Quinacrine pretreatment reduces microwave-induced neuronal damage by stabilizing the cell membrane
2018-04-04XuefengDingYanWuWenruiQuMingFanYongqiZhao
Xue-feng Ding, Yan Wu, Wen-rui Qu, Ming Fan, Yong-qi Zhao
1 Department of Cognitive Sciences, Beijing Institute of Basic Medical Sciences, Beijing, China
2 Hand & Foot Surgery and Reparative & Reconstructive Surgery Center, Orthopedic Hospital of the Second Hospital of Jilin University,Changchun, Jilin Province, China
Funding: This work was supported by the Integrated Drug Discovery Technology Platform of National Science and Technology Major Projects for “Major New Drugs Innovation and Development”, No. 2012ZX09J12201-005; the National Natural Science Foundation of China, No.31071042, 31200822; and a grant of Beijing Natural Science Foundation, No. 5122033.
Introduction
Microwaves are widely used in daily life. Their effects, both thermal and non-thermal, have been studied extensively(Mausset-Bonnefont et al., 2004; Brillaud et al., 2007; Zuo,2014; Deshmukh et al., 2016; Cantres-Rosario et al., 2017;Tan et al., 2017). One of the most important characteristics of microwaves is their thermal effect, which we harness for heating and cooking food, and which greatly facilitates our life (Hermann et al., 1997; Sinha et al., 2008; de Tommaso et al., 2009; Zhao et al., 2012; Xiong et al., 2013; Deshmukh,2015; Masuda et al., 2015; Xiong et al., 2015; Hinrikus et al., 2017). However, under some conditions, this thermal effect may damage human organs and the central nervous system (Ono et al., 2004; Campisi et al., 2010; Wang et al.,2015; Zhao et al., 2017; Zhi et al., 2017). Therefore, it is important to find effective drugs against microwave-induced heat injury. Quinacrine (6-chloro-9-[(4-diethylamino)-1-methyl-butyl]-amino-2-ethoxyacridine) was once widely used for treating protozoan infections, Giardia intestinalis,Taenia saginata, and malaria (Lalle, 2010; Meyer zu Hörste et al., 2010; Ehsanian et al., 2011; Growe et al., 2013; Haseman et al., 2015; Kodera et al., 2017). During World War II,quinacrine was used as a first-line antimalarial drug, saving many lives. Today, although quinacrine is no longer used as an antimalarial drug in higher-income countries, as more effective substituted compounds have been found, research on this drug is ongoing (Chauhan and Srivastava, 2001; Kalia and Dutz, 2007; Manna et al., 2016). Recently, quinacrine was found to stabilize the cell nucleus by binding to DNA and other nuclear proteins (Reyes et al., 2001; Hossain et al.,2008; McNamee et al., 2016). It is also an inhibitor of phospholipase-A2 (PLA2), which is responsible for hydrolyzing membrane phospholipids to release free fatty acids and lysophospholipids. So quinacrine may also be involved in stabilizing cell membranes (Markaverich et al., 2007; Ortiz et al.,2014; Sharma A et al., 2017). Furthermore, mounting evidence suggests quinacrine has a protective effect against heat injury,including that caused by microwaves (Zhao et al., 2004; Gao et al., 2009). Indeed, our previously published study showed that quinacrine protected animals against microwave-induced heat injury (Gao et al., 2009). However, the molecular mechanism of this process remains to be elucidated.
In the present study, we pretreated neurons derived from PC12 cells with quinacrine, then exposed them to microwave irradiation, to explore the role of quinacrine in microwave-induced heat damage to neurons.
Materials and Methods
PC12 cell culture and neuronal differentiation
PC12 cells (1.5 × 104) were cultured in Dulbecco’s modi fied Eagle’s medium (DMEM) containing 10% fetal bovine serum and maintained at 37°C with 5% CO2in a tissue culture incubator for up to 8 days (Fujino et al., 2013; Tarjányi et al., 2013). The cultured PC12 cells were transferred into poly-D-lysine-coated dishes (35 mm) and cultured in DMEM containing 1% fetal bovine serum and 1% horse serum (Gibco Life Technologies, Grand Island, NY, USA), before being induced with retinoic acid to differentiate into neuronal cells.About 7—8 days after induction, the induced cells formed a network of neurites. Next, the differentiated cells were characterized with antibodies against neuron-specific enolase and neuro filament (Sigma, St. Louis, MO, USA) as neuronal markers (Scheibe et al., 1991; Sakimura et al., 1995).
PC12 cell pretreatment with quinacrine before microwave irradiation
Quinacrine (low concentration, 20 mM; high concentration, 40 mM) was applied to differentiated PC12 cells and the dishes were immediately sealed with Parafilm to prevent bacterial contamination. The sealed dishes were put into a homemade microwave generator as described previously (Wang et al., 2015; Zhao et al., 2017; Zhi et al.,2017). In brief, the 2.9 GHz generation system consisted of a pulse microwave generator (BZJ1500M-300W, Glory MV Electronics, China) and a 45 dB gain power amplifier(VE1079A, Beijing Vacuum Electronics Research Institute,China). Microwave energy was transmitted by a rectangular waveguide and a 12 dB standard-gain horn antenna to an anechoic chamber (12.5 × 9.7 × 7.5 m3). The diagonal of the antenna was 17.2 cm. The interior walls of the anechoic chamber were covered with 500 mm and 300 mm pyramidal microwave absorbers to minimize reflections (> 45 dB). The emitted power was measured using a power sensor(N1921A, Keysight, Santa Rosa, CA, USA) connected to a directional coupler at one port of a circulator. The peak and average power densities were measured using a calibrated waveguide antenna, a power meter (N1912A, Keysight) and a power sensor (N1921, Keysight). Microwave pulses were delivered at 50 pps, 100 pps and 300 pps, respectively, with a pulse width of 500 ns. The peak power densities for the three exposure groups were 200 W/cm2. The average power densities were 5, 10 and 30 mW/cm2. The output power of the radiation source for the three exposure groups was 3 MW. The cells were irradiated with 50 mW/cm2microwaves for 3 or 6 hours. Untreated differentiated cells were used as the negative control. Microwave conditions were as follows:temperature, 15–25°C; humidity, 30–40%; frequency, 2.856 GHz; pulse length, 500 ns; dishes 2 meters from radiation source. The cover of the dish did not affect microwave transmission (Marchiarullo et al., 2013).
Flow cytometry
After irradiation, cell apoptosis and necrosis were examined using an annexin-V flow cytometry kit (Thermo Fisher Scienti fic, New York, NY, USA) as described previously (Qiao et al., 2014; Dai et al., 2015).
Membrane surface ultrastructure assay using atomic force microscopy
Atomic force microscopy was performed as previously described (Zhang et al., 2017; Marsh et al., 2018). In brief, the pretreated PC12 cells were fixed with 1.25% glutaraldehyde solution for 30 minutes at 4°C and washed three times with 0.9% NaCl. The treated cells were scanned using an atomic force microscope (SPM-9500J3, Shimadzu, Kyoto, Japan) at 25°C and images were acquired using the contact mode and 125 mm × 125 mm scanner at a rate of 2 Hz. Images were processed with specific atomic force microscopy software(SPM online and offline software; Shimadzu) (Yingge et al.,2003; Plodinec et al., 2015). Membrane integrity was analyzed using Image-Pro Plus software (Media Cybernetics,Rockville, MD, USA). The darker the membrane in the image, the more severe the damage.
Western blot assay
After microwave exposure, cells were immediately harvested and lysed using radioimmune precipitation assay lysis buffer(Sigma). Protein samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. After blocking with 5% skim milk in phosphate-buffered saline (PBS), the membrane was incubated with polyclonal antibody against HSP70 (1:1,000;Abcam, Cambridge, UK) and polyclonal β-actin (1:1,000;Abnova, Taipei, China) for 2 hours at room temperature.Peroxidase-coupled detection was performed using enhanced chemiluminescence (Amersham Biosciences, Little Chalfont, UK) (Huang et al., 2013). The relative expression level of HSP70 was calculated using Quantity One software(Bio-Rad, Hercules, CA, USA) as the gray value ratio of the HSP70 and actin bands.
Immunohistochemistry
Seven days after induction with retinoic acid, the cells were fixed with 4% paraformaldehyde/PBS at 4°C overnight. To block non-speci fic binding, the cells were incubated with 5%goat serum in PBS (pH 7.4) for 1 hour at room temperature,and then with rabbit polyclonal anti-neuron-speci fic enolase(NSE) and anti-neuro filament (NF) antibodies (1:500; Sigma) in PBS containing 5% goat serum and 0.1% Triton X-100 at 4ºC overnight. After three washes in PBS, the cells were incubated with goat anti-rabbit secondary antibody (Abcam)conjugated with horseradish peroxidase for 2 hours at room temperature. Immunoreactivity was examined using diaminobenzidine as a chromogen. NSE- and NF-immunoreactive cells were counted in five randomly placed visual fields in each group, the ratio of the number of NSE- or NF-positive cells and the number of total cells was calculated as a percentage of neuronal cells. The mean percentage for each group was used in the final analysis.
Statistical analysis
Statistical analyses were performed using Prism 5.0 software(GraphPad Software, San Diego, CA, USA). Comparisons between three or more groups were made using one-way analysis of variance and the Tukey’s post-hoc test, as described previously (Fan et al., 2015; Long et al., 2015). Data are presented as the mean ± SEM. P ≤ 0.05 was considered statistically signi ficant.
Results
PC12 cells were successfully induced to differentiate into neurons
PC12 cells differentiated into neuron-like cells after induction. Long neurites were observed in most induced cells(Figure 1). Immunohistochemistry showed that more than 90% of differentiated cells were positive for NSE and more than 70% were positive for NF, con firming that the induced cells could be used for the subsequent experiment.
Quinacrine pretreatment reduced the apoptosis andnecrosis rates of microwave-exposed cells
To understand the effect of quinacrine on microwave-induced neuronal apoptosis and necrosis, induced PC12 cells were exposed to microwave radiation, with or without pretreatment with quinacrine. The cells were then examined using flow cytometry. Compared with the control group,cells irradiated for either 3 or 6 hours showed signi ficantly more neuronal apoptosis and necrosis (Figure 2). However,compared with the microwave-only group, cells pretreated with low- or high-concentration quinacrine showed signi ficantly less apoptosis and necrosis after 3- and 6-hour irradiation. In cells exposed to 3-hour irradiation (Figure 2A–C), both concentrations of quinacrine signi ficantly reduced microwave-induced apoptosis, with no signi ficant difference between the concentrations; however, the rate of necrosis was signi ficantly lower after high-concentration quinacrine pretreatment than after low-dose quinacrine (P < 0.01). This might be due to the different processes involved in necrosis and apoptosis. In addition, apoptosis and necrosis caused by 6-hour microwave irradiation (Figure 2D) were lower in cells pretreated with low- or high-concentration quinacrine,with no signi ficant difference in effect between the two concentrations.
Quinacrine pretreatment reduced membrane damage caused by microwave irradiation
To understand the mechanism underlying the protective effect of quinacrine on microwave-exposed neuronal cells,we examined membrane integrity using atomic force microscopy. For both irradiation durations, cells pretreated with high-concentration quinacrine showed markedly less membrane injury than non-pretreated cells (Figure 3), suggesting that quinacrine protects neurons by stabilizing the cell membrane.
Quinacrine reduced microwave-induced neuronal membrane damage by increasing HSP70 expression
To further investigate the molecular mechanism underlying the protective effect of quinacrine, we examined the expression of HSP70, a commonly used heat injury marker, using western blots. HSP70 expression did not differ signi ficantly between non-irradiated cells, cells irradiated for 3 hours,and cells pretreated with low-concentration quinacrine and irradiated for 3 hours; however, high-concentration quinacrine resulted in signi ficantly higher HSP70 expression than in control cells (Figure 4). In contrast, 6-hour microwave irradiation resulted in signi ficantly lower HSP70 expression (P< 0.05); this may be caused by the greatly enhanced neuronal apoptosis and necrosis. Both low- and high-concentration quinacrine rescued this decrease in HSP70 expression (P <0.05), with no signi ficant difference found between concentrations (Figure 4). These results indicate that quinacrine may promote neuronal cell survival by regulating HSP70 expression, though it may be not the only mediator in the signaling pathway.
Discussion

Figure 1 PC12 cells were successfully induced into neuronal cells.
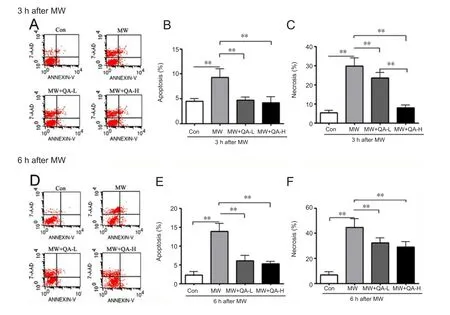
Figure 2 Quinacrine reduced microwave-induced neuronal cell apoptosis and necrosis.

Figure 3 Quinacrine pretreatment reduced membrane injury caused by microwave exposure.
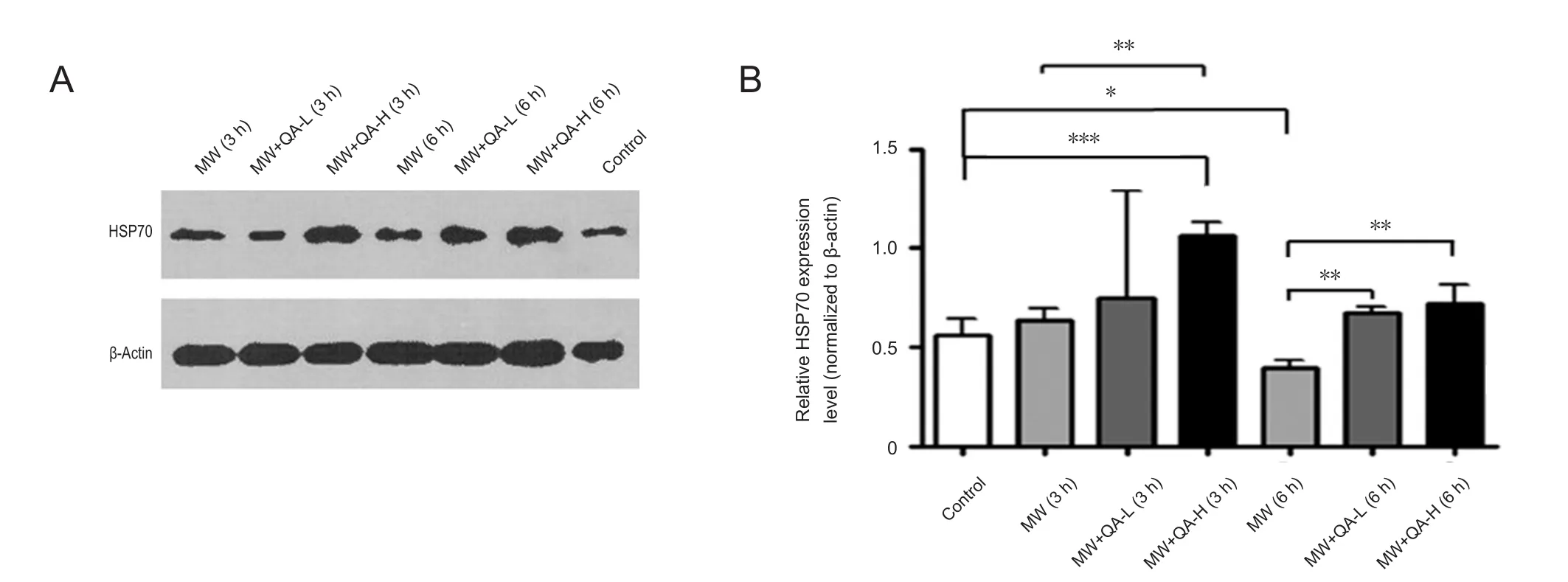
Figure 4 Quinacrine pretreatment reduced microwave-induced neuronal membrane injury by increasing HSP70 expression.
Due to the extensive use of microwaves in our daily life and work, there is increasing concern about the interactions of electromagnetic radiation with human health, in particular brain health (Zhao et al., 2012; Deshmukh et al., 2015; Xiong et al., 2015). The thermal effect is one of the most important effects of microwaves. It is this effect that is harnessed to make our lives easier, but excessive microwave radiation may cause neuronal heat injury and affect human brain function. Our previous study showed that quinacrine administration reduced microwave-induced heat injury to the hippocampus (Gao et al., 2009). Here, to characterize the molecular mechanism, we explored the effects of quinacrine on microwave-induced heat injury in PC12 cells differentiated into neurons after induction with retinoic acid. PC12 cells differentiated into NSE- and NF-immunoreactive cells,and outgrowth of neurites indicated successful establishment of the neuronal cell model. Subsequent investigation showed that microwave radiation under our conditions caused neuronal apoptosis and necrosis, consistent with previously published studies (Martin et al., 2009; Motawi et al.,2014). However, in the presence of quinacrine, this damage was markedly decreased, further supporting our previous study in an animal model. Subsequent analysis showed that there was no significant difference in apoptosis reduction between low- and high-concentration quinacrine at either 3-or 6-hour microwave irradiation, indicating that low-dose quinacrine is enough to prevent neuronal cell apoptosis in this cell model. For 3-hour irradiation, the high concentration of quinacrine reduced the rate of necrosis more than the low concentration, and quinacrine even reduced the rate of necrosis to a similar level as control (non-exposed) cells. In the 6-hour exposure groups, no signi ficant difference in cell necrosis rate was detected between low- and high-concentration quinacrine, although the necrosis rate in the quinacrine treatment groups was significantly higher than that in the control group, indicating that the necrosis caused by 6-hour microwave irradiation might be not reversible. Because PLA2 hydrolyzes membrane phospholipids to release free fatty acids and lysophospholipids, whereas quinacrine is an inhibitor of PLA2, quinacrine may reduce cell necrosis by inhibiting PLA2 (Markaverich et al., 2007; Ortiz et al., 2014).Further investigations on cell membrane integrity using atomic force microscopy showed that the membrane structure in cells exposed to 6-hour microwave irradiation was worse than that in 3-hour groups. In addition, high-concentration quinacrine had a better effect on stabilizing membrane structure than low-concentration quinacrine in the 3-hour groups. In the 6-hour groups, low-concentration quinacrine had better protective effects on membrane structure. HSP70 is a protector in heat stroke injury (Horowitz and Robinson, 2007; Kim et al., 2012; Liu et al., 2016; Shi et al., 2017). To find the related molecular mechanism involved in this process, we examined HSP70 expression levels using western blots. In the 3-hour groups, quinacrine at high, but not low, concentration resulted in a signi ficant upregulation of HSP70 expression. In the 6-hour irradiation groups, both low-dose and high-dose quinacrine unregulated HSP70 expression compared with irradiation alone. However, 6-hour,but not 3-hour, irradiation caused signi ficant downregulation of HSP70 compared with the control group, which also supports the flow cytometry results. Therefore, HSP70 may be in-volved in regulating the protective effect of quinacrine on microwave-induced neuronal injury. However, this effect may be limited to certain cell lines, as data from other labs have demonstrated that quinacrine can induce apoptosis in SGC-7901 cells, metastatic breast cancer stem cells, and human leukemia K562 cells (Wu et al., 2012; Changchien et al., 2015;Siddharth et al., 2016). Therefore, the roles of quinacrine in different cell types or cell lines should be explored in future.
In summary, we induced differentiation of PC12 cells into neurons and exposed the neurons to microwave irradiation.We found that quinacrine protected the cells against thermal damage from microwaves by upregulating HSP70 expression and stabilizing the cell membrane structure via PLA2 inhibition. These results will inform discussions on new uses of quinacrine in thermal brain damage.
Author contributions:XFD and YW prepared the paper. YW performed the experiments. YQZ and MF designed the study. WRQ provided constructive suggestion. All authors approved the final version of the paper.
Con flicts of interest:The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential con flict of interest.
Financial support:This work was supported by the Integrated Drug Discovery Technology Platform of National Science and Technology Major Projects for “Major New Drugs Innovation and Development”,No. 2012ZX09J12201-005; the National Natural Science Foundation of China, No. 31071042, 31200822; and a grant of Beijing Natural Science Foundation, No. 5122033. The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Brillaud E, Piotrowski A, de Seze R (2007) Effect of an acute 900 MHz GSM exposure on glia in the rat brain: a time-dependent study. Toxicology 238:23-33.
Campisi A, Gulino M, Acquaviva R, Bellia P, Raciti G, Grasso R, Musumeci F, Vanella A, Triglia A (2010) Reactive oxygen species levels and DNA fragmentation on astrocytes in primary culture after acute exposure to low intensity microwave electromagnetic field. Neurosci Lett 473:52-55.
Cantres-Rosario YM, Acevedo-Mariani FM, Pérez-Laspiur J, Haskins WE, Plaud M, Cantres-Rosario YM, Skolasky R, Méndez-Bermúdez I, Wojna V, Meléndez LM (2017) Microwave & magnetic proteomics of macrophages from patients with HIV-associated cognitive impairment. PLoS One 12:e0181779.
Changchien JJ, Chen YJ, Huang CH, Cheng TL, Lin SR, Chang LS(2015) Quinacrine induces apoptosis in human leukemia K562 cells via p38 MAPK-elicited BCL2 down-regulation and suppression of ERK/c-Jun-mediated BCL2L1 expression. Toxicol Appl Pharmacol 284:33-41.
Chauhan PM, Srivastava SK (2001) Present trends and future strategy in chemotherapy of malaria. Curr Med Chem 8:1535-1542.
Dai XJ, Li N, Yu L, Chen ZY, Hua R, Qin X, Zhang YM (2015) Activation of BV2 microglia by lipopolysaccharide triggers an inflammatory reaction in PC12 cell apoptosis through a toll-like receptor 4-dependent pathway. Cell Stress Chaperones 20:321-331.
de Tommaso M, Rossi P, Falsaperla R, Francesco Vde V, Santoro R,Federici A (2009) Mobile phones exposure induces changes of contingent negative variation in humans. Neurosci Lett 464:79-83.
Deshmukh PS, Megha K, Nasare N, Banerjee BD, Ahmed RS, Abegaonkar MP, Tripathi AK, Mediratta PK (2016) Effect of low level subchronic microwave radiation on rat brain. Biomed Environ Sci 29:858-867.
Deshmukh PS, Nasare N, Megha K, Banerjee BD, Ahmed RS, Singh D,Abegaonkar MP, Tripathi AK, Mediratta PK (2015) Cognitive impairment and neurogenotoxic effects in rats exposed to low-intensity microwave radiation. Int J Toxicol 34:284-290.
Ehsanian R, Van Waes C, Feller SM (2011) Beyond DNA binding-a review of the potential mechanisms mediating quinacrine’s therapeutic activities in parasitic infections, in flammation, and cancers. Cell Commun Signal 9:13.
Fan K, Li D, Zhang Y, Han C, Liang J, Hou C, Xiao H, Ikenaka K, Ma J (2015) The induction of neuronal death by up-regulated microglial cathepsin H in LPS-induced neuroin flammation. J Neuroin flammation 12:54.
Fujino K, Ogura Y, Sato K, Nedachi T (2013) Potential neuroprotective effects of SIRT1 induced by glucose deprivation in PC12 cells. Neurosci Lett 557:148-153.
Gao JT, Liu SH, Yan YE, Wu Y, Wu HT, Xing C, Ge XM, Wang H,Zhao YQ, Fan M (2009) Quinacrine protects neuronal cells against heat-induced injury. Cell Biol Int 33:874-881.
Growe RG, Luster MI, Fail PA, Lippes J (2013) Quinacrine-induced occlusive fibrosis in the human fallopian tube is due to a unique in flammatory response andmodi fication of repair mechanisms. J Reprod Immunol 97:159-166.
Haseman JK, Growe RG, Zeiger E, McConnell EE, Luster MI, Lippes J(2015) A critical examination of the mode of action of quinacrine in the reproductive tract in a 2-year rat cancer bioassay and its implications for human clinical use. Regul Toxicol Pharmacol 71:371-378.
Hermann DM, Hossmann KA (1997) Neurological effects of microwave exposure related to mobile communication. J Neurol Sci 152:1-14.
Hinrikus H, Bachmann M, Karai D, Lass J (2017) Mechanism of low-level microwave radiation effect on nervous system. Electromagn Biol Med 36:202-212.
Horowitz M, Robinson SD (2007) Heat shock proteins and the heat shock response during hyperthermia and its modulation by altered physiological conditions. Prog Brain Res 162:433-446.
Hossain M, Giri P, Kumar GS (2008) DNA intercalation by quinacrine and methylene blue: a comparative binding and thermodynamic characterization study. DNA Cell Biol 27:81-90.
Huang X, Sun J, Rong W, Zhao T, Li DH, Ding X, Wu LY, Wu K,Schachner M, Xiao ZC, Zhu LL, Fan M (2013) Loss of cell adhesion molecule CHL1 improves homeostatic adaptation and survival in hypoxic stress. Cell Death Dis 4:e768.
Joyner JJ, Yadav BK (2015) Microwave assisted dehulling of black gram(Vigna mungo L). J Food Sci Technol 52:2003-2012.
Kalia S, Dutz JP (2007) New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther 20:160-174.
Kim N, Kim JY, Yenari MA (2012) Anti-in flammatory properties and pharmacological induction of Hsp70 after brain injury. In flammopharmacology 20:177-185.
Kodera S, Gomez-Tames J, Hirata A, Masuda H, Arima T, Watanabe S (2017) Multiphysics and thermal response models to improve accuracy of local temperature estimation in rat cortex under microwave exposure. Int J Environ Res Public Health doi: 10.3390/ijerph14040358.
Lalle M (2010) Giardiasis in the post genomic era: treatment, drug resistance and novel therapeutic perspectives. Infect Disord Drug Targets 10:283-294.
Liu Y, Wang XC, Hu D, Huang SR, Li QS, Li Z, Qu Y (2016) Heat shock protein 70 protects PC12 cells against ischemia-hypoxia/reoxygenation by maintaining intracellular Ca(2+) homeostasis. Neural Regen Res 11:1134-1140.
Long AN, Owens K, Schlappal AE, Kristian T, Fishman PS, Schuh RA(2015) Effect of nicotinamide mononucleotide on brain mitochondrial respiratory de ficits in an Alzheimer’s disease-relevant murine model. BMC Neurol 15:19.
Manna D, Ghosh R (2016) Effect of radiofrequency radiation in cultured mammalian cells: a review. Electromagn Biol Med 35:265-301.Marchiarullo DJ, Sklavounos AH, Oh K, Poe BL, Barker NS, Landers JP (2013). Low-power microwave-mediated heating for microchip-based PCR. Lab Chip 13:3417-3425.
Markaverich BM, Crowley J, Rodriquez M, Shoulars K, Thompson T(2007) Tetrahydrofurandiol stimulation of phospholipase A2, lipoxygenase, and cyclooxygenase gene expression and MCF-7 human breast cancer cell proliferation. Environ Health Perspect 115:1727-1731.
Marsh BP, Chada N, Sanganna Gari RR, Sigdel KP, King GM (2018)The hessian blob algorithm: precise particle detection in atomic force microscopy imagery. Sci Rep 8:978.
Martin D, Cinca S, Margaritescu I, Neagu M, Iacob N, Ighigeanu D,Matei C, Craciun G, Manaila E, Chirita DA, Moisescu M (2009) Cell investigations simultaneously with exposure to 2.45 GHz microwaves. J Microw Power Electromagn Energy 43:21-25.
Masuda H, Hirota S, Ushiyama A, Hirata A, Arima T, Kawai H, Wake K, Watanabe S, Taki M, Nagai A, Ohkubo C (2015) No dynamic changes in blood-brain barrier permeability occur in developing rats during local cortex exposure to microwaves. In Vivo 29:351-357.
Mausset-Bonnefont AL, Hirbec H, Bonnefont X, Privat A, Vignon J,de Sèze R (2004) Acute exposure to GSM 900-MHz electromagnetic fields induces glial reactivity and biochemical modi fications in the rat brain. Neurobiol Dis 17:445-454.
McNamee JP, Bellier PV, Konkle AT, Thomas R, Wasoontarajaroen S, Lemay E, Gajda GB (2016) Analysis of gene expression in mouse brain regions after exposure to 1.9 GHz radiofrequency fields. Int J Radiat Biol 92:338-350.
Meyer zu Hörste G, Mausberg AK, Korth C, Stüve O, Kieseier BC (2010)Quinpramine-a promising compound for treating immune-mediated demyelination of the nervous system. Drug News Perspect 23:287-294.
Motawi TK, Darwish HA, Moustafa YM, Labib MM (2014) Biochemical modi fications and neuronal damage in brain of young and adult rats after long-term exposure to mobile phone radiations. Cell Biochem Biophys 70:845-855.
Ono T, Saito Y, Komura J, Ikehata H, Tarusawa Y, Nojima T, Goukon K, Ohba Y, Wang J, Fujiwara O, Sato R (2004) Absence of mutagenic effects of 2.45 GHz radiofrequency exposure in spleen, liver, brain,and testis of lacZ-transgenic mouse exposed in utero. Tohoku J Exp Med 202:93-103.
Ortiz ME, Bühler MI, Zelarayán LI (2014) Involvement of PLA2, COX and LOX in Rhinella arenarum oocyte maturation. Zygote 22:440-445.
Plodinec M, Lim RY (2015) Nanomechanical characterization of living mammary tissues by atomic force microscopy. Methods Mol Biol 1293:231-246.
Qiao D, Xu J, Le C, Huang E, Liu C, Qiu P, Lin Z, Xie WB, Wang H(2014) Insulin-like growth factor binding protein 5 (IGFBP5) mediates methamphetamine-induced dopaminergic neuronapoptosis.Toxicol Lett 230:444-453.
Reyes S, Herrera LA, Ostrosky P, Sotelo J (2001) Quinacrine enhances carmustine therapy of experimental rat glioma. Neurosurgery 49:969-973.
Sakimura K, Kushiya E, Ogura A, Kudo Y, Katagiri T, Takahashi Y(1995) Upstream and intron regulatory regions for expression of the rat neuron-speci fic enolase gene. Brain Res Mol Brain Res 28:19-28.
Scheibe RJ, Ginty DD, Wagner JA (1991) Retinoic acid stimulates the differentiation of PC12 cells that are de ficient in cAMP-dependent protein kinase. J Cell Biol 113:1173-1182.
Sharma A, Kesari KK, Saxena VK, Sisodia R (2017) The influence of prenatal 10 GHz microwave radiation exposure on a developing mice brain. Gen Physiol Biophys 36:41-51.
Shi P, Sun LL, Lee YS, Tu Y (2017) Electroacupuncture regulates the stress-injury-repair chain of events after cerebral ischemia/reperfusion injury. Neural Regen Res 12:925-930.
Siddharth S, Nayak D, Nayak A, Das S, Kundu CN (2016) ABT-888 and quinacrine induced apoptosis in metastatic breast cancer stem cells by inhibiting base excision repair via adenomatous polyposis coli. DNA Repair (Amst) 45:44-55.
Sinha RK, Aggarwal Y, Upadhyay PK, Dwivedi A, Keshri AK, Das BN(2008) Neural network-based evaluation of chronic non-thermal effects of modulated 2450 MHz microwave radiation on electroencephalogram. Ann Biomed Eng 6:839-851.
Tan S, Wang H, Xu X, Zhao L, Zhang J, Dong J, Yao B, Wang H, Zhou H, Gao Y, Peng R (2017) Study on dose-dependent, frequency-dependent, and accumulative effects of 1.5 GHz and 2.856 GHz microwave on cognitive functions in Wistar rats. Sci Rep 7:10781.
Tarjányi O, Berta G, Harci A, Bacsa EB, Stark B, Pap M, Szeberényi J,Sétáló G Jr (2013) The role of Src protein in the process formation of PC12 cells induced by the proteasome inhibitor MG-132. Neurochem Int 63:413-422.
Wang H, Peng R, Zhou H, Wang S, Gao Y, Wang L, Yong Z, Zuo H,Zhao L, Dong J, Xu X, Su Z (2013) Impairment of long-term potentiation induction is essential for the disruption of spatial memory after microwaveexposure. Int J Radiat Biol 89:1100-1107.
Wang LF, Wei L, Qiao SM, Gao XN, Gao YB, Wang SM, Zhao L, Dong J, Xu XP, Zhou HM, Hu XJ, Peng RY (2015) Microwave-induced structural and functional injury of hippocampal and PC12 cells is accompanied by abnormal changes in the NMDAR-PSD95-CaMKII pathway. Pathobiology 82:181-194.
Wang LF, Li X, Gao YB, Wang SM, Zhao L, Dong J, Yao BW, Xu XP,Chang GM, Zhou HM, Hu XJ, Peng RY (2015) Activation of VEGF/Flk-1-ERK pathway induced blood-brain barrier injury after microwave exposure. Mol Neurobiol 52:478-491.
Wu X, Wang Y, Wang H, Wang Q, Wang L, Miao J, Cui F, Wang J(2012) Quinacrine inhibits cell growth and induces apoptosis in human gastric cancer cell line SGC-7901. Curr Ther Res Clin Exp 73:52-64.
Xiong L, Sun CF, Zhang J, Gao YB, Wang LF, Zuo HY, Wang SM,Zhou HM, Xu XP, Dong J, Yao BW, Zhao L, Peng RY (2015) Microwave exposure impairs synaptic plasticity in the rat hippocampus and PC12 cells through over-activation of the NMDA receptor signaling pathway. Biomed Environ Sci 28:13-24.
Yingge Z, Xia J, Lan S (2003) The relations between neurite development and the subcellular structures of hippocampal neuron somata.J Struct Biol 144:327-336.
Zhang W, Chen Y, Xia X, Chu J (2017) Material discrimination and mixture ratio estimation in nanocomposites via harmonic atomic force microscopy. Beilstein J Nanotechnol 8:2771-2780.
Zhao L, Li J, Hao YH, Gao YB, Wang SM, Zhang J, Dong J, Zhou HM,Liu SC, Peng RY (2017) Microwave-induced apoptosis and cytotoxicity of NK cells through ERK1/2 signaling. Biomed Environ Sci 30:323-332.
Zhao L, Peng RY, Wang SM, Wang LF, Gao YB, Dong J, Li X, Su ZT(2012) Relationship between cognition function and hippocampus structure after long-term microwave exposure. Biomed Environ Sci 25:182-188.
Zhao YQ, Wu Y, Liu SH, Ge XM, Ding AS, Fan M (2004) Protective effect of Quinacrine on striatum neurons from heat treatment injury.Zhongguo Ying Yong Sheng Li Xue Za Zhi 20:319-323.
Zhi WJ, Peng RY, Li HJ, Zou Y, Yao BW, Wang CZ, Liu ZH, Gao XH,Xu XP, Dong J, Zhao L, Zhou HM, Wang LF, Hu XJ (2018) Microwave radiation leading to shrinkage of dendritic spines in hippocampal neurons mediated by SNK-SPAR pathway. Brain Res 1679:134-143.
Zuo H, Lin T, Wang D, Peng R, Wang S, Gao Y, Xu X, Li Y, Wang S,Zhao L, Wang L, Zhou H (2014) Neural cell apoptosis induced by microwave exposure through mitochondria-dependent caspase-3 pathway. Int J Med Sci 11:426-435.
杂志排行
中国神经再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Cerebral ischemia and neuroregeneration
- SNARE complex in axonal guidance and neuroregeneration
- Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease have impaired microglial function and defective repair of myelin damage
- The relaxin peptide family – potential future hope for neuroprotective therapy? A short review
- Roles of neural regeneration in memory pharmacology
