An experimental study of interaction between pure water and alkaline feldspar at high temperatures and pressures
2018-03-28TaoLiHepingLiLipingXu
Tao Li•Heping Li•Liping Xu
1 Introduction
Alkaline feldspar is widely distributed in the crust and in certain depth plates and is one of the most important ore forming minerals in many sedimentary rocks,magmatic rocks,and metamorphic rocks.At the same time,sediment compaction diagenetic process,magmatic,metamorphic dehydration,precipitation,plate subduction and seawater seepage,and other geological processes produce hightemperature and high-pressure fluid in the crust even in the upper mantle.Therefore,the interaction between alkaline feldspar and water fluid of high temperatures and high pressures is common interaction between earth materials inside the earth.Identifying the interaction between the two is an important basic work to explore the related geodynamic processes and associated diagenesis and mineralization(Gautier et al.1994;Hellmann 1994;Knauss and Wolery 1986;Hinman 2013).Feldspar and water fluid interaction experiment under the condition of high temperature and high pressure is the essential means to understand the reaction of the chemicals inside the earth.
Multiple studies on simulation experiments have been performed globally aiming at thermodynamics and dissolution kinetics of feldspar-like minerals(Adriaens et al.1999;Chardon et al.2006;Crund well 2015a,b,2016;Lu et al.2013;Fu et al.2009;Zhang et al.2009;Zhu and Lu 2013;Lu et al.2015).Previous studies mainly focused on the dissolution mechanism and kinetics of feldspar,so,there is a research blank on feldspar dissolution products.
Therefore,we have conducted a series of autoclave experiments of feldspar dissolution and secondary mineral precipitation in different conditions.The temperature ranged between 250 and 500°C,the pressure was 8–50 MPa,the pH values of the original solutions were 3.0 and 5.5.After the experiments,several techniques were used to test the samples such as Scanning Electron Microscopy(SEM),X-ray Fluorescence(XRF),X-ray diffraction(XRD),and Electron Microprobe Analysis(EMPA).
2 Experimental method
2.1 Sample preparation and characterization
The feldspar samples were obtained from a cocoa sea 3 series pegmatite area,XinJiang,China.Sampling location was located in 47°14′34.0′′N,90°00′54.0′′E.One part of the sample was cut to fl akes with a length of about 20 mm,width of about 15 mm,thickness of about 2 mm.The other part of the sample was ground with an agate mortar and pestle and then sieved to obtain the fraction between 74 and 150 um.The two parts of the feldspars were ultrasonically cleaned at least fi ve times in methanol until the methanol was clear.Then,the feldspars were ultrasonically cleaned at least three times in pure water until the water was clear.The chemical compositions of these cleaned feldspars as determined by Electron Microprobe Analysis(EMPA)are listed in Table 1.
The solutions used in this study were distilled water with the pH values of 3.0 and 5.7.This study did not consider the effect of CO2.
2.2 Experimental apparatus
The multi-function autoclave used in this work was made of stainless steel and titanium alloy double-layer structure.The outer autoclave body was made of stainless steel 1Cr18Ni9Ti,the interlining was made of titanium alloy TC11.The main sections of the autoclave include doublelayer structure autoclave body,stainless steel stoppers and nuts,red copper sealing rings,and titanium alloy pressure rings.
A schematic diagram of the multi-function autoclave experimental platform used in this study is shown in Fig.1.
The chemical composition of the sample was determined by PW1404/10 X-Ray Fluorescence(XRF),2263D/Max-2200 X-ray diffraction(XRD),and EPMA-1600 Electron microprobe analysis(EMPA).The surface mor-phology of the sample was observed by JSM-6440LV Scanning Electron Microscopy(SEM).The chemical composition of the residual solution after the reaction was tested by 96-750ICP-AES and Cary-50 ultraviolet spectrophotometer.

Table 1 Chemical composition(EMPA)of the alkali-feldspar used in this study(wt%)

Fig.1 Schematic diagram of multi-function autoclave experimental platform.1 Manual pump;2 capillary tube;3,5 high-pressure manometer;4,9 high-pressure valve;6 pressure sensor;7 sensor cable;8 computer;10 fixed link;11 stopper;12 nut;13 pressure ring;14 autoclave body;15 sample;16 Stopper for temperature and pressure measurement;17 sealing ring;18 Multimeter;19 Thermocouple;20 temperature control unit
2.3 Experimental steps
First,pour into 50 mL distilled water in the reaction autoclave.Second,put the feldspar,which was packed by titanium wire mesh,in the vertical autoclave.Third,heat the autoclave to the temperatures of 250,300,400,and 500°C.Fourth,keep the target temperature for 168 h.
After the reaction,invert the autoclave for 180°,cooled to room temperature,take out the sample and dip it in stilled water to soak out the soluble salts,dry at 105°C and weigh the matter,tested by XRF,XRD,and EMPA.Draw out the solution with a pipetting gun and transferred to a Te fl on crucible for chemical analysis.
3 Results and discussions
3.1 Mass analysis
The mass loss of the samples after the reaction is listed in Table 2.From Table 2,we found that the mass loss increased with the rise of temperature under the same pH value,which indicated the feldspar dissolved during the process of reaction and the degree of dissolution was positively correlated with temperature and pressure.The mass loss decreased with the rise of pH value in acidic pH ranges which instructed that the degree of dissolution was deepened along with the reduction of pH.
3.2 Changes of Surface morphology
The surface morphology of the sample was observed by SEM both before and after the reaction.From Fig.2,we can see that the surface of the sample before the reaction was smooth and had no obvious alteration phenomenon,the two main minerals in it were potassium feldspar(the part of light color)and albite(the part of deep color).
Compared the surface morphology of the products under different experimental conditions(Fig.3)with the initial feldspar samples,we found that different degrees of alteration happened after the reactions.The degree of alteration related to the temperature,pressure,and pH value.When the temperature was 300°C,the feldspar altered slightly,dissolution pores were found on the particle surface and there were no fi llings between the dissolution pores.When the temperature increased to 400,450,and 500°C,we found that the feldspar dissolved obviously,there were irregular corrosion marks on the surface,particularly in the direction of the cleavage fracture location.Flake or pine needle mineral aggregates which were made up of Al,Si,and O formatted between the dissolution pores.
Due to the inconsistent solubility of feldspar,the dissolution of minerals occurred fi rst in the high energyposition of the mineral surface.With the increase of temperature,the dissolution of the four elements Na,K,Al,and Si was enhanced.After the reaction to a certain extent,the feldspar surface was covered by residual secondary minerals which prevented the feldspar from contacting with aqueous solution sufficiently and led to a reduction of the ability of continuous dissolution.Meanwhile,ion concentration in the solution escalated with the increase of reaction temperature and the extension of reaction time.When the ions concentration in the solution increased to a certain extent,the dissolution rate was reduced.Therefore,in the process of interaction between geological fluid andsurrounding rocks,a continuous flow of fluid in and out is the key to the migration and enrichment of ore-forming elements.
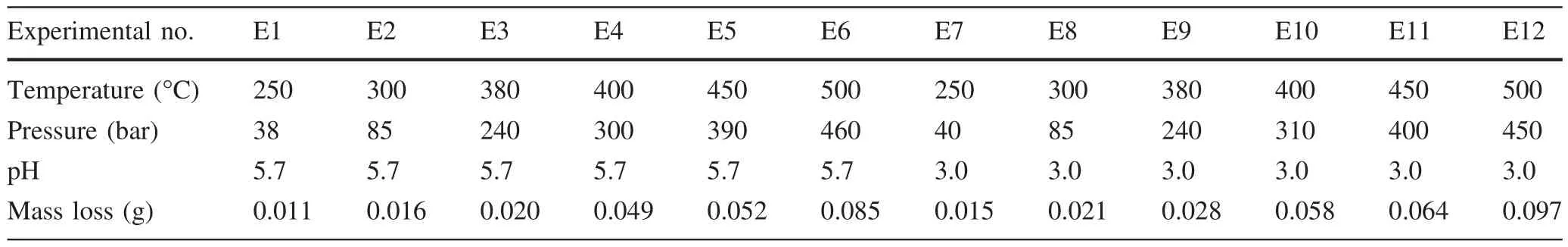
Table 2 Mass loss of the samples after reaction

Fig.2 Surface morphology of feldspar before reaction(SEM)
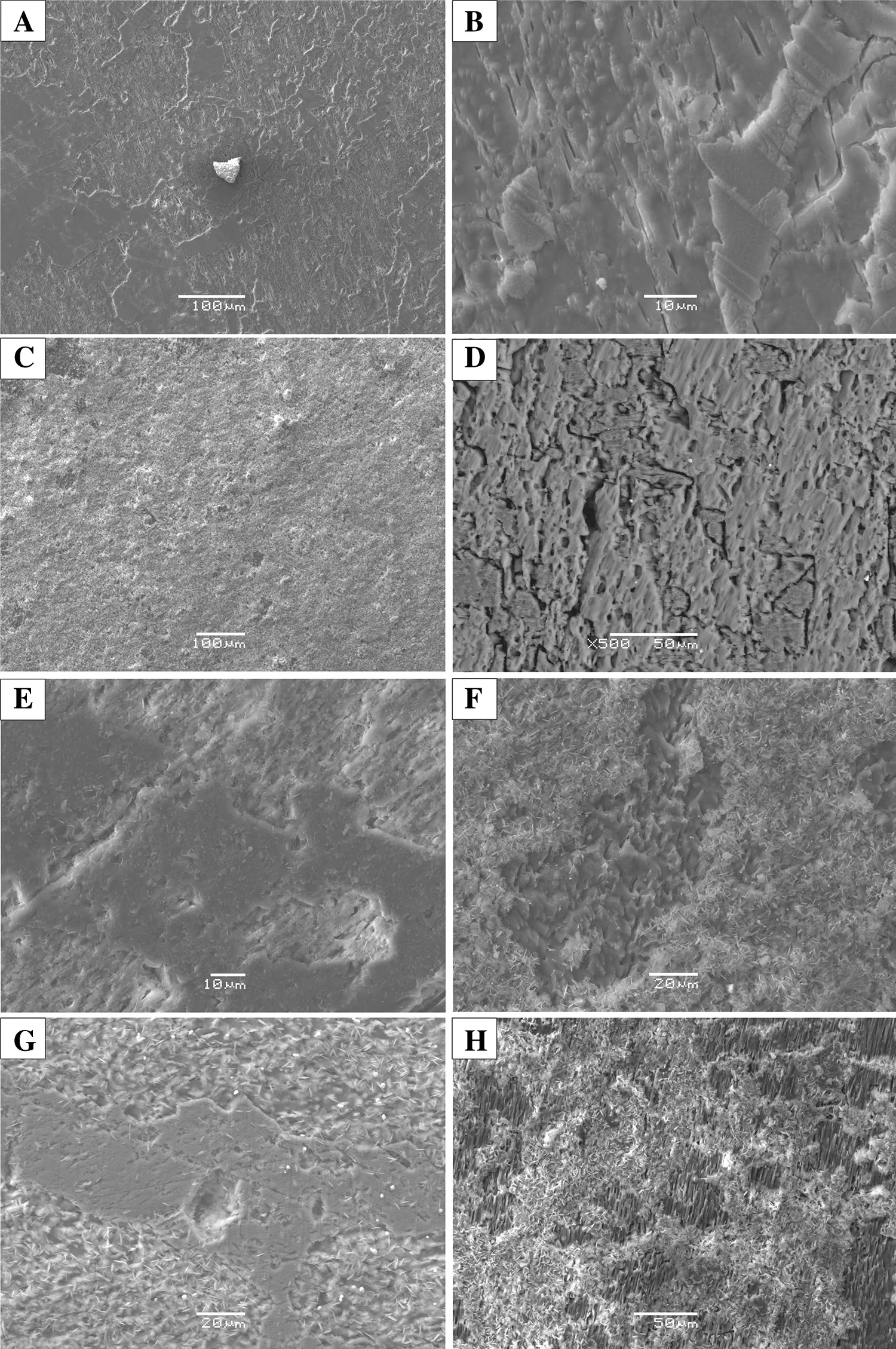
Fig.3 Surface morphology of feldspar after reaction(SEM).a 300 °C,pH=5.7;b 300 °C,pH=3.0;c 400°C,pH=5.7;d 400 °C,pH=3.0;e 450 °C,pH=5.7;f 450°C,pH=3.0;d 500 °C,pH=5.7;h 500 °C,pH=3.0

Table 3 Main oxide content of the samples(XRF)
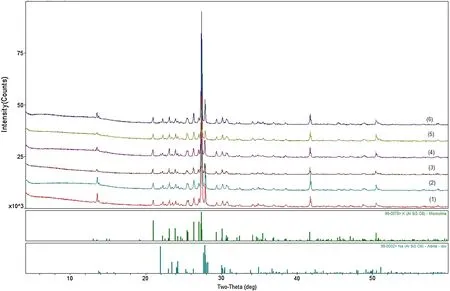
Fig.4 X-ray diffraction patterns of the sample.1 Original sample;2 300 °C,pH=5.7;3 300 °C,pH=3.0;4 400 °C,pH=5.7;5 400 °C,pH=3.0;6 500°C,pH=5.7
3.3 Changes of the sample chemical composition
The main oxide contents of the samples tested by X-ray fluorescence spectrometry before and after the reaction are listed in Table 3.Samples of feldspar dissolved inconsistent under different conditions of temperature,pressure and pH value.
X-ray diffraction patterns of the sample before and after reaction are shown in Fig.4.Before the reaction,the main minerals in samples were potassium feldspar and albite.After the reaction,the main mineral phases were potassium feldspar and albite still,new mineral phases were not detected.The possible reason is that the new mineral content is very small.
Because of albite and potassium,feldspar dissolution ability wasdifferent.Albite and potassium feldspar remaining in the residual solid sample were different also.So,the products analysis was carried out by EMPA to determine the chemical components of different mineral phases after the reaction.The results of EMPA are listed in Table 4.
By Table 4,we found that the chemical compositions of part of the minerals were the same as the origin feldspars,and the other part of the mineral’s chemical compositions changed obviously.When the temperature was lower,Na decreased more rapidly,which indicated that Na was the firstelement dissolved into the solution during the process of dissolution.
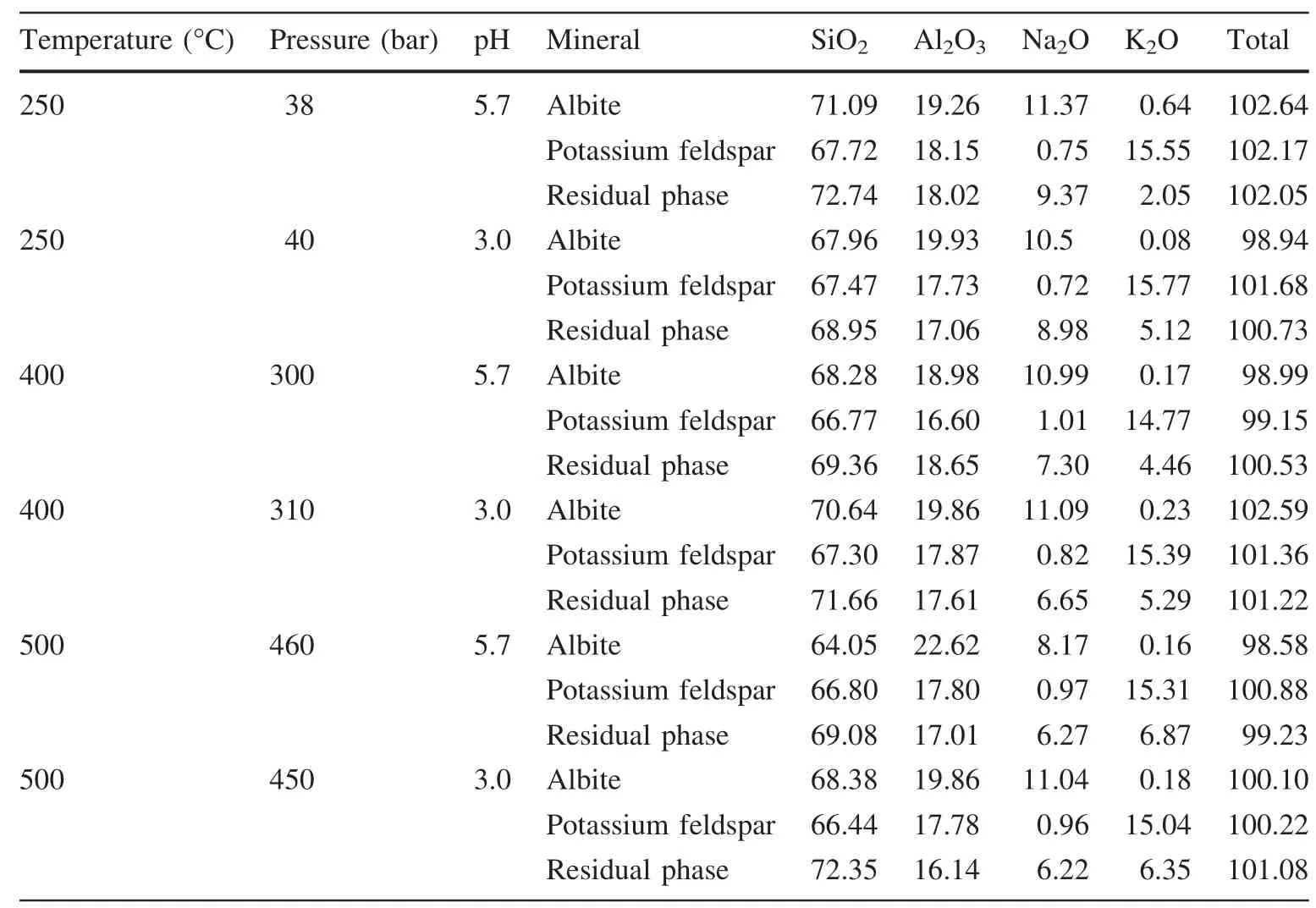
Table 4 Chemical composition of feldspar after the reaction(EMPA)
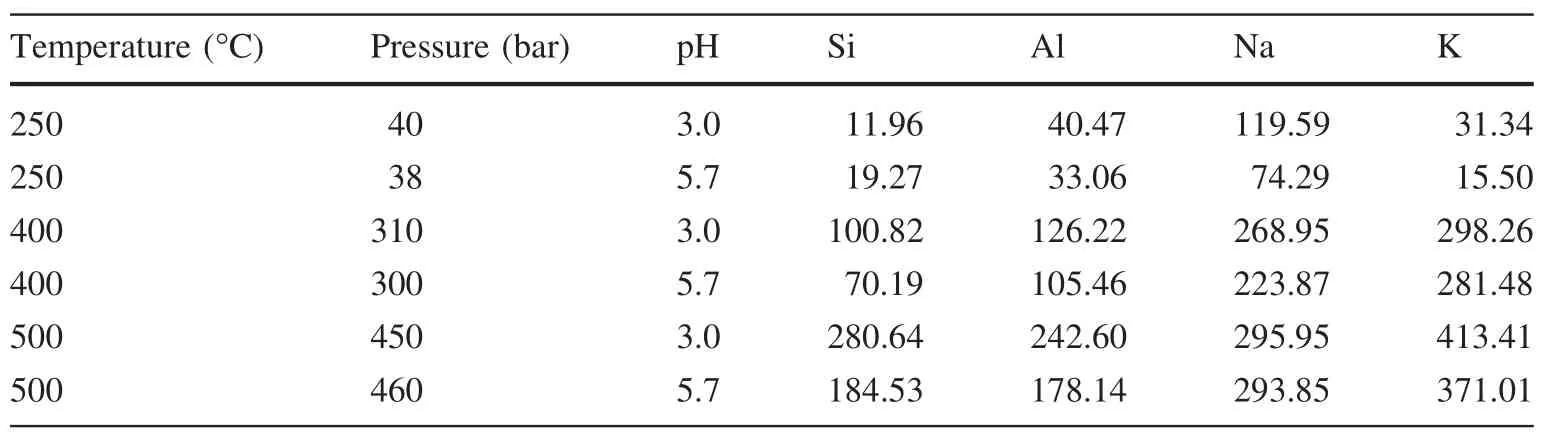
Table 5 Ion concentration in fluid after the reaction(mg/L)
3.4 Changes of ion concentration
The ions concentrations after reaction are listed in Table 5.Ion concentration scatter plots(Fig.5)were drawn according to Table 5.Under the same pH value condition,the ions in the solution all increased with the rise of temperature,which indicated that temperature can promote feldspar dissolution.Under the same reaction temperature,the ions in the solution decreased with the increase of pH value,which indicated that lower temperature is a benefit for feldspar dissolution.When the reaction temperature was 250°C,the concentration of Na+was higher than that of K+.We can draw a conclusion that al bite is easier to dissolve in water than potassium feldspar.

Fig.5 Ion concentration scatter plot after the reaction in different conditions.a pH=3.0;b pH=5.7
4 Conclusions
1. There are no altered minerals below the critical point,but there are needle-like and sheet altered minerals observed above the critical point.And the higher the temperature is,the more altered minerals are produced.
2. The law that people have grasped below the critical point about the in fl uence of temperature,pressure,and pH value on the alkaline feldspar dissolution behavior is still held above the critical point.Namely,the degree of dissolution of alkali feldspar enhances with the increase of temperature and pressure,in the acidic range decreased with the increase in pH value of the solution.
3. Due to the experimental techniques of autoclave flip 180 degrees—sharp quenching and based on electron microprobe analysis of mineral new formed,theoretical analysis has determined that the new altered minerals distributed on the island dissolution surface of feldspar are products of precipitation on a feldspar surface after saturation of the relative ion concentration in water fluid.
AcknowledgementsThis study is financed by the Fund from the Ministry of Science and Technology of People’s Republic of China under the grant number XDB18000000 and by Major State Research Development Program of China under Grant Nos.2016YFC0601101 and 2016YFC0600109.
Adriaens A,Goossens D,Pijpers A,Van TG,Gijbels R(1999)Dissolution study of potassium feldspars using hydrothermally treated sanidine as an example.Surf Interface Anal 27:8–23
Chardon ES,Livens FR,Vaughan DJ(2006)Reactions of feldspar surfaces with aqueous solutions.Earth Sci Rev 78:1–26
Crund well FK(2015a)The mechanism of dissolution of the feldspars:part I.dissolution at conditions far from equilibrium.Hydro metallurgy 151:151–162
Crund well FK(2015b)The mechanism of dissolution of the feldspars:part I dissolution at conditions close to equilibrium.Hydro metallurgy 151:163–171
Crund well FK(2016)The mechanism of dissolution of minerals in acidic and alkaline solutions:part V.surface charge and zeta potential.Hydro metallurgy 161:174–184
Fu Q,Lu P,Hiromi K,Robert D,Xu HF,Seyfried WE,Zhu C(2009)Coupled alkali-feldspar dissolution and secondary mineral precipitation in batch systems:1.New experiments at 200°C and 300 bars.Chem Geol 258:125–135
Gautier JM,Oelkers EH,Schott J(1994)Experimental study of K-feldspar dissolution rates as a function of chemical affinity at 150°C and pH 9.Geochim Cosmochim Acta 58:4549–4560
Hellmann R(1994)The albite-water system:part I.the kinetics of dissolution as a function of pH at 100,200 and 300°C.Geochim Cosmochim Acta 58:595–611
Hinman NW(2013)Water-rock interaction and life.Procedia Earth Planet Sci 7:354–359
Knauss KG,Wolery TJ(1986)Dependence of albite dissolution kinetics on pH and time at 25°C and 70°C.Geochim Cosmochim Acta 50:2481–2497
Lu P,Fu Q,Seyfried WE,Hedges SW,Soong Y,Jones K,Zhu C(2013)Coupled alkali feldspar dissolution and secondary mineral precipitation in batch systems:2.New experiments with supercritical CO2 and implications for carbon sequestration.Appl Geochem 30:75–90
Lu P,Konishi H,Oelkers E,Zhu C(2015)Coupled alkali feldspar dissolution and secondary mineral precipitation in batch systems:5.Results of K-feldspar hydrolysis experiments.Chin J Geochem 34:1–12
Zhang Y,Zeng J,Zhang S,Wang Y,Zhang S,Jiang H,Zhou S(2009)Experimental study of interaction between fluid and alkaline feldspar.J Geochem Explor 101:126
Zhu C,Lu P(2013)The Coupling of dissolution and precipitation reactions as the main contributor to the apparent field-lab rate discrepancy.Procedia Earth Planet Sci 7:948–952
杂志排行
Acta Geochimica的其它文章
- Heterogeneous Mg isotopic composition of the early Carboniferous limestone:implications for carbonate as a seawater archive
- Diffusion in garnet:a review
- Genesis of tuff interval and its uranium enrichment in Upper Triassic of Ordos Basin,NW China
- Constraints of molybdenite Re–Os and scheelite Sm–Nd ages on mineralization time of the Kukaazi Pb–Zn–Cu–W deposit,Western Kunlun,NW China
- Identification of bacterial fossils in marine source rocks in South China
- Timing of mineralization at the Shihu gold deposit in the middle segment of the Taihang Mountain,China
