Progesterone modulates mTOR in the hippocampus of mice after traumatic brain injury
2018-04-04RichardJustinGarlingLoraTalleyWattsShaneSpragueMuratDigicaylioglu
Richard Justin Garling, Lora Talley Watts Shane Sprague, Murat Digicaylioglu
1 Department of Neurosurgery, Wayne State University, Detroit, MI, USA
2 School of Medicine, The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
3 Department of Neurosurgery, The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
Introduction
Traumatic brain injury (TBI) affects roughly 1.7 million people per year in the United States, yet there are currently no pharmacological treatments aside from symptom management (Carney, 2017; Taylor et al., 2017). TBI is a multifocal injury that occurs in two phases. The primary injury is caused by the physical insult which causes the head trauma such as a high speed collision, fall, or other causes of head trauma. This is followed by the inflammatory response or secondary injury which occurs in the following hours to days and peaks at 72 hours (Chesnut et al., 1993; Jones et al.,1994). During secondary injury, cytokines and in flammatory cells are released and patients are increasingly susceptible to states of hypoxia (Chesnut et al., 1993; Jones et al., 1994).When the brain is injured, nutrients and energy sources for neurons become depleted and alternative sources must be utilized or the neurons will not survive. The mammalian or mechanistic target of rapamycin (mTOR) is an intracellular serine protein kinase that neurons rely upon to thrive and respond during injury. mTOR senses changes in nutrient levels and cellular energy status from the surrounding environment and integrates these signals into a metabolic response (Laplante and Sabatini, 2012). The ability to sense changes in the cellular microenvironment via the mTOR signaling pathway allows for cell survival during injury.The mTOR pathway has been shown to play a pivotal role in numerous pathologic disease states such as stroke, TBI,glioblastoma multiforme (GBM), ovarian hyperstimulation syndrome, and hypoxia due to its ability to respond to changes in the cellular microenvironment via regulators of the mTOR pathway (Chen et al., 2007; Liu et al., 2008;Garling et al., 2014; Atif et al., 2015; Kosmas et al., 2015).Identification of potential mTOR modulating drugs may prove bene ficial in elucidating potential treatments for TBI,a known modulator of mTOR.
mTOR is responsible for sensing energy and nutrient status in the cellular environment and is a regulator of cellular metabolism (Laplante and Sabatini, 2012). Under normal conditions, mTOR is primarily activated by phosphorylation at the Serine 2448 and Serine 2481 sites (Foster and Fingar, 2010).Once phosphorylated, mTOR forms one of two complexes:mTOR complex 1 (MTORC1) chiefly composed of Serine 2448 phosphorylated mTOR, or mTOR complex 2 (MTORC2),primarily composed of Serine 2481 phosphorylated mTOR(Fletcher et al., 2013). Though these two complexes both contain mTOR, they are functionally distinct. MTORC1 has been shown to sense nutrient status and regulate cellular metabolism (Laplante and Sabatini, 2012). The activation of MTORC1 stimulates cell growth and metabolism, protein synthesis, and lipid synthesis (Sengupta et al., 2010; Laplante and Sabatini, 2012). MTORC2 is less well studied and believed to be involved in the Akt signaling pathway (Copp et al., 2009;Laplante and Sabatini, 2012). When activated, MTORC2 has been shown to play a role in cell survival and cytoskeletal organization (Laplante and Sabatini, 2012).
Several regulators of mTOR have been identi fied including rapamycin, TBI, hypoxia, cellular energy states, and growth factors (Laplante and Sabatini, 2012). Rapamycin and growth factors enact their effects on mTOR directly and via membrane receptors respectively (Inoki et al., 2002, 2003; Yip et al., 2010). mTOR is able to sense states of hypoxia, and low energy through its upstream regulator adenosine monophosphate activated protein kinase (AMPK). AMPK senses decreased levels of cellular oxygen and energy status, such as the accumulation of adenosine monophosphate, or AMP,which is associated with decreased energy status (Inoki et al., 2003, 2006; Gwinn et al., 2008; Laplante and Sabatini,2012). Once low levels of oxygen or increased levels of AMP are sensed, AMPK is activated and inhibits MTORC1 in two ways: direct inhibition of MTORC1 or phosphorylation of the heterodimer tuberous sclerosis 1/ tuberous sclerosis 2(TSC1/2), which then directly inhibits MTORC1 (Inoki et al.,2003; Gwinn et al., 2008; Laplante and Sabatini, 2012). When MTORC1 is inhibited via direct inhibitors, AMPK or TSC1/2,the complex can no longer signal to its downstream targets and has been shown to decrease cell growth and metabolism,protein synthesis, and lipid synthesis (Sengupta et al., 2010;Laplante and Sabatini, 2012).
Progesterone (P4) is a hydrophobic steroid hormone that has been shown to have applications in an array of pathologies. Recently, P4 has been found to be a regulator of the mTOR pathway (Lee et al., 2012; Foster et al., 2014; Atif et al.,2015; Kosmas et al., 2015). P4 suppresses the mTOR pathway and mTOR expression in regulatory T cells thereby inducing regulatory T cell expression (Lee et al., 2012). Another study found that during early pregnancy, P4 levels are low and mTOR signaling is relatively unaffected (Foster et al., 2014).However, when P4 levels increased signi ficantly in late pregnancy, mTOR expression was significantly down regulated(Inoki et al., 2002). These studies suggest progesterone may be a key modulator of mTOR following injury. In addition to the effects of P4 on mTOR in the myometrium and regulatory T cells, the effects of P4 on the expression of mTOR in neural tissue have just begun to be explored. The goal of this study was to determine whether P4 modulates mTOR expression in the hippocampus of mice with TBI.
Materials and Methods
Animals
All protocols were approved by the Institutional Animal Care and Committee of the UTHSCSA (Committee Approval Number 13034X). C57B1/6 mice (22–25 g, male)(Charles River Laboratories, Wilmington, MA, USA) were randomized to sham, sham + P4, TBI, and TBI + P4 groups.
Controlled closed skull injury model
A pneumatic impact device (Precision Systems and Instrumentation, Alexandria, VA, USA) was used to generate a moderate TBI leaving the skull and dura matter intact (Talley Watts et al., 2013). The mice were anesthetized with isoflurane (3% induction, 1% maintenance) in 100% oxygen. A body temperature of 37°C was maintained using a temperature-controlled heated surgical table. A small midline incision was made on the scalp using aseptic surgical techniques. A 5 mm stainless steel disc was positioned on the skull and fixed using superglue on the right parietal surface of the skull between bregma and lamda overlying the somatosensory cortex.The mouse was then positioned on a stage directly under the pneumatic impact tip. A calibrated impact was delivered at 4.5 m/s at a depth of 2 mm. The sham mice underwent all interventions, including affixing of the steel disc, until pneumatic impact. Scalp incisions were closed using 4-0 nylon braided interrupted sutures and antibiotic ointment was subsequently applied to the incision. Mice were placed in a Thermo-Intensive Care Unit (Braintree Scientific Inc, model FV-1,Braintree, MA, USA; 37°C; 27% O2) and monitored until fully awake and moving freely. Three days post TBI, mice were anesthetized under isoflurane, sacrificed, brains harvested,and prepared accordingly for the assay to be performed.
P4 treatments
To assess the effects of P4 on mTOR, C57B1/6 mice were treated with 8 mg/kg progesterone 2-hydroxypropyl-beta-cyclodextrin complex (Sigma-Aldrich, St. Louis, MO,USA) or vehicle (2-hydroxypropyl-beta-cyclodextrin)(Sigma-Aldrich) at 1 (intraperitoneal), 6, 24, and 48 hours(subcutaneous) post closed skull TBI or sham (uninjured)(Djebaili et al., 2005). 2-Hydroxypropyl-beta-cyclodextrin was used to make progesterone water soluble given its large hydrophobic structure. The progesterone 2-hydroxypropyl-beta-cyclodextrin complex was diluted with saline (20 mg/mL) to form a stock solution. Mice were weighed prior to each administration of P4 and doses were adjusted accordingly using the 8 mg/kg conversion. Further, to ensure delivery of entire sample, each dose was brought up in 100 μL of normal saline just prior to administration. Doses for the vehicle (2-hydroxypropyl-beta-cyclodextrin complex)were calculated and administered in the same manner.
Western blot analysis
At 72 hours post injury, mice were anesthetized using isoflurane (Henry Schein Animal Health, Dublin, OH, USA)administered intranasally via the thermo-intensive care unit and subsequently decapitated. The brain was removed,placed on ice, and dissected into impacted and non-impacted hippocampus. The isolated tissue was rapidly homogenized in chilled homogenization buffer (0.32 M Sucrose, 1 mM EDTA, 1 M Tris-HCl, pH 7.8) with Phospho-STOP on ice using a Wheaton glass dounce (20 strokes). The homogenate was transferred to a 2 mL tube. Samples were vortexed and centrifuged (~11,000 × g, 10 minutes, 41°C) and the supernatant was collected. Protein concentration was determined using the Bichoninic Acid Protein assay using a 1:50 dilution following the manufacture instructions (Pierce,Dallas, TX, USA).
5 μg protein equivalents were added to lithium dodecyl sulfate-polyacrylamide and placed in a heat block for 10 minutes at 70°C. Samples were loaded on a 10% Bis-Tris precast gel (Invitrogen, Carlsbad, CA, USA) and electrophoresis was performed at 100 V for 2—2.5 hours. Proteins were transferred onto a nitrocellulose membrane (Invitrogen, Carlsbad, CA, USA) overnight (4°C at 10 V) and then blocked (0.1% Tween-20/5% bovine serum albumin prepared in Tris Buffered Saline, 1 hour, room temperature). Membranes were then incubated with primary antibodies overnight (12—14 hours, 4°C) with gentle agitation. The following antibodies were used: anti-phosphorylated mTOR (serine 2448), anti-mTOR (total) (polyclonal rabbit, 1:1,000 dilution, Cell Signaling, Danvers, MA, USA), and β-actin-HRP(1:50,000 dilution, Cell Signaling, Danvers, MA, USA). All primary antibodies were diluted in TBS/0.1% Tween-20/5%bovine albumin serum (BSA). After a 15-minute washing step (0.1% Tween-20 in PBS), the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies against appropriate species (1:50,000 dilution, Cell Signaling)in blocking buffer (1 hour, room temperature). Protein bands were visualized using SuperSignal West Femto or enhanced chemiluminescence (ECL) substrate system following manufacture directions (Pierce) and quanti fied using ImageJ soft-ware (NIH, Bethesda, MD, USA).
Immuno fluorescence
At 72 hours after injury, mice were anesthetized with iso flurane, perfused, and fixed with 4% paraformaldehyde (PFA)in 5% sucrose. The brains were harvested as described above,sectioned at 25 μm, and placed on gelatin-coated slides. The slides were dried at 37°C overnight. Next, the slides were permeabilized (100% 4°C methanol, 10 minutes), washed with 1× PBS for 5 minutes, and unspecific binding of antibodies were blocked with 1:2 dilution of Chemiblocker(Millipore, Billerica, MA, USA) for 1 hour. Brain slices were probed with primary antibodies and incubated overnight at 4°C. The following antibodies were used: anti-mTOR (#2983,1:100, Cell Signaling), anti-NeuN (#MAB377X, Millipore).After a washing step (1× PBS, 5 minute wash for three times), slices were incubated with AlexaFluor-conjugated secondary antibodies (anti rabbit for detection of mTOR;and anti-mouse for NeuN detection) (1:1,000, 1 hour, room temperature, PBS/5% BSA; Molecular Probes, Eugene, OR,USA). Coverslips were then mounted onto slides with Prolong Gold Antifade reagent with DAPI (Molecular Probes).Stained slices were visualized with an Olympus FV-1000 confocal microscope (Olympus America Inc., Center Valley,PA, USA) and images captured with FluoView v. 5.0 software (Olympus America Inc.).
Statistical analysis
GraphPad Prism software (GraphPad Software Inc, Version 6, La Jolla, CA, USA) was used to carry out all statistical analyses. One-way analysis of variance was used to compare the differences among three or more groups. The Tukey’s post hoc test was applied following initial analysis of variance. The signi ficance level was set at P < 0.05. Data are presented as the mean ± SEM.
Results
P4 decreased total mTOR levels in the hippocampus inmice after TBI
To test whether P4 altered mTOR expression in the hippocampus, total mTOR levels were first determined using western blot analysis following sham injury. Total mTOR expression was found to be signi ficantly decreased in the sham P4 group compared to the sham vehicle (V) group at 49.4± 7.4% and 57.3 ± 18.6% in the contralateral (P = 0.001, n =3) and ipsilateral (P = 0.048, n = 3) hippocampi respectively(Figures 1and2). Next, we determined the effect of P4 after TBI injury. The expression of total mTOR was signi ficantly decreased to 41.7± 8.3% and 25.0 ± 12.2% of sham V on the contralateral side in the TBI V (P < 0.05, n = 3) and TBI P4 (P< 0.05, n = 3) groups, respectively. Total mTOR expression decreased to 59.9 ± 31.7% when comparing TBI P4 to TBI V on the contralateral side, but this was not found to be statistically signi ficant (P = 0.23, n = 3). In the ipsilateral hippocampus, TBI V was signi ficantly decreased compared to sham V (P< 0.05) at 61.8 ± 8.6% (Figure 2). However, TBI P4 was not found to be signi ficantly different from sham V or TBI V in the ipsilateral hippocampus (P > 0.05).
P4 increased p-mTOR/mTOR ratio in contralateral hippocampus in mice after TBI
To determine the effect of P4 on mTOR phosphorylation at the serine 2448 site, mice were treated with P4 at 1 (intraperitoneal), 6, 24, and 48 hours (subcutaneous) after TBI and analyzed using western blot analysis 72 hours after TBI.The ratio of p-mTOR/mTOR signi ficantly increased to 450± 128% (P < 0.05) in the TBI P4 group compared to sham V in the contralateral hippocampus (Figure 3). Further,p-mTOR/mTOR in the TBI P4 group was found to be signi ficantly increased to 310 ± 116 % (P < 0.05) compared to TBI/V (Figure 3). Sham P4 and TBI/V were not found to be signi ficantly changed compared to sham V (P > 0.05;Figure3). In the ipsilateral hippocampus, no signi ficant changes in p-mTOR/mTOR were found between any groups compared to sham V (P > 0.05;Figure 4).
mTOR immunoreactivity was decreased in the hippocampus following TBI and treatment with P4
Immuno fluorescence was used to determine if this change in mTOR could be shown qualitatively in the CA3 region of the hippocampus. mTOR concentrations were localized to the cytoplasm of neurons in the hippocampus (Figure 5). mTOR was seen in the periphery of the cell and spared the central portion of the cell which was filled by the neuronal nuclear marker Neu-N.Figure 5show the CA3 region of the contralateral and ipsilateral sides of the hippocampus respectively.Data are presented as 60× images of single neurons labeled with mTOR, NeuN, and DAPI. Specifically, cytoplasmic mTOR levels appeared to be decreased in all groups compared to sham in the contralateral hippocampus (Figure 5A).In the ipsilateral hippocampus, cytoplasmic mTOR levels in the sham/P4 and TBI/V groups appeared to be decreased compared to those in the sham group (Figure 5B).
Discussion
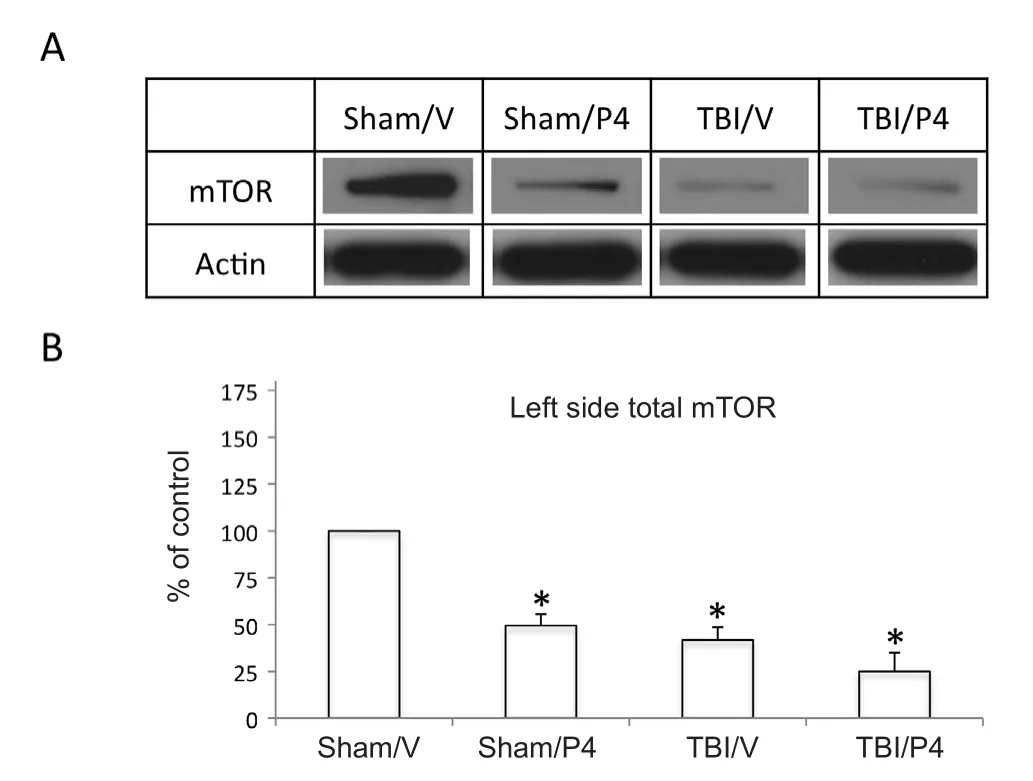
Figure 1 Progesterone (P4) treatment decreased mechanistic target of rapamycin (mTOR) protein levels and TBI further decreased mTOR protein levels in the contralateral hippocampus.

Figure 2 Progesterone (P4) decreased mechanistic target of rapamycin (mTOR) protein levels in sham treated group and traumatic brain injury (TBI) decreased mTOR levels in ipsilateral hippocampus.

Figure 3 Progesterone (P4) increased ratio of phosphorylated to non-phosphorylated mechanistic target of rapamycin (p-mTOR/mTOR) in contralateral hippocampus after traumatic brain injury(TBI).
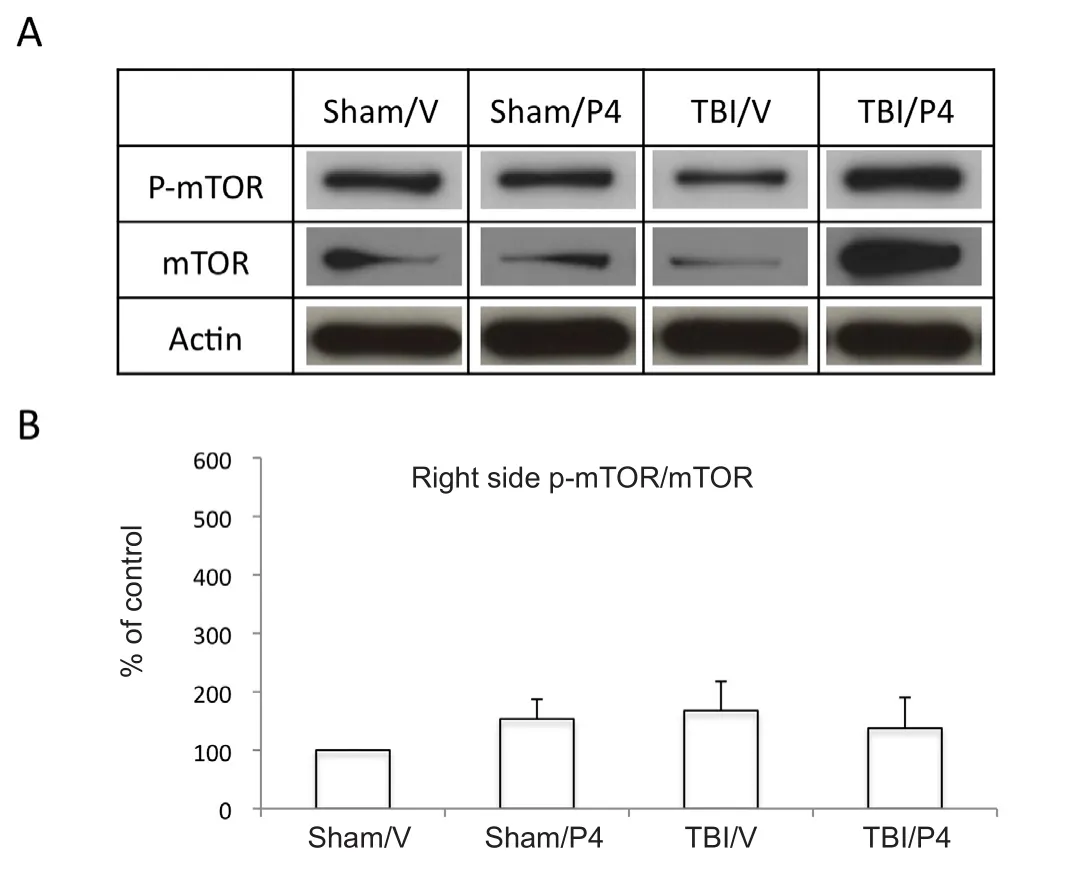
Figure 4 Progesterone (P4) has no signi ficant effect on ratio of phosphorylated to non-phosphorylated mechanistic target of rapamycin (p-mTOR/mTOR) in ipsilateral hippocampus after traumatic brain injury (TBI).
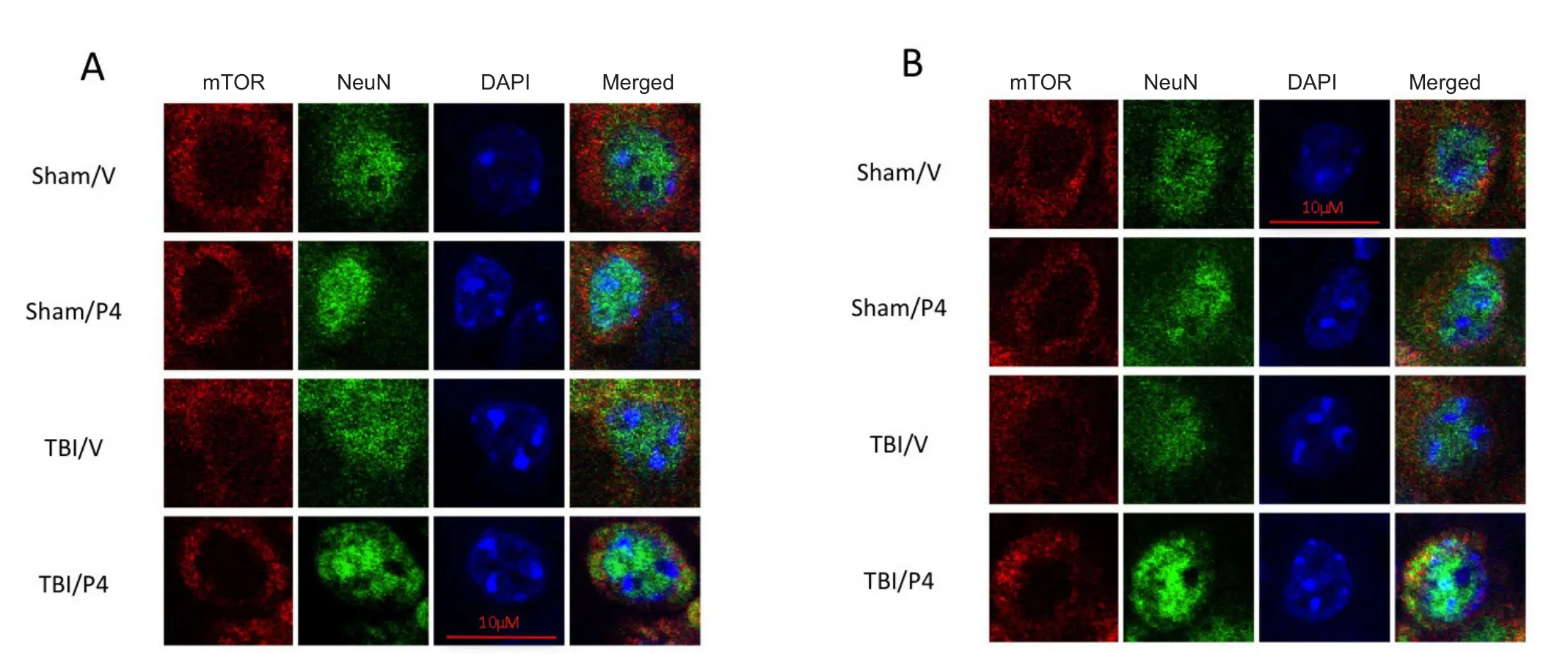
Figure 5 Mechanistic target of rapamycin (mTOR) immunoreactivity in the CA3 region of the hippocampus decreased after traumatic brain injury (TBI) and treatment with progesterone (P4).
Modulation of the mTOR is vital when nutrients and energy sources in the cellular microenvironment become limited.To date, several nutrients, growth factors, and pathologic states have been found to signi ficantly modulate the mTOR pathway. Previously, modulation of mTOR by P4 in neural tissue had only been shown in studies investigating P4 effects on mTOR pathway in the treatment of glioblastoma multiforme (Atif et al., 2015). In that study, P4 was found to suppress the mTOR pathway and thus decrease the growth of human GBM cells and increase survival in mice (Atif et al., 2015). Previous work by our lab showed that modulation of Akt expression by P4 could be extrapolated to neural cells of the hippocampus of mice undergoing TBI (Garling et al., 2014). The hippocampus was chosen because TBI is a diffuse injury that affects all regions of the brain. The hippocampus, basal ganglia, and cortex are highly susceptible to anoxic brain injury (Cervós-Navarro and Diemer, 1991).However, the cerebral cortex in humans contains 6 layers and speci fically layers 3, 5, and 6 are susceptible to hypoxia.Further, the cerebral cortex is a diffuse area and would be highly prone to sampling error when trying to isolate. The basal ganglia are not easily de finable on gross dissection of mouse brain and would also have high sampling error. The hippocampus is easily isolated in a mouse model and thus provided an area of brain highly susceptible to hypoxia and easy to isolate in its entirety. Our current study expanded upon this previous work to determine whether mTOR expression in the hippocampus could be suppressed by P4 in a congruent model. We found that P4 is a signi ficant modulator of mTOR phosphorylation and suppressor of mTOR expression in the hippocampus of mice with TBI.
Phosphorylation of mTOR at the serine 2448 site allows for the formation of MTORC1. Under normal conditions, when MTORC1 is activated, cellular growth and metabolism, protein synthesis, and lipid synthesis are stimulated (Sengupta et al., 2010; Laplante and Sabatini, 2012). In our study, the ratio of p-mTOR/mTOR was found to be significantly increased compared to sham V and TBI V in the contralateral hippocampus. However, no signi ficant differences were found between groups in the ipsilateral hippocampus. The increase in p-mTOR/mTOR observed in the contralateral rather than the ipsilateral hippocampus in the TBI P4 group appears to occur due to significant suppression of overall mTOR expression but a reciprocal increase in phosphorylation of the remaining mTOR proteins. Overall, P4 treated animals had decreased levels of mTOR and TBI further decreased mTOR levels. This is consistent with our hypothesis given that TBI and P4 have been found in other experimental models to be suppressors of mTOR expression. Further, the TBI V and sham P4 groups were found to have signi ficant decreases in expression of total mTOR in the ipsilateral hippocampus. The overall decrease in mTOR expression seen in the contralateral hippocampus may be a potential mechanism to decrease cellular growth and metabolism, protein synthesis, and lipid synthesis, that would normally be stimulated by activation of MTORC1, and conserve these vital resources for the more injured ipsilateral hippocampus. This appears to be further supported by the fact our study shows that expression of total mTOR in the hippocampus is decreased in the presence of P4 alone, as seen in our uninjured sham P4 groups bilaterally in the hippocampus. However, future studies with TBI outcome measures are needed to verify this hypothesis.
P4 is a steroid hormone with significant modulatory and suppressive effects on mTOR phosphoryaltion and expression (Lee et al., 2012; Foster et al., 2014; Atif et al.,2015; Kosmas et al., 2015). We explored the effects of P4 on mTOR expression in the hippocampus of mice undergoing right-sided TBI or sham injury. TBI induces a significant amount of hypoxia and both TBI and hypoxia have been shown to modulate mTOR independent of P4 adminis-tration (Chi et al., 2006; Hellewell et al., 2010; Feng et al.,2012). When P4 was administered to TBI or sham injured mice, we found that P4 was a signi ficant inhibitor of mTOR expression in the contralateral hippocampus. In addition, P4 was found to significantly increase phosphorylation of the remaining mTOR proteins once mTOR expression had been significantly decreased. We believe these findings may be protective mechanisms to conserve nutrients and energy for the more severely injured ipsilateral hippocampus.
Limitations of this study included additional time points,investigation of further brain areas, and behavioral outcome measures, which were out of the scope of this paper. Given that stressors such as hypoxia and TBI are proven modulators of mTOR in neural and glial cells, the suppressive and modulatory effects of progesterone on mTOR expression and phosphorylation should be further explored with additional time points and outcome measures as a potential neuroprotective pathway in TBI.
Author contributions:RJG, LTW, and MD formulated the hypotheses,organized and designed the studies. RJG performed experiments and analyzed the data. MD contributed to reagents and supplies necessary for the study. RJG, LTW and MD wrote and edited the article and figures.All authors approved the final version of this paper.
Conflicts of interest:The author declare that no competing financial interests exist.
Financial support:None.
Research ethics:This study was approved by the Institutional Animal Care and Committee of the UTHSCSA (Committee Approval Number 13034X).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewer:Seema K. Tiwari-Woodruff, UCR School of Medicine, USA.
Atif F, Yousuf S, Stein DG (2015) Anti-tumor effects of progesterone in human glioblastoma multiforme: role of PI3K/Akt/mTOR signaling.J Steroid Biochem Mol Biol 146:62-73.
Carney N, Totten AM, O’reilly C, Ullman JS, Hawryluk GW, Bell MJ,Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM (2017)Guidelines for the management of severe traumatic brain injury.Neurosurgery 80:6-15.
Chen S, Atkins CM, Liu CL, Alonso OF, Dietrich WD, Hu BR (2007)Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab 27:939-949.
Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA (1993) The role of secondary brain injury in determining outcome from severe head injury. J Trauma 34:216-222.
Chi JH, Knudson MM, Vassar MJ, McCarthy MC, Shapiro MB, Mallet S, Holcroft JJ, Moncrief H, Noble J, Wisner D, Kaups KL, Bennick LD, Manley GT (2006) Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J Trauma 61:1134-1141.
Copp J, Manning G, Hunter T (2009) TORC-speci fic phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res 69:1821-1827.
Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG (2005) The neurosteroids progesterone and allopregnanolone reduce cell death,gliosis, and functional de ficits after traumatic brain injury in rats. J Neurotrauma 22:106-118.
Feng JF, Zhao X, Gurkoff GG, Van KC, Shahlaie K, Lyeth BG (2012)Post-traumatic hypoxia exacerbates neuronal cell death in the hippocampus. J Neurotrauma 29:1167-1179.
Fletcher L, Evans TM, Watts LT, Jimenez DF, Digicaylioglu M (2013)Rapamycin treatment improves neuron viability in an in vitro model of stroke. PLoS One 8:e68281.
Foster HA, Davies J, Pink RC, Turkcigdem S, Goumenou A, Carter DR, Saunders NJ, Thomas P, Karteris E (2014) The human myometrium differentially expresses mTOR signalling components before and during pregnancy: evidence for regulation by progesterone. J Steroid Biochem Mol Biol 139:166-172.
Foster KG, Fingar DC (2010) Mammalian target of rapamycin (mTOR):conducting the cellular signaling symphony. J Biol Chem 285:14071-14077.
Garling RJ, Watts LT, Sprague S, Fletcher L, Jimenez DF, Digicaylioglu M (2014) Does progesterone show neuroprotective effects on traumatic brain injury through increasing phosphorylation of Akt in the hippocampus? Neural Regen Res 9:1891-1896.
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A,Vasquez DS, Turk BE, Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30:214-226.
Hellewell SC, Yan EB, Agyapomaa DA, Bye N, Morganti-Kossmann MC (2010) Post-traumatic hypoxia exacerbates brain tissue damage: analysis of axonal injury and glial responses. J Neurotrauma 27:1997-2010.
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577-590.
Inoki K, Li Y, Zhu T, Wu J, Guan KL (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4:648-657.
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q,Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955-968.
Jones PA, Andrews PJ, Midgley S, Anderson SI, Piper IR, Tocher JL,Housley AM, Corrie JA, Slattery J, Dearden NM (1994) Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol 6:4-14.
Kosmas IP, Kitsou C, Lazaros L, Markoula S, Peschos D, Mynbaev O,Tournaye H, Prapas N, Prapas I, Zikopoulos A, Galani V, Georgiou I(2015) Everolimus, an mTOR pathway inhibitor, is highly successful on ovarian hyperstimulation syndrome by reducing ovarian weight and progesterone levels: a preclinical experimental randomized controlled study. Gynecol Endocrinol 31:702-707.
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274-293.
Lee JH, Lydon JP, Kim CH (2012) Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J Immunol 42:2683-2696.
Liu L, Wise DR, Diehl JA, Simon MC (2008) Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem 283:31153-31162.
Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40:310-322.
Talley Watts L, Sprague S, Zheng W, Garling RJ, Jimenez D, Digicaylioglu M, Lechleiter J (2013) Purinergic 2Y1 receptor stimulation decreases cerebral edema and reactive gliosis in a traumatic brain injury model. J Neurotrauma 30:55-66.
Taylor CA, Bell JM, Breiding MJ, Xu L (2017) Traumatic brain injury-related emergency department visits, hospitalizations, and deaths- United States, 2007 and 2013. MMWR Surveill Summ 66:1-16.
Yip CK, Murata K, Walz T, Sabatini DM, Kang SA (2010) Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell 38:768-774.
杂志排行
中国神经再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Cerebral ischemia and neuroregeneration
- SNARE complex in axonal guidance and neuroregeneration
- Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease have impaired microglial function and defective repair of myelin damage
- The relaxin peptide family – potential future hope for neuroprotective therapy? A short review
- Roles of neural regeneration in memory pharmacology
