proliferative potential were induced by Math1 gene and few were differentiated into new hair cells
After culturing for 7—10 days, there were very few proliferating supporting cells in the postnatal mice vestibular epithelial sheets. BrdU was used to detect dividing supporting cells at the beginning of the culture, and almost no BrdU-positive hair cells were found in the sensory region of the epithelia.When induced by Math1gene transfer, a small number of the cells differentiated into new hair cells (Figure 4A). The ratio of new hair cells to transfected cells was 9.4%. In the control group, Ad-EGFP vectors were used under the same conditions, but no new hair cells were seen (Figure 4B).
Cell division seldom occurred during or after new hair cell regeneration induced by Math1
New hair cells induced by Math1gene transfer were visible in the non-sensory region as previously reported (Huang et al., 2009). To determine whether new hair cells in the non-sensory region originated from the proliferating cells in the non-sensory region, BrdU was added at the beginning of the culture according to protocol-1 (Figure 1A). New hair cells were labeled with BrdU in the non-sensory region of the samples treated with Ad-Math1-EGFP (Figure 5). In other samples, to confirm cell division was required during new hair cell differentiation, BrdU was added after the administration of Ad-Math1-EGFP according to protocol-2 (Fig-ure 1B). Very few hair cells were labeled with BrdU in the non-sensory region of samples treated with Ad-Math1-EGFP(Figure 5). These two experiments showed that cell division always occurred before Math1transfection but not during or after Math1transfection.
Discussion
Cells with high proliferative potential generated new cells induced by Math1
Cells with high proliferative potential could generate hair cells by Math1gene transfer. However, the underlying mechanism remains unclear. During embryogenesis, Math1is expressed just before or during the timepoint when prosensory cells begin to leave the cell cycle, and these prosensory cells(target cells of Math1) still have a high proliferative capability. RB1 and P27kip1 are thought to contribute to prosensory cells leaving the cell cycle (Chen and Segil, 1999; Löwenheim et al., 1999; Mantela et al., 2005; Liu and Zuo, 2008). When these genes are knocked out or knocked down, prosensory cells delay leaving the cell cycle and excessive new hair cells are formed. This might be because excessive hair cells remain in the cell cycle of prosensory cells for longer, resulting in greater numbers of new prosensory cells and the generation of new hair cells. Nevertheless, a lower number of proliferating prosensory cells results in fewer new hair cells.The Notch signaling pathway plays an important role in the differentiation of hair cells and supporting cells by mediating“lateral inhibition”. Hes1 is one of the effectors that mediates this effect (Zheng et al., 2000; Zine et al., 2001); however, it contributes to the adequate proliferation of sensory precursor cells via the potential transcriptional downregulation of p27Kip1 (Murata et al., 2009). In Hes1-/-mice, prosensory cells with low proliferative potential for upregulated p27kip1 led to a low efficiency of hair cell differentiation despite normal Math1expression. Izumikawa et al. (2008) confirmed that Ad-Math1-EGFP was administered into media tympanic scala after killing all hair cells and no differentiated cells were found despite the overexpression of Math1. This study also demonstrated that the target cell is an important factor for Math1induced hair cell differentiation.
Math1-induced hair cell regeneration in vitro was a process of trans-differentiation
In Mesh, the explanation for cell transdifferentiation is a naturally occurring phenomenon where terminally differentiated cells dedifferentiate to the point where they can switch cell lineage. The cells then differentiate into other cell types. Generally speaking, no cell division occurs during this process. In birds, hair cell regeneration occurs through two pathways: regeneration through supporting cell division and regeneration through direct transdifferentiation without cell division (Stone and Cotanche, 2007). During the embryonic stage, prosensory cells begin to differentiate into hair cells when these cells retreat from the cell cycle (Ruben, 1967;Chen et al., 2002). No cell division is found during hair cell differentiation, indicating cell transdifferentiation is the main process involved. BrdU is used to detect the cells that have experienced the S phase. In our study, BrdU was added after Ad-Math1-EGFP was added to the culture medium, and cell transdifferentiation was expected to occur simultaneously. Most new hair cells were negative for BrdU, showing that new hair cell differentiation was independent of cell division. This was consistent with previously published results(White et al., 2006; Yang et al., 2013).
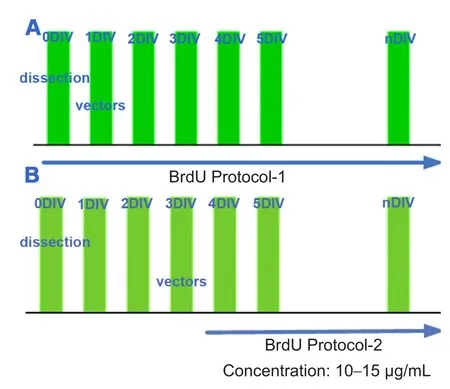
Figure 1 Protocol of vestibular epithelia labeling and transfection.
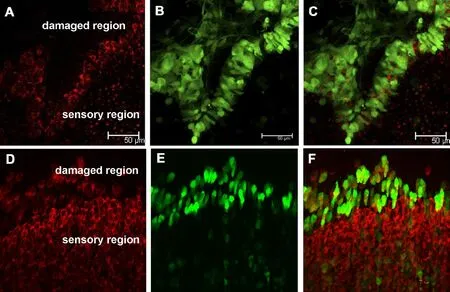
Figure 3 Cultured damaged utricle sensory epithelia transfected with Ad-Math1-EGFP at different time points.
However, in this study, very few new hair cells were positive for BrdU under protocol-2 and new hair cells with two nuclei were also commonly found. Cell division might occur just before, during or after hair cell differentiation and thus explain these BrdU-positive new hair cells. Most new hair cells came from high proliferative cells, and this cell differentiation is independent of cell proliferation; therefore, cell division often starts before transdifferentiation. Images of many hair cells with two nuclei and new cell division were occasionally captured, but this was insufficient to determine whether division occurred after cell differentiation.
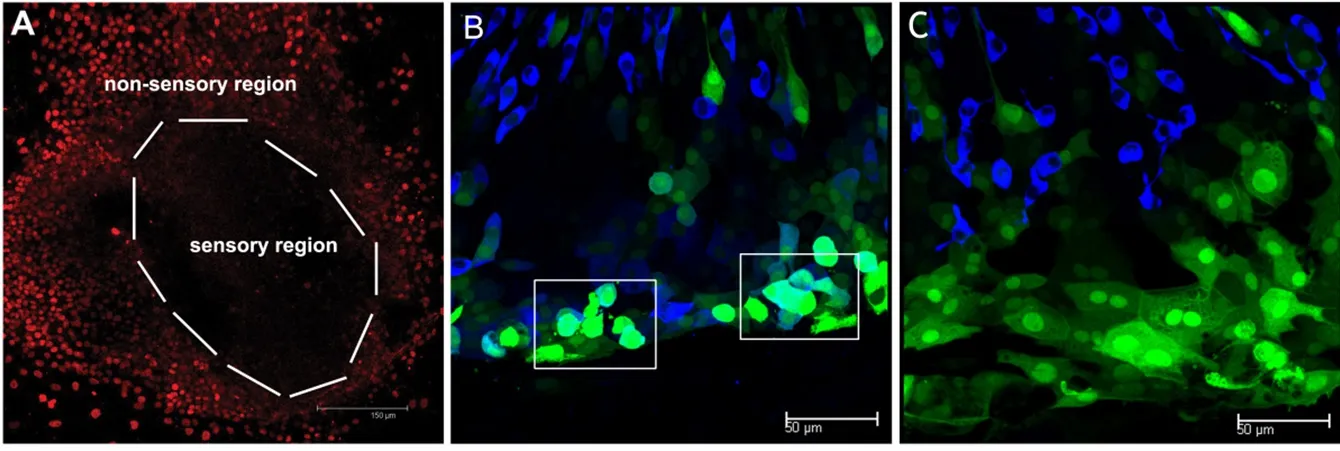
Figure 2 High proliferative cells and new hair cells in the nonsensory region are induced by Math1.

Figure 4 Proliferation of cultured utricle treated with Ad-Math1-EGFP or Ad-EGFP at 10 days in vitro.

Figure 5 Cell division tracked by BrdU after new hair cell regeneration induced by Math1 for 10 days.
Conclusions
Proliferative stage of vestibular epithelial cells is associated with their capability to differentiate into new hair cells by Math1gene transfer in vitro, but this regeneration is independent of cell proliferation.
Author contributions:YBH and FLC conceived and designed the study.YBH, RM, JMY, ZH, NC, ZG, DR and JW performed the experiments.YBH and RM wrote the paper. FLC reviewed and edited the paper. All authors approved the final version of the paper.
Con flicts of interest:None declared.
Financial support:The work was supported by the National Natural Science Foundation of China (NSFC), grant No. 81420108010, 81271084 to FLC, 81370022, 81570920, 81000413 to DR, 81200740 to JMY, 81200738 to NC, 81371093 to ZH, 81400460 to ZG, 81200739 to JW; 973 Program,grant No. 2011CB504500 and 2011CB504506; The Innovation Project of Shanghai Municipal Science and Technology Commission, grant No.11411952300 to FLC; and the Training Program of the Excellent Young Talents of the Shanghai Municipal Health System, grant No. XYQ2013084 to DR. Funders had no involvement in the study design; data collection,analysis, and interpretation; paper writing; or decision to submit the papaer for publication.
Research ethics:The study protocol was approved by the Chinese Science Academy Committee on Care and Use of Animals.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Chen P, Segil N (1999) p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 126:1581-1590.
Chen P, Johnson JE, Zoghbi HY, Segil N (2002) The role of Math1in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129:2495-2505.
Forge A, Li L, Corwin JT, Nevill G (1993) Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 259:1616-1619.
Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV (2008)Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 455:537-541.
Han Z, Yang JM, Chi FL, Cong N, Huang YB, Gao Z, Li W (2010) Survival and fate of transplanted embryonic neural stem cells by Atoh1 gene transfer in guinea pigs cochlea. Neuroreport 21:490-496.
Huang Y, Chi F, Han Z, Yang J, Gao W, Li Y (2009) New ectopic vestibular hair cell-like cells induced by Math1gene transfer in postnatal rats. Brain Res 1276:31-38.
Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y (2008)Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res 240:52-56.
Jeon SJ, Fujioka M, Kim SC, Edge AS (2011) Notch signaling alters sensory or neuronal cell fate speci fication of inner ear stem cells. J Neurosci 31:8351-8358.
Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y (2003)Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci 23:4395-4400.
Löwenheim H, Furness DN, Kil J, Zinn C, Gültig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner H-P (1999) Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of Corti. Proc Natl Acad Sci U S A 96:4084-4088.
Liu Z, Zuo J (2008) Cell cycle regulation in hair cell development and regeneration in the mouse cochlea. Cell Cycle 7:2129-2133.
Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U(2005) The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development 132:2377-2388.
Meyers JR, Corwin JT (2007) Shape change controls supporting cell proliferation in lesioned mammalian balance epithelium. J Neurosci 27:4313-4325.
Murata J, Ohtsuka T, Tokunaga A, Nishiike S, Inohara H, Okano H,Kageyama R (2009) Notch-Hes1 pathway contributes to the cochlear prosensory formation potentially through the transcriptional down-regulation of p27Kip1. J Neurosci Res 87:3521-3534.
Ruben RJ (1967) Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol:Suppl 220:221-244.
Staecker H, Praetorius M, Baker K, Brough DE (2007) Vestibular hair cell regeneration and restoration of balance function induced by math1gene transfer. Otol Neurotol 28:223-231.
Stone JS, Cotanche DA (2007) Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol 51:633-647.
Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT (1993)Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 259:1619-1622.
White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N (2006) Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441:984-987.
Woods C, Montcouquiol M, Kelley MW (2004) Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci 7:1310-1318.
Yang J, Cong N, Han Z, Huang Y, Chi F (2013) Ectopic hair cell-like cell induction by Math1 mainly involves direct transdifferentiation in neonatal mammalian cochlea. Neurosci Lett 549:7-11.
Zheng JL, Gao WQ (2000) Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci 3:580-586.
Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ (2000) Hes1 is a negative regulator of inner ear hair cell differentiation. Development 127:4551-4560.
Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F (2001) Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci 21:4712-4720.
杂志排行
中国神经再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Cerebral ischemia and neuroregeneration
- SNARE complex in axonal guidance and neuroregeneration
- Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease have impaired microglial function and defective repair of myelin damage
- The relaxin peptide family – potential future hope for neuroprotective therapy? A short review
- Roles of neural regeneration in memory pharmacology
