Effect of Zn deficiency and excessive bicarbonate on the allocation and exudation of organic acids in two Moraceae plants
2018-03-28KuanZhaoYanyouWu
Kuan Zhao•Yanyou Wu
1 Introduction
Zinc(Zn)deficiency is one of the most common micronutrient conditions adversely affecting plant growth in calcareous soils(Hajibol and et al.2005;Hoffl and et al.2006;Broadley et al.2010).It in fluences numerous biological processes,such as carbohydrate metabolism,reactive oxygen species,carbonic anhydrase activity,and phosphorus(P)–Zn interaction(Dickinson and Chang 2011;Gianquinto et al.2000;Ohki 1976;Rehman et al.2012).However,bicarbonate(HCO3-)toxicity is frequently associated with Zn deficiency in calcareous soils because excessive HCO3-inhibits the growth and stimulates physiological processes such as root-exuded organic acids in rice and wheat(Romera et al.1992;Wissuwa et al.2006).
Low-molecular weight organic acids,such as oxalic acid,malic acid,and citric acid,are the most abundant and widely distributed acids in plant organs,such as roots,stems,and leaves(Wang et al.2010).Derived from glycolysis,tricarboxylic acid cycle(TCA cycle),and anaplerotic pathway,these organic acidscombine with ammonium to form amino acids and then in fl uence photosynthesis,photorespiration,and other plant physiological processes(Arau´jo et al.2012;Sweetlove et al.2010).The metabolism of organic acids varies with plant species and genotypes,plant age,and nutritional status(Lo´pez-Bucio et al.2000).Organic acids accumulate in plant organs and are exuded by the roots into the rhizosphere;they also function in plant nutrition and environmental stresses(Fan et al.2007;Liu et al.2010a;Zeng et al.2008).Rootexuded organic acids,which are necessary in nutrient acquisition,metal detoxification,alleviation of anaerobic stress in roots,and pedogenesis,are mainly formed in the TCA cycle(Ryan et al.2001).Studies have been conducted to identify and determine the quantity of organic acids in root exudates with low concentrations of nutrients,such as P,iron(Fe),and Zn(Abadı´a et al.2002;Neumann and Ro¨mheld 1999;Nguyen et al.2003;Qin et al.2007).Variations in root-exuded organic acids are important mechanisms that function in response to environmental stresses(Bais et al.2006).
Under the dual effect of Zn deficiency and excessive HCO3-,organic acids highly accumulate in plant organs,and the release of root organic acids is influenced in different Zn genotypes of rice(Lee and Wool house1971;Roseetal.2011;Yang et al.1994).Low-molecular weight organic acids(predominantly malic and citric acids)excessively accumulate in roots of Zn-deficient rice because of excessive HCO3-,whereas Zn-sufficient genotypes can exude low-molecular weight organic acids from the roots(Hajibol and et al.2003,2005).The amount of root-exuded citrate and malate increases after a long-term exposure to high HCO3-and low Zn concentrations;furthermore,the amount of root exud ates of Zn-deficient rice is higher than that of Zn-sufficient genotypes(Hoffl and et al.2006;Rose et al.2011;Broadley et al.2010).Ollat et al.(2003)demonstrated that the concentrations of malic and citric acids differ in the roots of tolerant grapevine(Vitis)rootstock and in those of susceptible genotypes after 5 mM HCO3-is added under limited iron(Fe)conditions.However,few studies have established the association of organic acid levels with plant organs and root exudates.Furthermore,only a few studies have explored the effect of Zn deficiency,or excessive HCO3-,or both,on the sensitivity of organic acid allocation and exudation.Thus,allocation and exudation of organic acids were influenced as plant species-specific and nutrition-specific.
Broussonetia papyrifera and Morus alba,belonging to the family Moraceae,are characterized by high growth rate with different adaptabilities to low nutrient and excessive HCO3-environments.Some studies have shown that the two Moraceae plants have a great adaptability to low nutrient and excessive HCO3-environments in the Karst area of the Yunnan-Guizhou Plateau in southwest China.They have presented some mechanism of plants adaptability to Karst such as the mechanisms of biodiversity,photosynthesis,carbonic anhydrase (Liu etal.2010b,2011;Wu et al.2009,2011;Wu and Xing 2012).However,few studies have been concerned with the mechanism of organic acids allocation and exudation.
In the present study,the characteristics of both amounts of organic acids in the organs(root,stem,and leaf)and root exudates of B.papyrifera and M.alba were examined under the effect of Zn and HCO3-treatments.The modes of organic acid allocation and exudation of two mulberry plants in response to Zn and HCO3-stress were analyzed.Furthermore,the sensitivity of organic acid allocation and exudation to Zn deficiency,excessive HCO3-,and dual stress,respectively,was analyzed.
2 Methods
2.1 Hydroponics experiment
Seeds of the two Moraceae plants(B.papyrifera,Bp;M.alba,Ma)were surface sterilized for 5 min with 95%ethanol and for 30 min with 10%H2O2.These seeds were also washed with sterile water after each treatment.Afterward,the surface-sterilized seeds were sown and grown in 30 cm×30 cm plastic pots for 20 days.The seedlings were transferred into a modifi ed Hoagland nutrient solution(pH 8.0)containing(mM):KNO3,5.0;Ca(NO3)2·4H2O,4.0;NH4NO3,1.0;MgSO4·7H2O,1.0;H3BO3,0.05;MnSO4·4H2O,0.004;CuSO4·5H2O,0.005;ZnSO4·7H2O, 0.01; Fe(Na)EDTA, 0.03; (NH4)6-Mo7O24·4H2O,0.002.After 30 days,the plants were transferred to modified Hoagland nutrient solution with four different Zn and HCO3-concentrations obtained using ZnSO4·7H2O and sodium bicarbonate(NaHCO3)and were grown in a controlled environment with a photosynthetic photon fl ux density of 300 μmol quanta m-2s-1during a 14 h photo period,with a temperature of 25± 0.5°C,and a relative humidity of 55%± 2%.The four different treatments were prepared using the same composition of the nutrient solution with the following components:Zn-sufficiency conditions (+Zn0,with 0.02 mM ZnSO4·7H2O and without HCO3-;and+Zn10,with 0.02 mM ZnSO4·7H2O and 10 mM HCO3-);Zn-deficiency conditions(-Zn0,no ZnSO4·7H2O and no HCO3-;and-Zn10,without ZnSO4·7H2O and with 10 mM·HCO3-).Each treatment involved three plant seedlings and was performed in triplicate.The pH of all nutrient solutions was adjusted to 8.0 by using 1 MKOH before HCO3-addition.After 10 days of treatment time,root exud ates were collected from the two plants under sterile conditions.The organic acids of the plant organs(roots,stems,and leaves)were analyzed.The plant organ samples were dried at 105 °C for 30 min and at 70 °C until a constant weight was reached.Dry matter was then weighed.
2.2 Extraction of organic acids in plant organs(roots,stems,and leaves)
Organic acids were extracted from different plant organs according to a previously described method(Oliveira et al.2008).In brief,the fresh leaves(3 g)of the two plants pecies were cut into small pieces,extracted with 80 mL of 80%methanol for 6 h by using a Soxhlet extractor,and filtered.Extraction and filtration were repeated twice to extract the organic acids completely.Met hanolic extract was concentrated to dryness under a reduced pressure condition and at 40°C.The concentrated extract was re-dissolved in a solution containing0.1 m MHCl and then was stored in a freezer.
The fresh roots and stems(1 g)were ground in an ice bath with 5 mL of 0.2%meta-phosphoric acid and centrifuged for 15 min at 12500 r·min-1.The residues were extracted using 4 mL of 0.2%meta-phosphoric acid and centrifuged for another 15 min at 12500 r·min-1.The resulting supernatants were mixed,transferred to 10 mL polypropylene tubes,and stored in a freezer(Nisperos-Carriedo et al.1992).
All of the obtained samples were filtered using a 0.22 μm membrane before ion chromatography(IC)analysis was conducted.
2.3 Collection,separation,and purification of root exudates
After treatment,the plants were transferred to a 100 mL 10 mM calcium chloride(CaCl2)solution(pH 7.0)and cultured for6 h in sterile conditions.The solution containing root exudates was collected and passed through a cation exchange column(12 mm×15 mm) filled with 5 g of Amberlite IR-120B resin(H+form,Alfa Co.).Afterward,these exudates were passed through an anion exchange column(12 mm×15 mm) filled with 3 g of Dowex 1×8 resin(100 to 200 mesh;OH-form;Acros Co.).The organic acids retained on the anion exchange resin were eluted by 1 MHCl,dried using a rotary evaporator(40°C),and stored in a refrigerator at-20°C until analysis.
2.4 Analysis of organic acids in plant organs and root exudates
The contents and compositions of organic acids involved in citricacid(CA),malicacid(MA),oxalicacid(OA),succinic acid(SA)and tartaric acid(TA)in plant organs and root exudates were analyzed by IC(883 Basic IC plus,Metrohm,Swiss)coupled with a conductivity detector.The standards CA,MA,OA,SA and TA were obtained from Sigma-Aldrich-Fluka(ref.27488,02288,75688,S7501,and 251380,respectively).The fi ve kinds of organic acids were determined without requiring a regeneration solution for Metrohm suppression module(MSM).In this procedure,the following parameters were used:IC column,a Transgenomic Coregel COR-64H(7.8 mm i.d.×300 mm);eluent solution,0.4 mM·H2SO4; fl ow rate,0.6 mL·min-1;and injection volume,20 μL.The eluent solution and analyte were filtered using a 0.22 μm membrane and then ultrasonically degassed before use.
2.5 Statistical analysis
All experiments were performed in triplicate with the same treatment independently replicated.Statistical analyses of data were carried out by t-tests,one-way ANOVA,and bivariate correlations.significance was assigned at the P<0.05 level with Duncan’s test.All analyses were conducted using SPSS 17.0(SPSS Inc.,Chicago,IL,USA).
3 Results
3.1 Plant organs(roots,stems,leaves)biomass
The biomass of two Moraceae plants organs varied with the plant species and the stress of Zn and HCO3-(Table 1).Zn deficiency decreased the biomass of roots,stems and leaves when the nutrition contained HCO3-while HCO3-treatment increased the organs growth under Zn-sufficiency conditions and decreased their growth in Bp and Ma under Zn-deficiency conditions.Under the dual treatment of Zn deficiency and excessive HCO3-(-Zn10),the organs’growth of two plant species was remarkably decreased.
3.2 Effect of excessive HCO3-and Zn deficiency on organic acid accumulation in plant organs
Figure 1 shows that the total amount of organic acids in the organs of Bp and Ma increased when HCO3-concentration increased.UnderZn-sufficiency conditions,the total amount of organic acids in the organs(roots,stems,and leaves)of Bp was higher than that of Ma exposed to+Zn10 treatment(P<0.05),particularly in the leaves of the two Moraceae plants(Fig.1).By contrast,the total amounts of organic acids in the roots,stems,and leaves of Bp exposed to+Zn10 treatment increased by 34.48%,39.87%,and 96.24%,respectively (Fig.1a);the total amounts of organic acids in the roots,stems,and leaves of Ma were 55.15%,44.36%,and 47.14%,respectively(Fig.1b).Under Zn-deficiency conditions,the total amount of organic acids in plant organs under-Zn10 treatment was higher than that under-Zn0 treatment,particularly in theleaves of the two Moraceae plants.The increased ratios of organic acids in the leaves of Bp and Ma that were exposed to-Zn10 treatment were 169.71%and 67.24%,respectively(Fig.1).The total amount of organic acids did not significantly vary in the two Moraceae plants exposed to-Zn0 treatment compared with+Zn0 treatment.The total amount of organic acids significantly increased in the leaves of Bp,whereas no significant increase in the total amount of organic acids was observed in the organs of Ma exposed to-Zn10 treatment.
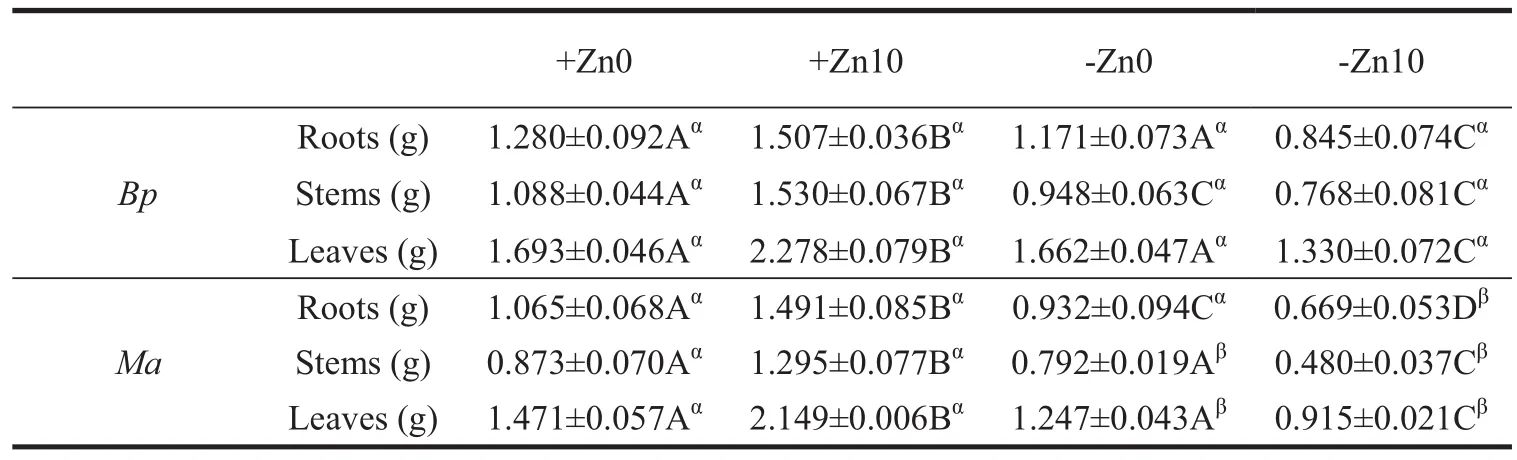
Table 1 Biomass of the two Moraceae plant organs(roots,stems,leaves)as affected by Zn and bicarbonate stress treatments

Fig.1 Organic acid content in the organs(roots,stems,and leaves)of the two Moraceae plants exposed to excessive HCO3-and Zn deficiency.Note:Blocks with bars indicate mean±SE.The different small letters indicate the significant difference of the total content of organic acids in four treatments under the same plant organs at P<0.05
3.3 Effect of HCO3-and Zn deficiency on root exuded organic acids
The amount of root-exuded organic acids increased under Zn deficiency and excessive HCO3-conditions;this increase varied with plant species(Fig.2).The total amount of rootexuded organic acids in the HCO3-addition was higher than that in no HCO3-under Zn-sufficiency or Zn-deficiency conditions.Regardless of the involvement of HCO3-,the total amounts of root-exuded organic acids in Zn-deficiency conditions were higher than that in Zn-sufficiency conditions;they did not significantly differ between Bp and Ma in Zndeficiency conditions except in-Zn10 treatment(P<0.05).In contrast,only the amount of OA in Bp significantly increased in+Zn10,-Zn0,and-Zn10 treatments.The increased values were 91.31%,194.32%,and 330.14%,respectively.By comparison,the amount of CA in Bp increased by 57.70%and 84.84%in-Zn0 and-Zn10 treatments,respectively(Fig.2a).The amount of organic acids in root exudations of Ma did not significantly vary in the treatments except in-Zn10 treatment(Fig.2b).
3.4 Allocation of organic acids among organs
The percentage of organic acids was decreased from aboveground parts(leaf and stem)to belowground parts(root)in the two Moraceae plants exposed to the fourtreatments(Table 2).The allocation of organic acids in the two Moraceae plants did not differ in+Zn0 treatment.The total amount of organic acids increased in the leaves of Bp exposed to+Zn10 treatment.Additionally,OA and CA in the leaves of Bp were accounted for approximately 50%of total amount of organic acids.In-Zn0 treatment,MA and TA were allocated at a greater extent in the leaves of Bp than in the roots and stems.SA in the roots of Bp was higher than that in the leaves and stems.The amount of organic acids in the organs of Ma did not significantly differ in+Zn10 and+Zn0 treatments.In-Zn10 treatment,OA,CA,and MA in the leaves of two Moraceae plants were higher than that of the stems or roots.The percentage of the total organic acids in the two Moraceae plants increased,particularly in the leaves of Bp exposed to-Zn10 treatment.

Fig.2 Organic acid content in the root exudates of the two Moraceae plants exposed to excessive HCO3-and Zn deficiency.Note:Blocks with bars indicate mean±SE.The different small letters indicate the significant difference of the total content of root-exuded organic acids in four treatments under the same plant species at P<0.05
3.5 Correlation of organic acids in plant organs and root exudates
The correlation data involved in the Pearson correlation coefficient and P value are presented in Table 3.The allocated organic acids exhibited a good correlation between the organs and root exudates of Ma exposed to the four treatments.By contrast,the allocated organic acids in the organs did not correlate with those in the root exudates of Bp in+Zn0 treatment;the organic acids in the leaves in+Zn10 treatment and in the roots in-Zn10 treatment did not correlate with those in the root exudates at P<0.05(Table 3).The fitted values as expressed by the linear curves are presented in Table 4.The organic acids allocated in plant organs had significance in Ma in four treatments at P<0.05.Except for the relationship of organic acids in roots and leaves under+Zn10 treatment and in roots and stems(leaves)under-Zn10 treatment,the organic acids allocated in plant organs of Bp had signi fi cant correlation in four treatments(Table 4).
4 Discussion
4.1 Organic acid accumulation and exudation in response to Zn deficiency or excessive HCO3-concentration
Organic acids accumulated in the organs of two Moraceae plants reveal the species-specific difference under Zn deficiency or excessive HCO3-stress.Zn deficiency inhibited the two plants’growth,regardless of whether or not the plant contained HCO3-.Under no HCO3-conditions,Zn deficiency induced root-exuded organic acids actively in two Moraceae plants.Bp was more sensitive than Ma because the capacity of root-exuded organic acids in Bp was stronger than that in Ma,and then much more nutrition such as P,Zn and necessary microelements can be utilized by Bp under Zn deficiency(Rengel 2015;Hoffl and et al.2006).Under HCO3-treatments,the bioaccumulation effect of organic acids did not reduce their root activity and actually accelerated the organic acids exudation in two plants.The two Moraceae plants had a slightly resistance ability on Zn deficiency.But Bp was more adaptable than Ma under-Zn10,which was characterized by its organs accumulating a large amount of organic acids in the leaves of Bp.The toxic effect of excessive organic acid accumulation did not inhibit the growth of Bp and did not significant damage its root activity;however the amount of root-exuded organic acids(dominantly OA and CA)in Bp was higher than that in Ma.Bp preferred to detoxify bioaccumulated metals by pumping them into epidermal vacuoles and storing them there as weakly bounded by organic acids(Sun et al.2006).

Table 2 Allocation percentage of organic acids per plant in the organs of the two Moraceae plants exposed to four different treatments(n=3)

Table 3 Correlation coefficient(Pearson)and P-value of the fi ve types of organic acids in plant organs(roots,stems,and leaves)and in root exudates exposed to four different treatments(n=5)

Table 4 Correlation coefficient(Pearson)of the fi ve types and P-value of organic acids among roots,stems,and leaves exposed to four different treatments(n=5)
The difference in organic acid responses to different Zn and HCO3-levels between Bp and Ma may be responsible for their different abilities in relation to Zn and bicarbonate accumulation and tolerance.Bp transferred higher amounts of OA and CA from the roots to the rhizosphere than Ma.OA and CA exhibited a stronger complexation ability than MA,SA,and TA with Ca2+and Fe3+to overcome nutrient deficiency in calcareous soils(Parker et al.1995;Wang et al.2015).OA and CA were the most active forms of carboncontaining compounds as photosynthetic intermediates translocated from aboveground parts to belowground parts in Bp(Kuzyakov and Domanski 2000).On the other hand,Bp exhibited a greater HCO3-use capacity than Ma,resulting in a decrease in the toxicity of HCO3-(Zhao and Wu 2017).Furthermore,inorganic carbon accumulation increased in Bp leaves and promoted photosynthesis of Bp(Wu and Xing 2012;Wu et al.2009).HCO3-treatment promoted the two plants’growth and organic acids accumulation in two plants organs under Zn-sufficiency conditions.While their root organic acid secretion did not significantly improve,the illustrated HCO3-use capacity was enhanced in two Moraceae plants,and Zn was a necessary element in influencing plant growth and development under natural conditions.
4.2 Production and translocation of organic acids
Organic acids in plants are mainly produced in the mitochondria via the TCA cycle in dark respiration and in the glyoxysome as the intermediates of the glyoxylate cycle in photorespiration(Lo´pez-Bucio et al.2000;Haichar et al.2014).Organic acids in plants are originally from photosynthetically fixed carbon (Jones1998).Leaves are the main organs involved in photosynthesis,and stems are the secondary organs involved in the same process.The amount of organic acids decreased from aboveground(leaf and stem)parts to belowground(root and rhizosphere)parts,and this can be interpreted to mean that root-exuded organic acids are likely derived from the leaves because these plant organs are the main parts involved in photosynthesis and the site of the primary metabolism of organic compounds(Hohmann-Marriott and Blankenship 2011;Jones et al.2009).Organic compounds were transferred from aboveground parts(leaf)to belowground parts(root)via stem.The variation of organic acids in the organs was similar to that in the root exudates of the two Moraceae plants.The leaves were the main organ in which organic acids were accumulated and allocated.The organic acids that accumulated in the leaves were involved in TCA cycle and glyoxylate cycle;these acids were primarily produced during photosynthesis(Baetz and Martinoia 2013).Organic acids were transferred from the leaves to the root.The composition and concentration of organic acids in the roots and root exudates also changed.The organic acid contribution of dark respiration to photorespiration increased from the leaves and stems to the root.
Therefore,the sources of organic acids differ in the leaves and the stems.In particular,organic acids in the leaves may have been derived from dark respiration and photorespiration.By comparison,organic acids in the stems,roots,and root exudates may have been derived from dark respiration and organic acid translocation from the leaves.
5 Conclusions
The allocated and exuded organic acids in plant organs and root exudates varied with species and environmental stresses.Root-exuded organic acids may have been originally derived from the leaves.The sources of organic acids differed in the leaves,stems,root,and root exudates.Leaves were the main organ involved in organic acid production.Organic acid accumulation,translocation,and exudation in the plant-soil system were in fluenced by Zn deficiency,excessive HCO3-,and both,respectively.An increase in organic acid in the leaves resulted in an increase in root-exuded organic acids.OA and CA were the key intermediates influencing organic acid translocation and exudation;they regulated nutrient deficiency in the rhizosphere of Bp under excessive HCO3-conditions.The results and information generated in this study are valuable for future effective con figuration of species diversity.
AcknowledgementsOur work was funded by the National Key Basic Research Program of China under Grant No.2013CB956701,the National Natural Science Foundation of China under Grant No.31070365,and a Funded by talents introduction of Anqing Normal University(No.14000100032),We thank valuable comments and suggestions of the anonymous reviewers.
Compliance with ethical standards
Conflict of interestThe authors declare that they have no con fl ict of interest.
Abadı´a J,Lo´pez-Milla´n AF,Rombola`A,Abadı´a A(2002)Organic acids and Fe deficiency:a review.Plant Soil 241:75–86
Arau´jo WL,Nunes-Nesi A,Nikoloski Z,Sweetlove LJ,Fernie AR(2012)Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues.Plant Cell Environ 35:1–21
Baetz U,Martinoia E(2013)Root exudates:the hidden part of plant defense.Trends Plant Sci 19:90–98
Bais HP,Weir TL,Perry LG,Gilroy S,Vivanco JM(2006)The role of root exudates in rhizosphere interactions with plants and other organisms.Annu Rev Plant Biol 57:233–266
Broadley MR,Rose T,Frei M,Pariasca-Tanaka J,Yoshihashi T,Thomson M,Hammond JP,Aprile A,Close TJ,Ismail AM,Wissuwa M(2010)Response to zinc deficiency of two rice lines with contrasting tolerance is determined by root growth maintenance and organic acid exudation rates,and not by zinctransporter activity.New Phytol 186:400–414
Dickinson BC,Chang CJ(2011)Chemistry and biology of reactive oxygen species in signaling or stress responses.Nat Chem Biol 7:504–511
Fan HL,Wang X,Zhou W(2007)Low molecular weight organic acids in rhizosphere and their effects on cadmium accumulation in two cultivars of amaranth(Amaranthus mangostanus L.).Sci Agric Sin 40:2727–2733
Gianquinto G,Abu-Rayyan A,di Tola L,Piccotino D,Pezzarossa B(2000)Interaction effects of phosphorus and zinc on photosynthesis,growth and yield of dwarf bean grown in two environments.Plant Soil 220:219–228
Haichar FEZ,Santaella C,Heulin T,Achouak W(2014)Root exudates mediated interactions belowground.Soil Biol Biochem 77:69–80
Hajiboland R,Yang XE,Ro¨mheld V(2003)Effects of bicarbonate and high pH on growth of Zn-efficient and Zn-inefficient genotypes of rice,wheat and rye.Plant Soil 250:349–357
Hajibol and R,Yang XE,Ro¨mheld V,Neumann G(2005)Effect of bicarbonate on elongation and distribution of organic acids in root and root zone of Zn-efficient and Zn-inefficient rice(Oryza sativa L.)genotypes.Environ Exp Bot 54:163–173
Hoffl and E,Wei CZ,Wissuwa M(2006)Organic anion exudation by lowland rice(Oryza sativa L.)at zinc and phosphorus deficiency.Plant Soil 283:155–162
Hohmann-Marriott MF,Blankenship RE(2011)Evolution of photosynthesis.Annu Rev Plant Biol 62:515–548
Jones DL(1998)Organic acids in the rhizosphere—a critical review.Plant Soil 205:25–44
Jones DL,Nguyen C,Finlay RD(2009)Carbon flow in the rhizosphere:carbon trading at the soil–root interface.Plant Soil 321:5–33
Kuzyakov Y,Domanski G(2000)Carbon input by plants into the soil-review.J Plant Nutr Soil Sci 163:421–431
Lee JA,Woolhouse HW(1971)The relationship of compartmentation of organic acid metabolism to bicarbonate ion sensitivity of root growth in calcicoles and calcifuges.New Phytol 70:103–111
Liu CC,Liu YG,Guo K,Zheng YR,Li GQ,Yu LF,Yang R(2010a)In fl uence of drought intensity on the response of six woody karst species subjected to successive cycles of drought and rewatering.Physiol Plantarum 139:39–54
Liu D,Liu AH,Wang JH,Zhang YZ,Wang YA,Zhang FS,Shu HR(2010b)Organic acids in apple trees and their effects on zinc uptake and distribution under zinc deficiency.Sci Agric Sin 43:3381–3391
Liu CC,Liu YG,Guo K,Fan DY,Li GQ,Zheng YR,Yu LF,Yang R(2011)Effect of drought on pigments,osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China.Environ Exp Bot 71:174–183
Lo´pez-Bucio J,Nieto-Jacobo MF,Ramı´rez-Rodrı´guez V,Herrera-Estrella L(2000)Organic acid metabolism in plants:from adaptive physiology to transgenic varieties for cultivation in extreme soils.Plant Sci 160:1–13
Neumann G,Ro¨mheld V(1999)Root excretion of carboxylic acids and protons in phosphorus-de fi cient plants.Plant Soil 211:121–130
Nguyen NT,Nakabayashi K,Thompson J,Fujita K(2003)Role of exudation of organic acids and phosphate in aluminum tolerance of four tropical woody species.Tree Physiol 23:1041–1050
Nisperos-Carriedo MO,Buslig BS,Shaw PE(1992)Simultaneous detection of dehydroascorbic,ascorbic and some organic acids in fruits and vegetables by HPLC.J Agric Food Chem 40:1127–1130
Ohki K(1976)Effect of zinc nutrition on photosynthesis and carbonic anhydrase activity in cotton.Physiol Plantarum 38:300–304
Oliveira AP,Pereira JA,Andrade PB,Valenta˜o P,Seabra RM,Silva BM(2008)Organic acids composition of Cydonia oblonga Miller leaf.Food Chem 111:393–399
Ollat N,Laborde W,Neveux M,Diakou-Verdin P,Renaud C,Moing A(2003)Organic acid metabolism in roots of various grapevine(Vitis)rootstocks submitted to iron de fi ciency and bicarbonate nutrition.J Plant Nutr 26:2165–2176
Parker DR,Chaney RL,Norvell WA(1995)Chemical equilibria models:applications to plant research.In:Schwab AP,Goldberg S,Loeppert RH(eds)In chemical equilibria and reaction models.Soil Science Society of America,Madison,pp 163–200
Qin R,Hirano Y,Brunner I(2007)Exudation of organic acid anions from poplar roots after exposure to Al,Cu and Zn.Tree Physiol 27:313–320
Rehman HU,Aziz T,Farooq M,Wakeel A,Rengel Z(2012)Zinc nutrition in rice production systems:a review.Plant Soil 361:203–226
Rengel Z(2015)Availability of Mn,Zn and Fe in the rhizosphere.J Soil Sci Plant Nutr 15:397–409
Romera FJ,Alcantara E,de la Guardia MD(1992)Effect of bicarbonate,phosphate and high pH on the reducing capacity of Fe-de fi cient sun flower and cucumber plants.J Plant Nutr 15:1519–1530
Rose MT,Rose TJ,Pariasca-Tanaka J,Widodo Wissuwa M(2011)Revisiting the role of organic acids in the bicarbonate tolerance of zinc-efficient rice genotypes.Funct Plant Biol 38:493–504
Ryan PR,Delhaize E,Jones DL(2001)Function and mechanism of organic anion exudation from plant roots.Annu Rev Plant Physiol Plant Mol Biol 52:527–560
Sun R,Zhou Q,Jin C(2006)Cadmium accumulation in relation to organic acids in leaves of Solanum nigrum L.as a newly found cadmium hyperaccumulator.Plant Soil 285:125–134
Sweetlove LJ,Beard KF,Nunes-Nesi A,Fernie AR,Ratcliffe RG(2010)Not just a circle: fl ux modes in the plant TCA cycle.Trends Plant Sci 15:462–470
Wang HC,Ma FF,Cheng LL(2010)Metabolism of organic acids,nitrogen and amino acids in chlorotic leaves of ‘Honeycrisp’apple(Malus domestica Borkh)with excessive accumulation of carbohydrates.Planta 232:511–522
Wang YL,Almvik M,Clarke N,Eich-Greatorex S,Øgaard AF,Krogstad T,Lambers H,Clarke JL(2015)Contrasting responses of root morphology and root-exuded organic acids to low phosphorus availability in three important food crops with divergent root traits.AoB Plants.doi:10.1093/aobpla/plv097
Wissuwa M,Ismail AM,Yanagihara S(2006)Effects of zinc de fi ciency on rice growth and genetic factors contributing to tolerance.Plant Physiol 142:731–741
Wu YY,Xing DK(2012)Effect of bicarbonate treatment on photosynthetic assimilation of inorganic carbon in two plant species of Moraceae.Photosynthetica 50:587–594
Wu YY,Liu CQ,Li PP,Wang JZ,Xing DK,Wang BL(2009)Photosynthetic characteristics involved in adaptability to Karst soil and alien invasion of paper mulberry (Broussonetia papyrifera(L.)Vent.)in comparison with mulberry(Morus alba L.).Photosynthetica 47:155–160
Wu YY,Xing DK,Liu Y(2011)The characteristics of bicarbonate used by plants.Earth Environ 39:273–277
Yang X,Ro¨mheld V,Marschner H(1994)Effect of bicarbonate on root growth and accumulation of organic acids in Zn-inefficient and Zn-efficient rice cultivars(Oryza sativa L.).Plant Soil 164:1–7
Zeng F,Chen S,Miao Y,Wu F,Zhang G(2008)Changes of organic acid exudation and rhizosphere pH in rice plants under chromium stress.Environ Pollut 155:284–289
Zhao K,Wu Y(2017)Effects of Zn deficiency and bicarbonate on the growth and photosynthetic characteristics of four plant species.PLoS ONE 12(1):e0169812.doi:10.1371/journal.pone.0169812
杂志排行
Acta Geochimica的其它文章
- Heterogeneous Mg isotopic composition of the early Carboniferous limestone:implications for carbonate as a seawater archive
- Diffusion in garnet:a review
- Genesis of tuff interval and its uranium enrichment in Upper Triassic of Ordos Basin,NW China
- Constraints of molybdenite Re–Os and scheelite Sm–Nd ages on mineralization time of the Kukaazi Pb–Zn–Cu–W deposit,Western Kunlun,NW China
- An experimental study of interaction between pure water and alkaline feldspar at high temperatures and pressures
- Identification of bacterial fossils in marine source rocks in South China
