运输过程中水质和鱼类生理指标的变化及运输控制策略
2018-03-30张云龙贺亚蒙张子涵丁淑荃杨启超张志强鲍传和沈志刚
张云龙 贺亚蒙 袁 娟 张子涵 丁淑荃 杨启超 张志强鲍传和 万 全 沈志刚
(1. 安徽农业大学动物科技学院, 合肥 230036; 2. 肥东县水产技术推广站, 肥东 231600;3. 华中农业大学水产学院, 武汉 430070)
在水产养殖中, 活体运输是鱼类经常需要面临的应激之一[1]。鱼类运输具有重要的意义, 主要应用于食用鱼销售[2,3]; 苗种转运至成鱼养殖设施[4];野生鱼类人工驯养[2,5]以及运输至研究机构中[6]等。出于不同的目的, 养殖鱼类在生活史的不同阶段均可能面临应激胁迫[7], 然而, 应激会导致鱼类氧化应激、细胞凋亡和免疫力下降[8—10], 进而导致病原感染和死亡。因此, 制定标准的鱼类运输操作规程以最小化应激反应对水产养殖业是至关重要的,可降低鱼类运输后的死亡率、提高经济效益。相关的科学研究也是以此为主要目的而进行的。本文综述了已有的相关资料, 旨在整合相关的文献信息, 分析鱼类运输过程中生理指标及水质的变化,并为鱼类运输操作标准规程的制定提供理论依据。
1 运输对水质的影响
鱼类的运输方式各不相同, 最主要的表现为运输时使用的容器, 其主要取决于鱼的种类、个体规格以及生态习性等。幼鱼以及个体规格较小的鱼类常用的运输容器为塑料袋, 如黑点无须■(Puntius filamentosus)[11]、翘嘴鲌(Culter alburnus)幼鱼[12,13]、斑点叉尾鲙(Ictalurus punctatus)幼鱼[14]等, 而一些规格较大的鱼则使用盒、箱子或桶等较大的容器, 如大西洋鳕(Gadus morhua)[15]、库鲁鲁■(Potamotrygon cf. histrix)[16]、刀鲚(Coilia nasus)[4]、巴鲣(Euthynnus affinis)[17]等, 甚至有使用网箱直接在海水中运输规格特别大的鱼类, 如蓝鳍金枪鱼(Thunnus thynnus)[3]。由此可见, 运输会将鱼类限制在一个小环境水体中, 因而水质对运输的成活率、效率以及鱼类福利起到了至关重要的作用。对黄尾■(Seriola lalandi)[18]和大西洋鲑(Salmo salar)[19]的研究中发现, 运输会造成鱼类代谢速率明显加快, 继而引起耗氧率增加和代谢废物排泄量的(CO2和氨氮)增加, CO2溶于水中又会导致水体pH降低。CO2作为一种代谢产物, 对鱼类具有很大的生理副作用。CO2在血浆中富集会导致血浆酸化, 降低血液的携氧能力和血红蛋白对氧气的亲和力[20]。运输过程中水质的恶化很大程度上受到运输密度、鱼的规格以及运输时间的影响。表 1中列出了一些鱼类运输过程对运输容器中水质的影响状况, 可见运输对容器中水质具有显著的影响, 常用评价指标有溶解氧、总氨氮及pH。
由于运输过程中水质的恶化会影响运输鱼类的成活率, 如何维持运输过程中的水质是急需解决的问题, 具有十分重要的应用价值。溶解氧是相对较为容易控制的水质指标之一, 开放容器中常用持续曝气的方法维持水体溶解氧[21], 密闭容器(如氧气袋)常用充纯氧来维持[12,13]。水体pH的降低则可通过添加缓冲剂来维持[22]。而鱼类运输过程中水体氨氮和CO2则并不是那么容易控制的, 且氨氮对鱼类具有较强的毒理作用[23,24], CO2对鱼类也具有明显的副作用[20]。运输过程中如果溶氧充足, 鱼类会进行有氧代谢消耗机体储存的糖原, 产生大量的CO2, 致使周围水体和血浆酸化; 如果溶氧不足, 鱼类则会进行无氧代谢, 产生大量的乳酸, 致使机体酸中毒。待机体储存糖原耗尽后, 鱼类则会代谢机体蛋白或氨基酸以维持能量供应, 这一过程则会产生氨。就此来说, CO2的大量释放以及水体的酸化在短途运输中会经常出现, 而氨氮的大量排泄则常出现在长途运输中。CO2会在鱼类运输开始后的24h内迅速上升, 随着运输时间的增加, CO2的排泄速率则明显下降, 而氨排泄速率则明显上升[25]。由此可见, CO2排泄速率这一指标在鱼类运输中具有非常重要的意义, 然而这一指标在现有研究中的应用却并不多[22]。此外, 随着运输时间的增加, 氨排泄速率会明显上升, 说明了长途运输中容器内水质调控的难度更大[26]。
2 运输对鱼类生理的影响
运输会对鱼类造成应激反应, 首先表现在生理指标的变化上, 造成鱼体外与体内稳态的变化[22]。鱼类对应激的生理反应可分为3个阶段, 第一阶段是神经系统接受外界应激信号并迅速分泌肾上腺素, 刺激鱼类头肾组织释放皮质醇[22], 随后皮质醇会调动体内储备的能量以提供调节渗透压所需的氧气和能量[38,39], 因此血浆皮质醇水平也成为了评价鱼类应激第一阶段最重要的指标之一。第二阶段主要涉及生理生化方面的代谢反应, 包括血浆和组织中代谢产物、血液学指标的变化以及应激蛋白(如热休克蛋白)的激活等[37,40—42], 研究鱼类应激第二阶段最常用的指标则是血糖含量。第三阶段主要涉及应激对鱼类造成的生长、繁殖、抗病力、代谢能力以及存活的影响[43,44], 然而有关鱼类运输应激第三阶段的研究却相当缺乏。表 2总结了运输应激对鱼类血浆中生理指标的影响。从表中可看出, 现有的研究主要针对于淡水鱼类的短途运输(运输时间<8h), 而有关海水鱼类以及长途运输(运输时间>8h)的报道则相对较少, 而且评价指标也以血浆皮质醇和血糖含量为主、缺乏应激第三阶段的研究。
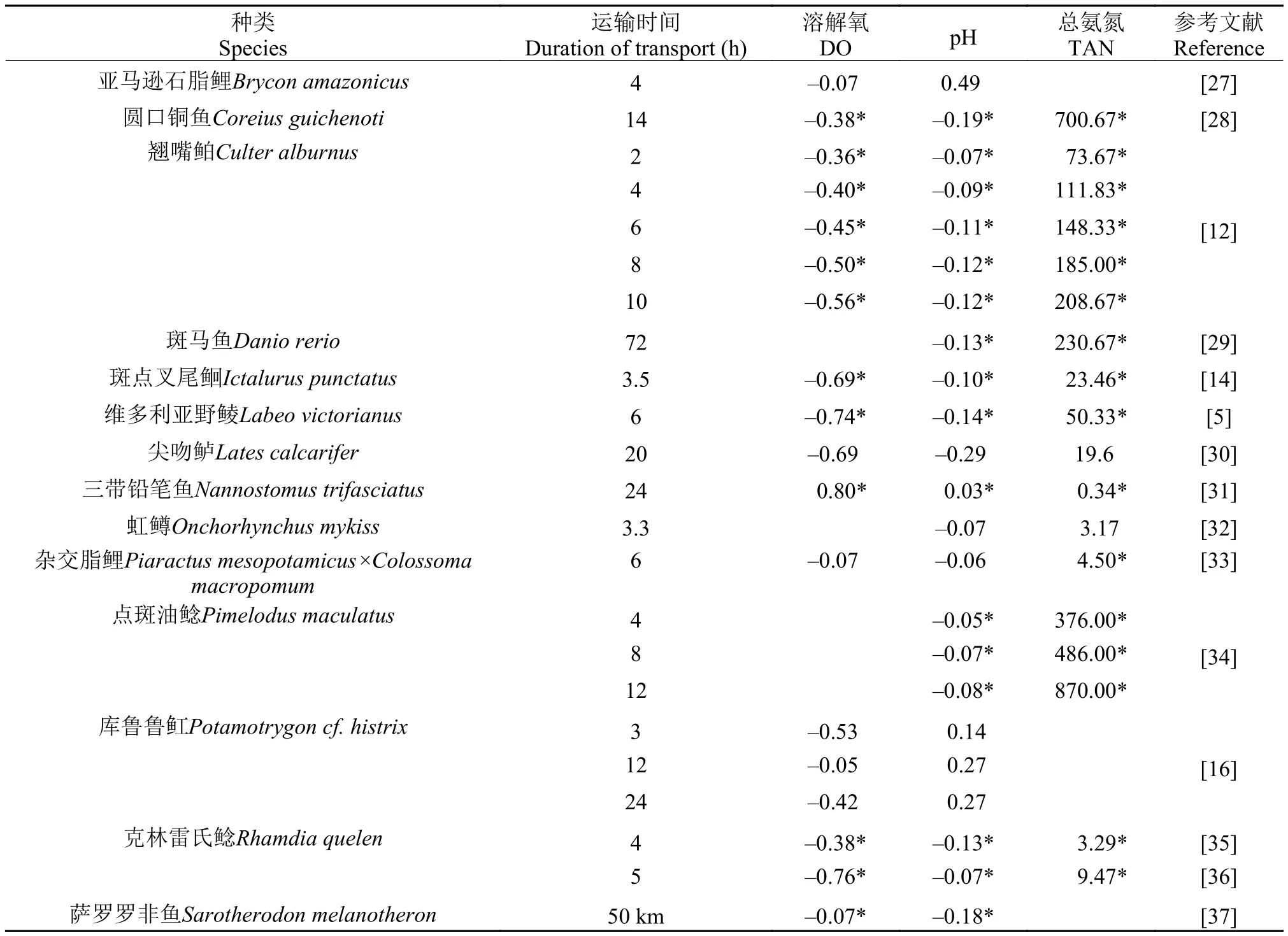
表 1 鱼类运输后水体中溶氧、pH及总氨氮的增加倍数Tab. 1 Fold changes in dissolved oxygen, pH and total ammonia nitrogen after live fish transport
2.1 运输应激第一阶段
从表 2可看出, 血浆皮质醇是最经典、最常用的鱼类应激评价指标之一, 由鱼类头肾组织分泌,受下丘脑-垂体-肾间轴调控[58]。鱼类在应激的初期阶段会迅速分泌皮质类固醇和儿茶酚胺, 尤其是肾上腺素[59], 但肾上腺素增加的持续时间却远比皮质醇增加的时间短, 因此血浆皮质醇被广泛用于鱼类应激的评价中。皮质醇的释放会对鱼类造成多种不利的生理影响, 如降低免疫力、刺激蛋白分解及引起糖异生反应等(图 1)[22,60,61]。然而, 运输应激会诱导鱼类血浆皮质醇含量显著增加, 如细鳞肥脂鲤(Piaractus mesopotamicus)[55]、斑点叉尾鲙[14]、大口黑鲈(Micropterus salmoides)[51]及翘嘴鲌[12]等(表2)。血浆皮质醇对外界应激因子的反应是非常迅速的, 一般10min之内即可发现显著的上升, 反应较慢的也在2—24h内迅速释放, 而且一旦外界应激因子消失, 血浆皮质醇会在24h内恢复到基础水平[22]。鱼体内皮质醇的消除主要在肝脏中进行[62], 皮质醇的消除速度可能与环境因子相关, 但应激、盐度、性成熟度、营养状况等因子会抑制鱼体内皮质醇的消除[63]。然而, 鱼类处于运输胁迫时, 其血浆皮质醇会迅速上升并持续相当长的时间(表 2), 甚至在整个运输过程中均保持非常高的水平[64]。从这个角度来看, 血浆皮质醇指标适用于鱼类短途(<8h)和长途(>8h)运输应激的评价。值得一提的是, 一些仔鱼及小型观赏鱼的血液采集是非常困难的, 通常的解决办法是通过合并血液样本或者测定全鱼皮质醇水平[12,29,49,65]。此外, 鱼类在运输之前通常需要手工搬运, 这也会对鱼类造成应激反应, 此时尽管还未开始运输, 其血液皮质醇水平仍然不能作为正常的基础水平。为了解决这些问题, 一些报道提供了许多措施, 主要可分为2大类, 一类是使用麻醉剂, 如间氨基苯甲酸乙酯甲磺酸盐(Tricaine methanesulphonate, MS-222)[13,28,51]、植物精油[56,66]、丁香提取物[67]等; 另一类是使用非应激手段(Non invasive methods), 其目的在于去除或最小化取样造成的应激胁迫, 如检测鱼类释放到水体中的皮质醇[15,68]及检测粪便中的皮质醇[69]等。然而, 鱼体内皮质醇的排出主要通过鳃组织[70], 因而检测粪便中的皮质醇含量的准确度并不够。总体来看, 皮质醇作为评价鱼类运输应激指标适用于短途和长途运输, 但其检测方法仍需进行改善, 检测鱼类释放至水体中的皮质醇是非常有效的非应激手段之一, 对采血不便的小型鱼类同样适用且可消除取样引起的应激反应。
2.2 运输应激第二阶段
鱼类在应激第一阶段会释放大量皮质醇, 诱导机体糖原分解和糖异生作用以应对应激胁迫(图 1),血糖含量及糖原分解的增加被认为是应激的第二阶段。肝脏和肌肉糖原含量作为应激评价指标的原理与血糖基本是一致的, 近年的报道也较多[52,56,65,71]。在通常情况下, 运输应激会导致鱼类血糖含量上升、糖原含量降低(表 2), 其原因主要是鱼类在应激状态下会消耗更多的能量。鱼类应激状态下血糖含量也受到多种因素的影响, 包括种类、生长阶段、营养状况等, 如营养状况会影响点带石斑鱼(Epinephelus coioides)寒冷胁迫下血糖的含量[72]。皮质醇的释放已被证实会刺激鱼类血糖含量的上升, 但塞内加尔鳎(Solea senegalensis)在中密度拥挤胁迫下血糖含量显著高于低密度组, 尽管其血浆皮质醇含量与低密度组无差异[73]。这种增加显然不是皮质醇诱导的, 可能是由于儿茶酚胺的释放引起的(图 1)[74,75]。因此, 要确认鱼类在运输应激下血糖增加是否由皮质醇诱导, 研究鱼类皮质醇的基础水平是非常重要的。从表 2中可看出, 随着运输时间的延长, 鱼类血糖含量呈现下降的趋势, 说明鱼体内贮存能量的耗尽。也就是说血糖作为鱼类长途运输应激评价指标的适用性并不如短途运输, 而且长途运输时血糖测定的间隔时间也应该更短, 有助于更直观的评价鱼类在应激时的生理应答。
乳酸是鱼体肌肉无氧代谢产物, 鱼类在应激条件下肌肉无氧代谢会显著增加, 导致血浆乳酸浓度升高, 如圆口铜鱼(Coreius guichenoti)[28]、大黄鱼(Larimichthys crocea)[65]、长吻裸颊鲷(Lethrinus miniatus)[76]等。鱼类运输后血浆乳酸浓度的升高说明了运输后机体供氧的不足, 如何保证运输期间充足的机体供氧是急需解决的关键问题之一。温度对鱼类应激条件下血浆乳酸含量可能起到某些特殊的作用[77,78], 温度对鱼类血浆乳酸含量的影响也是后续研究需要关注的方向之一。乳酸作为一种无氧代谢产物, 对机体是有危害的, 但对斑马鱼(Danio rerio)的研究发现乳酸在寒冷胁迫下可作为能量以维持机体生理稳态[79]。后续的研究可以关注乳酸在鱼类运输应激下是否具有类似的作用及其调节机制。
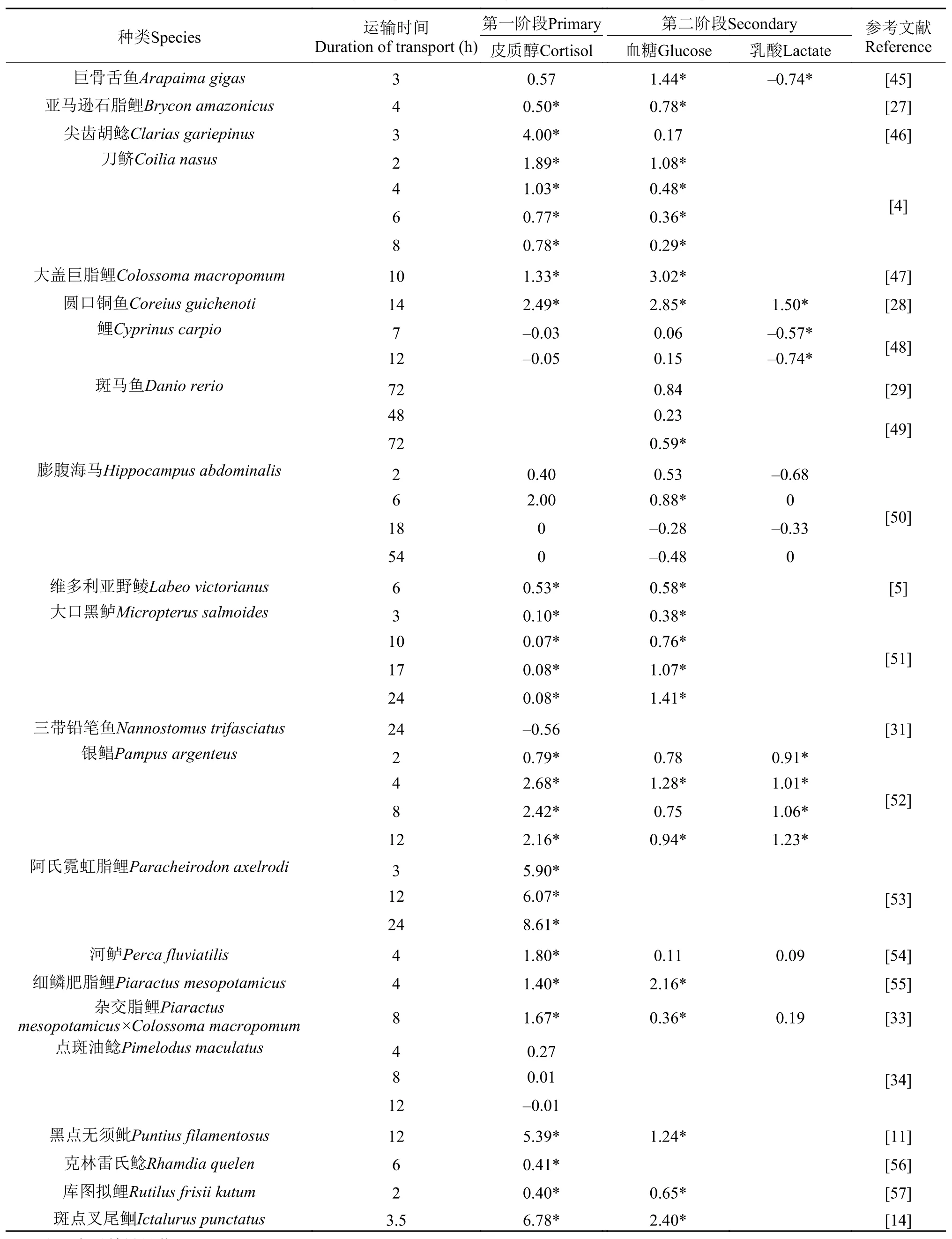
表 2 运输后鱼类血浆中皮质醇、葡萄糖及乳酸含量的增加倍数Tab. 2 Fold changes in plasma cortisol, glucose and lactate after transport

2.3 其他应激指标
由于外界环境的可变性, 鱼类血浆的渗透压也是可调节的, 但相对是较为稳定的。淡水鱼类的血浆渗透压为230—280 mOsmol/kg, 海水鱼类为300—350 mOsmol/kg, 两者相差并不大[22]。血浆渗透压作为鱼类运输应激评价指标的报道并不多, 可见维多利亚野鲮(Labeo victorianus)[5]、圆口铜鱼[28]、刀鲚[4]及库鲁鲁■[16]等。但其是反映鱼类自身内稳态的重要指标之一, 而且很大程度上受到鱼体内皮质醇的影响[80], 因此将血浆渗透压作为鱼类运输应激的评价指标是非常必要的。除此之外, 也有研究将血浆无机盐离子作为鱼类运输应激的评价指标之一, 报道较多的是血浆Na+离子、Cl-离子和K+离子, 因为这些离子对鱼类渗透压影响较大, 如维多利亚野鲮[5]、库鲁鲁■[16]、三带铅笔鱼(Nannostomus trifasciatus)[31]、细鳞肥脂鲤[55]、小锯盖鱼(Centropomus parallelus)[81]、克林雷氏鲶(Rhamdia quelen)[36]等。
血液学指标也被用于评价鱼类运输应激[27,37,41],但其对应激的响应时间较长, 用于评价鱼类长途运输应激和鱼类应激第二阶段可能更加合适。近年来, 随着研究手段的进步, 应用于评价鱼类运输应激的生理指标也越来越多, 如肌肉品质[14,82]、核苷酸含量[21]、性激素[57]、对氧磷酶活性[33,66]、血氨浓度[5]、耗氧率[12]、基因表达水平[9,83,84]、转录组学[49]等。这些指标在特定的研究条件和研究目的下, 可作为经典应激评价指标(皮质醇和血糖)的补充资料, 对真实反映特定状态下鱼类运输后的应激生理具有重要意义。
3 应用中降低运输应激的措施
鱼类运输在生产中应用十分广泛, 如何在运输过程中维持水质指标、尽可能的降低应激反应是相关研究重点关注的方向之一, 对提高运输成活率起到关键的作用, 常用的措施主要有i)增加水体溶解氧, ii)调整水体盐度, iii)添加缓冲剂, iv)氨处理措施, v)降低水温, vi)麻醉运输, vii)添加益生菌,viii)合理的运输密度。
3.1 增加水体溶解氧
溶解氧是鱼类运输过程中的重要限制因子之一, 理想的运输水体中溶解氧饱和度应为100%。氧气在水体中的溶解度受水温、气体组成、盐度、气压等因素的影响[25]。充氧是鱼类运输过程中最常用的措施之一, 常用的设备有氧气罐、增氧机及液氧等, 有关这几种充氧方式的优劣点可见Harmon的综述[25]。运输开始的0.5—1h内鱼类活动会异常激烈, 对氧气的消耗也非常大, 在此期间必须保证足够的水体溶解氧。然而, 水体中过饱和的溶解氧也存在一定的危害性, 如过高的溶解氧会以气泡的形式存在并导致鱼类的气泡病[85], 而且过饱和的溶解氧是否会对鱼类造成氧化应激反应也是需要关注的问题之一。
3.2 调整水体盐度
鱼类处于运输应激状态下, 由于肾上腺素的释放会导致鱼类鳃上皮的水通透性改变。在这种情况下会引起鱼类渗透压内稳态失调[86], 进而引起运输鱼类的死亡。淡水鱼类为高渗型, 需要抑制外源水的进入和体内无机盐离子的流失, 因此在淡水鱼类运输中通常选择加入适量的盐(如NaCl、NaHCO3和海水)以形成体内外等渗的环境, 有助于降低淡水鱼类运输过程中的应激反应, 对维多利亚野鲮[5]、刀鲚[4]、虹鳟[84]等淡水鱼类的研究结果均证实了这一结论。但在巨骨舌鱼(Arapaima gigas)幼鱼运输过程中加入NaCl不仅不会降低其运输应激反应, 还会导致其渗透压调节紊乱[45], 说明适量添加盐可降低淡水鱼类运输应激似乎是因种而异的, 针对不同种类的研究仍然是必要的。有关水体盐度对海水鱼类运输应激的研究并不多, 仅见军曹鱼(Rachycentron canadum)[7]和大黄鱼[65], 后续研究应关注这一问题。
3.3 添加缓冲剂
运输会造成鱼类代谢速率明显加快, 继而排泄大量的CO2溶于水中, 导致水体pH降低。由于运输用水体积有限, 添加缓冲剂可有效的缓解水体酸化。研究表明在运输容器中加入小苏打[87]、三甲醇氨基甲烷(Tris)[88]以及氢氧化镁微粒[15]可有效缓解运输导致的水体pH降低。由于运输过程中水体中氨的含量会显著增加(表 2), 而氨的存在形式很大程度上又取决于水体pH[89]。在偏碱性环境中氨主要以分子态(NH3)形式存在, 对水生动物毒性较大; 在偏酸性环境中主要以离子态()形式存在,毒性较小。因此, 在使用缓冲剂时还应考虑到水体中氨氮的含量, 以免造成氨中毒。
3.4 氨处理措施
氨是水体中常见的环境毒素之一, 可造成鱼类鳃结构异化[90]、脑组织损伤[91]、氧化应激[92]等毒理作用。因此, 运输导致的水体氨增加也是急需处理的问题之一。甲醛亚硫酸氢钠[87]及沸石[88]等氨还原剂均被证实可有效降低运输导致的水体氨累积。考虑到水体pH对氨毒性的影响, 氨还原剂和pH缓冲剂的同时使用更利于鱼类运输过程中水质的调控。
3.5 降低水温
对于鱼类这种变温动物来说, 降低水温可降低其消化酶活性、降低新陈代谢速率[93], 也就降低了鱼类的耗氧率、CO2和氨的排泄率, 还可增加氧气在水体中的溶解率[25]。在对圆口铜鱼的研究中发现降低水温可降低运输应激并提高水质[28]。但水温降低需要精细的操作规程, 并不能骤降, 而且各种鱼类对温度的适应性也各不相同, 基础资料的累积和规范化操作规程的制定是必要的。
3.6 麻醉运输
麻醉是鱼类运输中常用的降低应激措施之一,麻醉后鱼类的代谢速率、耗氧率、CO2和氨排泄率均有明显的降低趋势。MS-222[11,13,28,51]、丁香酚[35,94,95]、苯佐卡因[11,96]、喹哪啶[96]及2-苯氧乙醇[88]等麻醉剂已广泛应用于鱼类运输中, 并取得了良好的抗应激效果。某些植物提取的精油同样对鱼类具有一定的麻醉效果, 也可用于鱼类运输抗应激, 如丁香属[67]、过江藤属[33,35,66]、阿莱藤属[36,56,97]以及罗勒属[97]等属的植物。然而, 对小锯盖鱼的研究中发现植物精油尽管可以起到麻醉的作用, 但并不能降低其运输过程中的应激反应[81]。可见麻醉在鱼类运输中的作用并不都是积极的, 针对特定种类的研究仍然是必要的。此外, 麻醉剂对鱼类也是具有一定的生理副作用的, 如常用的MS-222可造成鱼类血液学指标的变化[98]。因此, 在鱼类运输过程中使用麻醉剂是需要充分的科研理论作为支撑的, 相关的理论研究也需要加强。
3.7 添加益生菌
近年来, 一些益生菌和免疫多糖类被应用于水产养殖中, 用于提高养殖鱼类的生长速度和免疫机能[99,100]。也有将益生菌和免疫多糖应用于鱼类运输中以维持机体内稳态、提高运输成活率, 如益生菌Efinol®L可降低阿氏霓虹脂鲤(Paracheirodon axelrodi)运输过程中代谢废物的排放量, 从而维持水质、提高运输成活率[53]; 饲料中添加β-1, 3葡聚糖投喂三带铅笔鱼7d可维持其运输过程中Na+和K+内平衡和水质指标[31]。然而, 添加益生菌对库鲁鲁魟等[16]运输并没有明显的有益作用。因此, 特定益生菌和免疫多糖对特定鱼类运输过程中的作用仍需要持续研究。
3.8 合理的运输密度
鱼类运输过程中的装载密度受到水质、运输时间、水温、规格和鱼的种类等因素影响[25], 运输密度反之又会影响鱼类运输过程中的应激反应和水质[12,34]。过高的运输密度会导致应激更强、代谢废物排放更多、水质恶化更快, 运输成活率显著降低; 过低的运输密度则意味着更高的运输成本。合理的运输密度是鱼类运输操作规程中重要的组成部分之一, 随着运输时间的延长, 运输密度应相应降低。
4 结论
运输在水产养殖业中是非常重要的, 鱼类标准化运输操作规程的制定有利于水产养殖业的健康发展。运输会对鱼类造成应激反应, 引起生理指标和水质的变化。尽管近年来鱼类运输越来越受到关注, 相关研究报道也逐年增加, 但与世界上种类繁多的经济鱼类和观赏鱼类相比, 这些研究的量仍明显不足。水质恶化(尤其是pH降低和氨累积)是鱼类运输中急需解决的关键问题, 现有的解决措施主要是通过添加缓冲剂、氨还原剂以及降低运输鱼类代谢来实现水质调控, 然而这些解决措施尚存在一定的不稳定性。运输引起的鱼类应激反应, 常用的评价指标有皮质醇和血糖。皮质醇仍然是经典的应激评价指标, 适用于鱼类短途和长途运输,而血糖则更加适用于评价短途运输应激。某些特殊的生理指标在特定的研究条件和研究目的下, 可作为皮质醇和血糖的补充资料, 对真实反映特定状态下鱼类运输后的应激生理具有重要意义。生理影响和水质影响在鱼类运输中是相互影响的, 水质恶化会导致鱼类生理学变化, 鱼类生理学活动又会影响水质。因此, 在鱼类运输相关研究中, 水质和生理指标都是需要评价的。
5 展望
鱼类标准化运输操作规程的制定是鱼类运输相关研究的核心任务, 后续的研究应围绕这一主题进行。以下几个方面应该是后续研究应该重点关注的: 第一, 不同鱼类的生态习性和不同生活史阶段对运输的响应; 第二, 鱼类运输过程中CO2的排泄率和水体中CO2含量应该被充分认识和重视; 第三, 针对特定鱼类其适宜的运输密度、运输时间、水温、盐度、麻醉剂种类和麻醉方式等抗应激措施应被充分评估; 第四, 更加多样化的应激评价指标(如抗氧化酶等)和更加科学的测定手段应被应用到后续研究中; 第五, 干法运输可以避免运输过程中水质的恶化, 且很大程度上消除了运输密度的限制, 某些气呼吸型鱼类如大鳞副泥鳅(Paramisgurnus dabryanus)[101]等, 可在空气中(需保持体表湿润)存活相当长的时间, 其干法运输的可行性值得深究。
参考文献:
[1]Meinelt T, Schreckenbach K, Pietrock M, et al. Humic substance - Part 1: dissolved humic substances (HS) in aquaculture and ornamental fish breeding [J]. Environmental Science and Pollution Research International,2008, 15(1): 17—22
[2]Marçalo A, Pousão-Ferreira P, Mateus L, et al. Sardine early survival, physical condition and stress after introduction to captivity [J]. Journal of Fish Biology, 2008,72(1): 103—120
[3]Reglero P, Balbín R, Ortega A, et al. First attempt to assess the viability of bluefin tuna spawning events in offshore cages located in an a priori favourable larval habitat [J]. Scientia Marina, 2013, 77(4): 585—594
[4]Xu G C, Du F K, Nie Z J, et al. Effects of 10‰ salinity to the plasma osmotic pressure, cortisol, glucose and liver glycogen in Colilia nasus stressed during loading and transportation [J]. Acta Hydrobiologica Sinica, 2015, 39(1): 66—72 [徐钢春, 杜富宽, 聂志娟,等. 10‰盐度对长江刀鲚幼鱼装载和运输胁迫中应激指标的影响. 水生生物学报, 2015, 39(1): 66—72]
[5]Oyoo-Okoth E, Cherop L, Ngugi C C, et al. Survival and physiological response of Labeo victorianus(Pisces: Cyprinidae, Boulenger 1901) juveniles to transport stress under a salinity gradient [J]. Aquaculture, 2011, 319(1-2): 226—231
[6]Corrêa L L, Souza G T R, Takemoto R M, et al. Behavioral changes caused by Austrodiplosomum spp. in Hoplias malabaricus from the São Francisco River,Brazil [J]. Parasitology Research, 2014, 113(2):499—503
[7]Stieglitz J D, Benetti D D, Serafy J E. Optimizing transport of live juvenile cobia (Rachycentron canadum): Effects of salinity and shipping biomass [J].Aquaculture, 2012, 364—365: 293—297
[8]Sun P, Chai X J, Yin F, et al. Responses of antioxidant system to transport stress in the liver of Nibea japonica [J]. Marine Fisheries, 2014, 36(5): 469—474 [孙鹏, 柴学军, 尹飞, 等. 运输胁迫下日本黄姑鱼肝脏抗氧化系统的响应. 海洋渔业, 2014, 36(5): 469—474]
[9]Du F, Xu G, Gao J, et al. Transport-induced changes in hypothalamic - pituitary - interrenal axis gene expression and oxidative stress responses in Coilia nasus [J].Aquaculture Research, 2016, 47(11): 3599—3607
[10]Cheng C H, Ye C X, Guo Z X, et al. Immune and physiological responses of pufferfish (Takifuku obscurus) under cold stress [J]. Fish & Shellfish Immunology, 2017, 64: 137—145
[11]Pramod P K, Ramachandran A, Sajeevan T P, et al.Comparative efficacy of MS-222 and benzocaine as anaesthetics under simulated transport conditions of a tropical ornamental fish Puntius filamentosus (Valenciennes) [J]. Aquaculture Research, 2010, 41(2):309—314
[12]Hu P P, Liu R P, Zhao Z B, et al. Effects of transportation time and density on whole-body cortisol concentrations, oxygen consumption rate and water quality of juvenile topmouth culter, Culter alburnus [J]. Acta Hydrobiologica Sinica, 2014, 38(6): 1190—1194 [胡培培, 刘汝鹏, 赵忠波, 等. 运输时间和密度对翘嘴鲌皮质醇、耗氧率及氧气袋内水质的影响. 水生生物学报, 2014, 38(6): 1190—1194]
[13]Zhao Z B, Hu P P, Liu R P, et al. Effect of transport time and MS-222 on cortisol, lactate levels of juvenile topmouth culter and the water quality indexes in O2-aerated bags [J]. Freshwater Fisheries, 2016, 46(2):94—98 [赵忠波, 胡培培, 刘汝鹏, 等. 运输时间和MS-222浓度对翘嘴鲌皮质醇、乳酸及氧气袋内水质的影响. 淡水渔业, 2016, 46(2): 94—98]
[14]Rafaey M M, Tian X, Tang R, et al. Changes in physiological responses, muscular composition and flesh quality of channel catfish Ictalurus punctatus suffering from transport stress [J]. Aquaculture, 2017,478: 9—15
[15]Treasurer J W. Changes in pH during transport of juvenile cod Gadus morhua L. and stabilisation using buffering agents [J]. Aquaculture, 2012, 330—333:92—99
[16]Brinn R P, Marcon J L, McComb D M, et al. Stress responses of the endemic freshwater cururu stingray(Potamotrygon cf. histrix) during transportation in the Amazon region of the Rio Negro [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2012, 162(2): 139—145
[17]Bar I, Dutney L, Lee P, et al. Small-scale capture,transport and tank adaptation of live, medium-sized Scombrids using “Tuna Tubes” [J]. Springer Plus,2015, 4: 604
[18]Moran D, Wells R M G, Pether S J. Low stress response exhibited by juvenile yellowtail kingfish (Seriola lalandi Valenciennes) exposed to hypercapnic conditions associated with transportation [J]. Aquaculture Research, 2008, 39(13): 1399—1407
[19]Tang S, Thorarensen H, Brauner C J, et al. Modeling the accumulation of CO2during high density, re-circulating transport of adult Atlantic salmon, Salmo salar,from observations aboard a sea-going commercial livehaul vessel [J]. Aquaculture, 2009, 296(1-2): 102—109
[20]Lim L C, Dhert P, Sorgeloos P. Recent developments and improvements in ornamental fish packaging systems for air transport [J]. Aquaculture Research, 2003,34(11): 923—935
[21]Anacleto P, Maulvault A L, Barrento S, et al. Physiological responses to depuration and transport of native and exotic clams at different temperatures [J]. Aquaculture, 2013, 408—409: 136—146
[22]Sampaio F D F, Freire C A. An overview of stress physiology of fish transport: changes in water quality as a function of transport duration [J]. Fish and Fisheries, 2016, 17(4): 1055—1072
[23]Pinto M R, Lucena M N, Faleiros R O, et al. Effects of ammonia stress in the Amazon river shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae) [J].Aquatic Toxicology, 2015, 170: 13—23
[24]Zhou X, Dong Y W, Wang F, et al. The effect of high ammonia concentration on gill structure alternation and expression of sod and hsp90 genes in grass carp, Ctenopharyngodon idella [J]. Acta Hydrobiologica Sinica,2013, 37(2): 321—328 [周鑫, 董云伟, 王芳, 等. 急性氨氮胁迫对于草鱼sod和hsp90基因表达及鳃部结构的影响. 水生生物学报, 2013, 37(2): 321—328]
[25]Harmon T S. Methods for reducing stressors and maintaining water quality associated with live fish transport in tanks: a review of the basics [J]. Reviews in Aquaculture, 2009, 1(1): 58—66
[26]Young F A, Kajiura S M, Visser G J, et al. Notes on the long-term transport of the scalloped hammerhead shark (Sphyrna lewini) [J]. Zoo Biology, 2002, 21(3):243—251
[27]de Abreu J S, Sanabria-Ochoa A I, Gonçalves F D, et al. Stress responses of juvenile matrinxã (Brycon amazonicus) after transport in a closed system under different loading densities [J]. Ciência Rural, 2008,38(5): 1413—1417
[28]Zhao J, Zhu Y, He Y, et al. Effects of temperature reduction and MS-222 on water quality and blood biochemistry in simulated transport experiment of largemounth bronze gudgeon, Coreius guichenoti [J]. Journal of the World Aquaculture Society, 2014, 45(5):493—507
[29]Dhanasiri A K S, Fernandes J M O, Kiron V. Acclimation of zebrafish to transport stress [J]. Zebrafish, 2013,10(1): 87—98
[30]Paterson B D, Rimmer M A, Meikle G M, et al.Physiological responses of the Asian sea bass, Lates calcarifer to water quality deterioration during simulated live transport: acidosis, red-cell swelling, and levels of ions and ammonia in the plasma [J]. Aquaculture, 2003, 218(1-4): 717—728
[31]Abreu J S, Brinn R P, Gomes L V, et al. Effect of beta 1,3 glucan in stress responses of the pencilfish (Nannostomus trifasciatus) during transport within the rio Negro basin [J]. Neotropical Ichthyology, 2014, 12(3):623—628
[32]Shabani F, Erikson U, Beli E, et al. Live transport of rainbow trout (Onchorhynchus mykiss) and subsequent live storage in market: Water quality, stress and welfare considerations [J]. Aquaculture, 2016, 453:110—115
[33]Sena A C, Teixeira R R, Ferreira E L, et al. Essential oil from Lippia alba has anaesthetic activity and is effective in reducing handling and transport stress in tambacu (Piaractus mesopotamicus×Colossoma macropomum) [J]. Aquaculture, 2016, 465: 374—379
[34]Braun N, Nuñer A P de O. Stress in Pimelodus maculatus (Siluriformes: Pimelodidae) at different densities and times in a simulated transport [J]. Zoologia, 2014,31(1): 101—104
[35]Becker A G, Parodi T V, Heldwein C G, et al. Transportation of silver catfish, Rhamdia quelen, in water with eugenol and the essential oil of Lippia alba [J].Fish Physiology and Biochemistry, 2012, 38(3):789—796
[36]Parodi T V, Cunha M A, Becker A G, et al. Anesthetic activity of the essential oil of Aloysia triphylla and effectiveness in reducing stress during transport of albino and gray strains of silver catfish, Rhamdia quelen[J]. Fish Physiology and Biochemistry, 2014, 40(2):323—334
[37]Akinrotimi O A, Ansa E J, Owhonda K N, et al. Effects of transportation stress on haematological parameters of blackchin tilapia Sarotherodon melanotheron [J]. Journal of Animal and Veterinary Advances,2007, 6(7): 841—845
[38]Peter M C S. The role of thyroid hormones in stress response of fish [J]. General and Comparative Endocrinology, 2011, 172(2): 198—210
[39]George N, Peter V S, Peter M C S. Physiologic implications of inter-hormonal interference in fish: lessons from the interaction of adrenaline with cortisol and thyroid hormones in climbing perch (Anabas testudineus Bloch) [J]. General and Comparative Endocrinology, 2013, 181: 122—129
[40]Weber III E S. Fish analgesia: pain, stress, fear aversion, or nociception [J]? Veterinary Clinics of North America: Exotic Animal Practice, 2011, 14(1): 21—32
[41]Gupta K, Gupta R, Bharat V. Haematological changes in Cyprinus carpio subjected to transportation stress[J]. Biosciences, Biotechnology Research Asia, 2010,7(1): 343—346
[42]Zhang Y, Xu G C, Du F K, et al. Molecular characterization of hsp70 and its response to transport stress in American shad (Alosa sapidissima) [J]. Chinese Journal of Zoology, 2016, 51(2): 268—280 [张勇, 徐钢春,杜富宽, 等. 美洲鲥hsp70的分子特征及其对运输应激的应答. 动物学杂志, 2016, 51(2): 268—280]
[43]Skomal G B, Mandelman J W. The physiological response to anthropogenic stressors in marine elasmobranch fishes: a review with a focus on the secondary response [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology,2012, 162(2): 146—155
[44]Kvamme B O, Gadan K, Finne-Fridell F, et al. Modulation of innate immune responses in Atlantic salmon by chronic hypoxia-induced stress [J]. Fish & Shellfish Immunology, 2013, 34(1): 55—65
[45]Gomes L C, Chagas E C, Brinn R P, et al. Use of salt during transportation of air breathing pirarucu juveniles (Arapaima gigas) in plastic bags [J]. Aquaculture,2006, 256(1—4): 521—528
[46]Manuel R, Boerrigter J, Roques J, et al. Stress in African catfish (Clarias gariepinus) following overland transportation [J]. Fish Physiology and Biochemistry,2014, 40(1): 33—44
[47]Gomes L C, Araujo-Lima C A R M, Roubach R, et al.Effect of fish density during transportation on stress and mortality of juvenile tambaqui Colossoma macropomum [J]. Journal of the World Aquaculture Society,2003, 34(1): 76—84
[48]Dobšíková R, Svobodová Z, Blahová J, et al. Stress response to long distance transportation of common carp(Cyprinus carpio L.) [J]. Acta Veterinaria Brno, 2006,75(3): 437—448
[49]Dhanasiri A K S, Fernandes J M O, Kiron V. Liver transcriptome changes in zebrafish during acclimation to transport-associated stress [J]. PLoS One, 2013, 8(6):e65028
[50]Wright K A, Woods C M C, Gray B E, et al. Recovery from acute, chronic and transport stress in the pot-bellied seahorse Hippocampus abdominalis [J]. Journal of Fish Biology, 2007, 70(5): 1447—1457
[51]Wang L J, Cheng S K, Zhang Y J, et al. Anesthetic effects of MS-222 in simulated transportation of largemouth bass (Micropterus salmoides) [J]. Journal of Shanghai Ocean University, 2015, 24(2): 235—241 [王利娟, 程守坤, 张饮江, 等. MS-222在加州鲈鱼模拟运输中的麻醉效果. 上海海洋大学学报, 2015, 24(2):235—241]
[52]Peng S M, Shi Z H, Li J, et al. Effect of transportation stress on serum cortisol, glucose, tissue glycogen and lactate of juvenile silver pomfret (Pampus argenteus)[J]. Journal of Fisheries of China, 2011, 35(6):831—837 [彭士明, 施兆鸿, 李杰, 等. 运输胁迫对银鲳血清皮质醇、血糖、组织中糖元及乳酸含量的影响. 水产学报, 2011, 35(6): 831—837]
[53]Gomes L C, Brinn R P, Marcon J L, et al. Bebefits of using the probiotic Efinol®L during transportation of cardinal tetra, Paracheirodon axelrodi (Schultz), in the Amazon [J]. Aquaculture Research, 2009, 40(2):157—165
[54]Acerete L, Balasch J C, Espinosa E, et al. Physiological responses in Eurasian perch (Perca fluviatilis, L.)subjected to stress by transport and handling [J].Aquaculture, 2004, 237(1-4): 167—178
[55]Feitosa K C de O, Povh J A, de Abreu J S. Physiological responses of pacu (Piaractus mesopotamicus)treated with homeopathic product and submitted to transport stress [J]. Homeopathy, 2013, 102(4):268—273
[56]Zeppenfeld C C, Toni C, Becker A G, et al. Physiological and biochemical responses of silver catfish,Rhamdia quelen, after transport in water with essential oil of Aloysia triphylla (L’Herit) Britton [J]. Aquaculture, 2014, 418—419: 101—107
[57]Nikoo M, Falahatkar B. Physiological responses in wild broodstocks of the Caspian Kutum (Rutilus frisii kutum) subjected to transportation stress [J]. Journal of Applied Animal Welfare Science, 2012, 15(4): 372—382
[58]Martínez-Porchas M, Martínez-Córdova L R, Ramos-Enriquez R. Cortisol and glucose: Reliable indicators of fish stress [J]? Pan-American Journal of Aquatic Sciences, 2009, 4(2): 158—178
[59]Cook K V, McConnachie S H, Gilmour K M, et al. Fitness and behavioral correlates of pre-stress and stressinduced plasma cortisol titers in pink salmon (Oncorhynchus gorbuscha) upon arrival at spawning grounds[J]. Hormones and Behavior, 2011, 60(5): 489—497
[60]Philip A M, Vijayan M M. Stress-immune-growth interactions: cortisol modulates suppressors of cytokine signaling and JAK/STAT pathway in rainbow trout liver [J]. PLoS One, 2015, 10(6): e0129299
[61]Faught E, Vijayan M M. Mechanisms of cortisol action in fish hepatocytes [J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2016, 199: 136—145
[62]Wilson J M, Vijayan M M, Kennedy C J, et al. β-Naphthoflavone abolishes interrenal sensitivity to ACTH stimulation in rainbow trout [J]. Journal of Endocrinology, 1998, 157(1): 63—70
[63]Mommsen T P, Vijayan M M, Moon T W. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation [J]. Reviews in Fish Biology and Fisheries, 1999, 9(3): 211—268
[64]Aupérin B, Baroiller J F. Teleost Fish Handling and Transport under Reduced Stress Conditions. In: Ozouf-Costaz C, Pisano E, Foresti F, et al (Eds.), Fish Cytogenetic Techniques [M]. New York: CRC Press. 2015,1—10
[65]Zhang W, Wang Y J, Li W M, et al. Effects of transportation density and salinity on cortisol, glycogen and lactate of large yellow croaker (Larimichthys crocea)juveniles [J]. Journal of Fisheries of China, 2014,38(7): 973—980 [张伟, 王有基, 李伟明, 等. 运输密度和盐度对大黄鱼幼鱼皮质醇、糖元及乳酸含量的影响. 水产学报, 2014, 38(7): 973—980]
[66]Hohlenwerger J C, Baldisserotto B, Couto R D, et al.Essential oil of Lippia alba in the transport of Nile tilapia [J]. Ciência Rural, 2017, 47(3): e20160040
[67]Balamurugan J, Kumar T T A, Prakash S, et al. Clove extract: A potential source for stress free transport of fish [J]. Aquaculture, 2016, 454: 171—175
[68]Leong H, Ros A F H, Oliveira R F. Effects of putative stressors in public aquaria on locomotor activity, metabolic rate and cortisol levels in the Mozambique tilapia Oreochromis mossambicus [J]. Journal of Fish Biology, 2009, 74(7): 1549—1561
[69]Turner J W Jr, Nemeth R, Rogers C. Measurement of fecal glucocorticoids in parrotfishes to assess stress [J].General and Comparative Endocrinology, 2003,133(3): 341—352
[70]Ellis T, James J D, Scott A P. Branchial release of free cortisol and melatonin by rainbow trout [J]. Journal of Fish Biology, 2005, 67(2): 535—540
[71]El-Khaldi A T F. Effect of different stress factors on some physiological parameters of Nile tilapia (Oreochromis niloticus) [J]. Saudi Journal of Biological Sciences, 2010, 17(3): 241—246
[72]Cheng A C, Chen C Y, Liou C H, et al. Effects of dietary protein and lipids on blood parameters and superoxide anion production in the grouper, Epinephelus coioides (Serranidae: Epinephelinae) [J]. Zoological Studies, 2006, 45(4): 492—502
[73]Costas B, Aragão C, Mancera J M, et al. High stocking density induces crowding stress and affects amino acid metabolism in Senegalese sole Solea senegalensis(Kaup 1858) juveniles [J]. Aquaculture Research,2008, 39(1): 1—9
[74]Wagner G N, Singer T D, McKinley R C. The ability of clove oil and MS-222 to minimize handling stress in rainbow trout (Oncorhynchus mykiss Walbaum) [J].Aquaculture Research, 2003, 34(3): 1139—1146
[75]Trenzado C E, Carrick T R, Pottinger T G. Divergence of endocrine and metabolic responses to stress in two rainbow trout lines selected for differing cortisol responsiveness to stress [J]. General and Comparative Endocrinology, 2003, 133(3): 332—340
[76]Currey L M, Heupel M R, Simpfendorfer C A, et al.Blood lactate loads of redthroat emperor Lethrinus miniatus associated with angling stress and exhaustive exercise [J]. Journal of Fish Biology, 2013, 83(5):1401—1406
[77]Lankford S E, Adams T E, Cech J J Jr. Time of day and water temperature modify the physiological stress response in green sturgeon, Acipenser medirostris [J].Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2003, 153(2):291—302
[78]Clark T D, Donaldson M R, Pieperhoff S, et al.Physiological benefits of being small in a changing world: responses of coho salmon (Oncorhynchus kisutch) to an acute thermal challenge and a simulated capture event [J]. PLoS One, 2012, 7(6): e39079
[79]Tseng Y C, Liu S T, Hu M Y, et al. Brain functioning under acute hypothermic stress supported by dynamic monocarboxylate utilization and transport in ectothermic fish [J]. Frontiers in Zoology, 2014, 11: 53
[80]Evans D H, Claiborne J B. Osmotic and ionic regulation in fishes. In: Evans D H. (Eds.), Osmotic and Ionic Regulation: Cells and Animals [M]. Boca Raton:CRC Press. 2008, 295—366
[81]Tondolo J S M, Amaral L de P, Simões L N, et al. Anesthesia and transport of fat snook Centropomus parallelus with the essential oil of Nectandra megapotamica(Spreng.) Mez [J]. Neotropical Ichthyology, 2013,11(3): 667—674
[82]Farrell A P, Tang S, Nomura M, et al. Toward improved public confidence in farmed fish: a Canadian perspective on fish welfare during marine transport [J].Journal of the World Aquaculture Society, 2010, 41(2):225—239
[83]Du F, Xu G, Li Y, et al. Glyoxalase 1 gene of Coilia nasus: molecular characterization and differential expression during transport stress [J]. Fisheries Science,2016, 82(5): 719—728
[84]Tacchi L, Lowrey L, Musharrafieh R, et al. Effects of transportation stress and addition of salt to transport water on the skin mucosal homeostasis of rainbow trout(Oncorhynchus mykiss) [J]. Aquaculture, 2015, 435:120—127
[85]Crosby T C, Hill J E, Martinez C V, et al. On-farm transport of ornamental fish [A]. UF/IFAS Extension,2014, Vol. FA-119, Fisheries and Aquatic Sciences Department, Florida
[86]Portz D E, Woodley C M, Cech Jr J J. Stress-associated impacts of short-term holding on fishes [J]. Reviews in Fish Biology and Fisheries, 2006, 16(2):125—170
[87]Treasurer J W. Remediation of ammonia accumulation during live transport of juvenile cod, Gadus morhua L.,and the effects of fast period on ammonia levels and water quality [J]. Aquaculture, 2010, 308(3-4):190—195
[88]Singh R K, Vartak V R, Balange A K, et al. Water quality management during transportation of fry of Indian major carps, Catla catla (Hamilton), Labeo rohita(Hamilton) and Cirrhinus mrigala (Hamilton) [J].Aquaculture, 2004, 235(1—4): 297—302
[89]Zhang Y L, Zhang H L, Wang L Y, et al. Impact factors of ammonia toxicity and strategies for ammonia tolerance in air-breathing fish: a review [J]. Acta Hydrobiologica Sinica, 2017, 41(5): 1157—1168 [张云龙, 张海龙, 王凌宇, 等. 氨氮对鱼类毒性的影响因子及气呼吸型鱼类耐氨策略. 水生生物学报, 2017,41(5): 1157—1168]
[90]Sinha A K, Matey V, Giblen T, et al. Gill remodeling in three freshwater teleosts in response to high environmental ammonia [J]. Aquatic Toxicology, 2014, 155:166—180
[91]Rodrigues R V, Schwarz M H, Delbos B, et al. Acute exposure of juvenile cobia Rachycentron canadum to nitrate induces gill, esophageal and brain damage [J].Aquaculture, 2011, 322—323: 223—226
[92]Ching B, Chew S F, Wong W P, et al. Environmental ammonia exposure induces oxidative stress in gills and brain of Boleophthalmus boddaerti (mudskipper) [J].Aquatic Toxicology, 2009, 95(3): 203—212
[93]Zhang Y L, Yao C L, Liu R P, et al. Effect of temperature and pH on digestive enzyme activities of Misgurnus anguillicaudatus [J]. Chinese Agricultural Science Bulletin, 2013, 29(35): 69—74 [张云龙, 姚昌林,刘汝鹏, 等. 温度、pH对泥鳅消化酶活力影响的研究. 中国农学通报, 2013, 29(35): 69—74]
[94]Fang X L, Ke C L, Li L D, et al. Concentrations of eugenol assisting for transport and handling of Ctenopharyngodon idellus [J]. Science and Technology of Food Industry, 2017, 38(17): 275—278, 318 [方晓磊, 柯常亮, 李刘东, 等. 丁香酚辅助鲜活草鱼处理和运输的研究剂量. 食品工业科技, 2017, 38(17): 275—278,318]
[95]Lin M, Wang Q, Xia Y, et al. Effects of two anesthetics on survival of juvenile Culter mongolicus during a simulated transport experiment [J]. North American Journal of Aquaculture, 2012, 74(4): 541—546
[96]Hasan M, Bart A N. Improved survival of rohu, Labeo rohita (Hamilton-Buchanan) and silver carp, Hypophthalmichthys molitrix (Valenciennes) fingerlings using low-dose quinaldine and benzocaine during transport[J]. Aquaculture Research, 2007, 38(1): 50—58
[97]Benovit S C, Gressler L T, Silva L de L. Anesthesia and transport of brazilian flounder, Paralichthys orbignyanus, with essential oils of Aloysia gratissima and Ocimum gratissimum [J]. Journal of the World Aquaculture Society, 2012, 43(6): 896—900
[98]Popovic N T, Strunjak-Perovic I, Coz-Rakovac R, et al.Tricaine methane-sulfonate (MS-222) application in fish anaesthesia [J]. Journal of Applied Ichthyology,2012, 28(4): 553—564
[99]Lazado C C, Caipang C M A, Estante E G. Prospects of host-associated microorganisms in fish and penaeids as probiotics with immunomodulatory functions [J]. Fish &Shellfish Immunology, 2015, 45(1): 2—12
[100]Munir M B, Hashim R, Chai Y H, et al. Dietary prebiotics and probiotics influence growth performance, nutrient digestibility and the expression of immune regulatory genes in snakehead (Channa striata) fingerlings[J]. Aquaculture, 2016, 460: 59—68
[101]Zhang Y L, Zhang H L, Wang L Y, et al. Changes of ammonia, urea contents and transaminase activity in the body during aerial exposure and ammonia loading in Chinese loach Paramisgurnus dabryanus [J]. Fish Physiology and Biochemistry, 2017, 43(2): 631—640
