北豆根根茎中生物碱类化学成分及其生物活性研究△
2018-03-21周艳丽赵旭李硕熙吕红梅王爱莉张艳
周艳丽,赵旭,李硕熙,吕红梅,王爱莉,张艳
(黑龙江中医药大学佳木斯学院,黑龙江 佳木斯 154007)
北豆根是防己科蝙蝠葛属植物蝙蝠葛Menispermum dauricum的干燥根茎,又名狗葡萄根、磨石豆根、山花子根、光光叶根[1]。主产于河北、山东、内蒙古、黑龙江、吉林、辽宁、四川及陕西等地。北豆根具有清热解毒、祛风止痛功能,用于咽喉肿痛、热毒泻痢、风湿痹痛[2]。近年来,药学工作者对北豆根的化学成分研究表明,北豆根中主要含有生物碱类化合物,该类化合物结构丰富多样,主要包括双苄基四氢异喹啉类、吗啡烷类、氧化异阿朴啡以及四氢异喹啉等类型;其中,双苄基四氢异喹啉类在北豆根生物碱中的含量最高[3]。该类化合物具有较广泛的药理作用,主要包括抗炎、抑菌、抗心率失常、抗心肌缺血、抑制血小板聚集、降血压、免疫增强等作用[4-7]。因此,对北豆根中生物碱类化合物继续进行更加系统的化学成分及生物活性研究,对于寻找活性单体化合物并明确其药效物质基础具有非常深远的意义。本文对购自河北省安国药材市场的北豆根中生物碱类化学成分及生物活性进行了系统研究,从该植物中分离并鉴定了10个化合物,并对分离得到的单体化合物进行了抗炎活性实验。
1 材料
Mercury-400型核磁共振仪(德国Bruker公司);Autospec-Ultima E TOF型质谱仪(美国Waters公司);Shimadzu LC-20AD型制备HPLC(日本岛津公司);碱性硅胶(200-300目,青岛海洋化工厂)。
北豆根药材于2015年7月购自河北省安国药材市场,经黑龙江中医药大学陈效忠副教授鉴定为防己科蝙蝠葛属植物蝙蝠葛Menispermum dauricum的干燥根茎。药材标本储存于本研究室。
2 提取和分离
将药材北豆根(5 kg)干燥,粉碎,用HCl水溶液(pH 3.0)渗漉提取3次。提取液调节pH至7.0,减压回收得干浸膏(200 g)。过碱性硅胶柱色谱,用二氯甲烷-甲醇系统(90∶10→10∶90,体积比)洗脱得到FrA~FrE 5个部位。FrB(30 g)过碱性硅胶柱色谱,用二氯甲烷-甲醇(90∶10→10∶90,体积比)洗脱得到FrB.1~FrB.10。FrB.5(5 g)过 ODS柱,以甲醇-水系统(10∶90→90∶5,体积比)为洗脱剂,得到FrB.5.1~FrB.5.20。FrB.5.7(300 mg)经高压制备液相色谱(甲醇-水-三氟乙酸,30∶70∶0.1,流速:5 mL·min-1)分离,得到化合物 1(10.0 mg)和 2(5.0 mg)。FrB.5.10(500 mg)经高压制备液相色谱纯化 (甲醇-水-三氟乙酸,40∶60∶0.1,流速:5 mL·min-1),得到化合物 3(2.0 mg),4(5.0 mg)和5(8.0 mg)。FrC(20 g)过 ODS柱,以甲醇-水(10∶90→90∶5,体积比)为洗脱剂,得到 FrC.1~FrC.25。FrC.7(300 mg)经制备型 HPLC(甲醇-水-三氟乙酸,30∶70∶0.1,流速:5 mL·min-1)分离,得到化合物6(8.0 mg)和7(12.0 mg)。FrC.15(500 mg)用制备型 HPLC(甲醇-水-三氟乙酸,45∶55∶0.1,流速:5 mL·min-1),纯化得到化合物8(5.0 mg),9(15.0 mg)和 10(21.0 mg)。
3 结构鉴定
化合物1:淡黄色粉末;ESI-MS m/z277[M+H]+;1H-NMR(CDCl3,400 MHz)δ:8.72(1H,d,J=5.2 Hz,H-2),7.62(1H,d,J=5.2 Hz,H-3),7.20(1H,s,H-4),8.59(1H,m,H-8),7.71(1H,m,H-9),7.84(1H,m,H-10),9.09(1H,m,H-11),4.14(3H,s,5-OMe);13C-NMR(CDCl3,100 MHz)δ:142.1(C-2),120.4(C-3),129.7(C-3a),119.8(C-3b),108.6(C-4),150.5(C-5),148.3(C-6),108.5(C-6a),184.2(C-7),133.1(C-7a),126.5(C-8),192.2(C-9),132.4(C-10),124.9(C-11),136.6(C-11a),143.6(C-11b),56.2(5-OMe)。以上数据与文献报道的化合物基本一致[8],故确定其结构为lakshminine,为首次从该植物中获得。
化合物2:白色粉末;ESI-MS m/z 277[M+H]+;1H-NMR(CDCl3,400 MHz)δ:7.08(1H,s,H-3),7.72(1H,d,J=5.0 Hz,H-4),8.81(1H,d,J=5.0 Hz,H-5),8.64(1H,dd,J=1.6,8.0 Hz,H-8),7.50(1H,m,H-9),7.75(1H,m,H-10),8.58(1H,m,J=7.9 Hz,H-11),4.13(3H,s,2-OMe),5.52(2H,s,1-NH2);13C-NMR(CDCl3,100 MHz)δ:139.9(C-1),107.2(C-1a),123.0(C-1b),152.5(C-2),104.3(C-3),133.1(C-3a),123.6(C-4),143.5(C-5),144.0(C-6a),182.6(C-7),132.5(C-7a),129.7(C-8),126.9(C-9),133.8(C-10),124.9(C-11),136.2(C-11a),56.5(2-OMe)。以上数据与文献报道的波谱数据基本一致[8],故确定其结构为telazoline,为首次从该植物中获得。
化合物3:白色粉末;ESI-MS m/z 427[M+H]+;1H-NMR(CDCl3,400 MHz)δ:8.87(1H,d,J=5.1 Hz,H-2),7.65(1H,d,J=5.1 Hz,H-3),7.34(1H,s,H-4),8.46(1H,d,J=2.7 Hz,H-8),7.57(1H,dd,J=2.7,8.8 Hz,H-10),9.45(1H,d,J=8.8 Hz,H-11),3.84(3H,s,OMe-5),3.85(3H,s,OMe-9),13.32(1H,br,N1′-Me),4.27(2H,m,H-2′),3.12(2H,m,H-3′),7.43(2H,d,J=8.4 Hz,H-5′和 H-9′),7.24(2H,d,J=8.4 Hz,H-6′和 H-8′);13C-NMR(CDCl3,100 MHz)δ:142.4(C-2),119.7(C-3),130.5(C-3a),119.2(C-3b),111.7(C-4),153.4(C-5),151.0(C-6),106.6(C-6a),181.9(C-7),135.3(C-7a),108.4(C-8),161.2(C-9),121.7(C-10),123.3(C-11),130.5(C-11a),142.9(C-11b),55.7(5-OMe),55.9(9-OMe),49.4(C-2′),37.5(C-3′),130.0(C-4′),130.8(C-5′),116.7 C-6′),157.9(C-7′),116.7(C-8′),130.7(C-N9′)。以上数据与文献报道的化合物基本一致[9],故鉴定其为 daurioxoisoporphine A,为首次从该植物中获得。
化合物4:白色粉末;ESI-MS m/z 337[M+H]+;1H-NMR(CDCl3,400 MHz)δ:8.92(1H,d,J=5.2 Hz,H-2),7.60(1H,d,J=5.2 Hz,H-3),8.44(1H,d,J=2.7 Hz,H-8),7.55(1H,dd,J=2.7,8.8 Hz,H-10),9.41(1H,d,J=8.8 Hz,H-11),4.09(3H,s,4-OMe),3.92(3H,s,5-OMe),3.84(3H,s,9-OMe);13C-NMR(CDCl3,100 MHz)δ:142.1(C-2),115.3(C-3),126.4(C-3a),120.5(C-3b),151.1(C-4),141.3(C-5),153.6(C-6),103.5(C-6a),182.1(C-7),135.3(C-7a),108.7(C-8),161.5(C-9),121.1(C-10),127.9(C-11),130.4(C-11a),144.1(C-11b),60.8(4-OMe),61.3(5-OMe),55.7(9-OMe)。以上数据与文献报道的波谱数据基本一致[9],故鉴定其结构为 daurioxoisoporphine B,为首次从该植物中获得。
化合物5:白色粉末;ESI-MS m/z 330[M+H]+;1H-NMR(CDCl3,400 MHz)δ:6.73(1H,s,H-1),6.84(1H,s,H-4),6.22(1H,s,H-5),3.39(1H,dd,J=17.6,14.0 Hz,H-8a),2.46(1H,dd,J=17.6,4.4 Hz,H-8b),2.90(1H,brd,J=5.8 Hz,H-9),3.10(1H,d,J=18.0 Hz,H-10a),2.65(1H,dd,J=18.0,5.9 Hz,H-10b),2.46(1H,m,H-14),2.13(1H,m,H-15a),1.52(1H,m,H-15b),2.50(1H,m,H-16a),2.13(1H,m,H-16b),3.91(3H,s,3-OMe),3.69(3H,s,6-OMe),2.36(3H,s,N-Me);13C-NMR(CDCl3,100 MHz)δ:114.3(C-1),144.3(C-2),145.2(C-3),106.4(C-4),121.6(C-5),151.0(C-6),194.9(C-7),39.3(C-8),56.8(C-9),27.1(C-10),130.5(C-11),132.2(C-12),36.4(C-13),40.2(C-14),36.5(C-15),45.9(C-16),56.3(3-OMe),55.1(6-OMe),42.9(N-Me)。以上数据与文献报道的核磁数据基本一致[10],故确定其为scrodentoside A,为首次从该植物中获得。
化合物6:白色粉末;ESI-MS m/z 589[M+H]+;1H-NMR(CDCl3,400 MHz)δ:8.20(1H,d,J=5.9 Hz,H-3),7.54(1H,d,J=5.9 Hz,H-4),7.25(1H,s,H-5),7.69(1H,s,H-8),4.53(1H,d,J=12.7 Hz,Ha-1),4.13(1H,d,J=12.7 Hz,Ha-2),7.17(1H,d,J=1.6 Hz,H-10),6.96(1H,d,J=8.6 Hz,H-13),7.04(1H,dd,J=1.6,8.6 Hz,H-14),4.64(1H,m,H-1′),2.62(3H,s,2′-N-CH3),3.52(1H,m,H-3′a),3.06(1H,m,H-3′b),3.06(1H,m,H-4′a),2.90(1H,m,H-4′b),7.25(1H,s,H-5′),3.38(1H,m,Hα′-1),2.96(1H,m,Hα′-2),7.06(1H,dd,J=8.3,1.8 Hz,H-10′),7.21(1H,dd,J=8.3,2.5 Hz,H-11′),6.64(1H,dd,J=8.5,2.5 Hz,H-13′),7.43(1H,dd,J=8.5,1.8 Hz,H-14′),5.68(1H,brs,OCH2O-a),5.50(1H,brs,OCH2O-b),3.79(3H,s,6-OCH3),3.88(3H,s,12-OCH3);13C-NMR(CDCl3,100 MHz)δ:160.7(C-1),141.7(C-3),137.9(C-4a),120.8(C-4b),107.7(C-5),156.6(C-6),146.9(C-7),123.4(C-8a),119.7(C-8b),42.5(C-a),132.9(C-9),122.7(C-10),150.4(C-11),150.7(C-12),114.6(C-13),125.2(C-14),61.2(C-1′),42.0(2′-N-CH3),45.5(C-3′),24.4(C-4′),127.2(C-4′a),105.1(C-5′),150.0(C-6′),135.6(C-7′),139.9(C-8′),122.7(C-8′a),42.4(C-α′),136.9(C-9′),131.7(C-10′),122.6(C-11′),158.2(C-12′),122.5(C-13′),132.3(C-14′),102.7(OCH2O),56.8(6-OCH3),56.9(12-OCH3)。以上数据与文献对比波谱数据基本一致[11],故确定其结构为(+)-1,3,4-dehydrocepharanthine,为首次从该植物中获得。
化合物7:白色粉末;ESI-MS m/z 605[M+H]+;1H-NMR(CDCl3,400 MHz)δ:8.21(1H, d,J=6.0 Hz,H-3),7.56(1H,d,J=6.0 Hz,H-4),7.25(1H,s,H-5),7.71(1H,s,H-8),4.52(1H,d,J=13.8 Hz,Ha-1),4.15(1H,d,J=13.8 Hz,Ha-2),7.21(1H,brs,H-10),6.90(1H,d,J=8.6 Hz,H-13),7.02(1H,dd,J=1.6,8.6 Hz,H-14),4.98(1H,m,H-1′),3.32(3H,s,2′-N-CH3),3.95(1H,m,H-3′a),3.61(1H,m,H-3′b),3.39(1H,m,H-4′a),3.28(1H,m,H-4′b),6.57(1H,s,H-5′),4.45(1H,brd,J=12.6 Hz,Hα′-1),2.79(1H,dd,J=11.4,12.6 Hz,Hα′-2),7.53(1H,dd,J=8.6,2.4 Hz,H-10′),6.61(1H,dd,J=8.6,2.4 Hz,H-11′),7.21(1H,m,H-13′),7.04(1H,dd,J=8.5,2.4 Hz,H-14′),5.71(1H,brs,OCH2O-a),5.53(1H,brs,OCH2O-b),3.77(3H,s,6-OCH3),3.82(3H,s,12-OCH3);13C-NMR(CDCl3,100 MHz)δ:160.8(C-1),141.8(C-3),138.1(C-4a),120.9(C-4b),108.0(C-5),156.2(C-6),146.2(C-7),120.9(C-8a),120.0(C-8b),42.6(C-a),132.8(C-9),122.9(C-10),150.3(C-11),150.9(C-12),114.9(C-13),132.0(C-14),75.7(C-1′),56.9(2′-N-CH3),60.1(C-3′),27.2(C-4′),105.2(C-5′),125.1(C-4′a),151.5(C-6′),136.3(C-7′),139.0(C-8′),120.9(C-8′a),39.1(C-α′),135.2(C-9′),132.3(C-10′),122.4(C-11′),158.9(C-12′),122.9(C-13′),132.0(C-14′),103.5(OCH2O),57.0(6-OCH3),57.1(12-OCH3)。以上数据与文献对比基本一致[11],故确定其为(+)-1,3,4-dehydrocepharanthine-2′β-N-oxide,为首次从该植物中获得。
化合物8:白色粉末;ESI-MS m/z 609[M+H]+;1H-NMR(CDCl3,400 MHz)δ:3.58(1H, d,J=8.0 Hz,H-1),3.28(1H,m,H-3a),2.89(1H,m,H-3b),2.85(1H,m,H-4a),2.45(1H,m,H-4b),6.23(1H,s,H-5),2.72(1H,dd,J=14.4,8.0 Hz,Ha-1),2.21(1H,d,J=14.4 Hz,Ha-2),6.79(1H,d,J=1.2 Hz,H-10),6.88(1H,d,J=8.4 Hz,H-13),6.91(1H,dd,J=8.4,1.2 Hz,H-14),2.29(3H,s,2-NMe),3.88(3H,s,6-OMe),3.68(1H,dd,J=4.0,2.8 Hz,H-1′),3.12(1H,m,H-3′a),2.57(1H,m,H-3′b),3.09(1H,m,H-4′a),3.62(1H,m,H-4′b),6.65(1H,s,H-5′),6.81(1H,s,H-8′),3.37(1H,dd,J=16.4,4.0 Hz,Hα′-1),3.04(1H,dd,J=16.4,2.8 Hz,Hα′-2),7.33(1H,brd,J=8.0 Hz,H-10′),7.09(1H,brd,J=8.0 Hz,H-11′),7.09(1H,brd,J=8.0 Hz,H-13′),7.32(1H,brd,J=8.0 Hz,H-14′),5.08(1H,d,J=12.4 Hz,H-15′a),4.59(1H,d,J=12.4 Hz,H-15′b),2.49(3H,s,2′-NMe),3.81(3H,s,6′-OMe);13C-NMR(CDCl3,100 MHz)δ:61.4(C-1),45.1(C-3),24.2(C-4),129.8(C-4a),102.9(C-5),150.6(C-6),132.7(C-7),146.8(C-8),119.5(C-8a),40.9(C-a),135.3(C-9),118.0(C-10),144.4(C-11),144.9(C-12),115.1(C-13),124.6(C-14),43.0(2-NMe),56.1(6-OMe),63.8(C-1′),52.8(C-3′),29.7(C-4′),131.8(C-4′a),112.5(C-5′),148.8(C-6′),143.5(C-7′),117.4(C-8′),129.9(C-8′a),36.5(C-α′),139.8(C-9′),130.5(C-10′),128.8(C-11′),134.9(C-12′),128.8(C-13′),130.5(C-14′),76.9(C-15′),43.9(2′-NMe),56.0(6′-OMe)。以上数据与文献报道的波谱数据基本一致[12],故确定该化合物为cissampentine A,为首次从该植物中获得。
化合物9:白色粉末;ESI-MS m/z623[M+H]+;1H-NMR(CDCl3,400 MHz)δ:3.60(1H,d,J=8.4 Hz,H-1),3.19(1H,m,H-3a),2.74(1H,m,H-3b),2.85(1H,m,H-4a),2.43(1H,m,H-4b),6.17(1H,s,H-5),2.66(1H,dd,J=14.4,8.4 Hz,Ha-1),2.19(1H,d,J=14.4 Hz,Ha-2),6.78(1H,d,J=2.0 Hz,H-10),6.79(1H,d,J=8.0 Hz,H-13),6.87(1H,dd,J=8.0,2.0 Hz,H-14),2.17(3H,s,2-NMe),3.88(3H,s,6-OMe),3.78(3H,s,12-OMe),3.58(1H,dd,J=3.6,3.2 Hz,H-1′),3.02(1H,m,H-3′a),2.46(1H,m,H-3′b),2.98(1H,m,H-4′a),2.51(1H,m,H-4′b),6.55(1H,s,H-5′),6.63(1H,s,H-8′),3.32(1H,dd,J=16.4,3.6 Hz,Hα′-1),2.89(1H,dd,J=16.4,3.2 Hz,Hα′-2),7.19(1H,brd,J=8.0 Hz,H-10′),6.96(1H,brd,J=8.0 Hz,H-11′),6.96(1H,brd,J=8.0 Hz,H-13′),7.20(1H,brd,J=8.0 Hz,H-14′),4.99(1H,d,J=12.0 Hz,H-15′a),4.45(1H,d,J=12.0 Hz,H-15′b),2.42(3H,s,2′-NMe),3.72(3H,s,6′-OMe);13C-NMR(CDCl3,100 MHz)δ:61.0(C-1),44.9(C-3),24.5(C-4),129.8(C-4a),102.7(C-5),150.6(C-6),132.5(C-7),146.4(C-8),118.9(C-8a),40.1(C-a),135.3(C-9),111.8(C-10),145.9(C-11),148.2(C-12),118.6(C-13),123.8(C-14),42.7(2-NMe),56.0(6-OMe),55.7(12-OMe),63.8(C-1′),52.8(C-3′),29.6(C-4′),131.1(C-4′a),112.2(C-5′),148.5(C-6′),143.5(C-7′),116.3(C-8′),129.8(C-8′a),37.6(C-α′),139.5(C-9′),130.5(C-10′),128.6(C-11′),134.5(C-12′),128.7(C-13′),130.5(C-14′),76.8(C-15′),43.9(2′-NMe),55.6(6′-OMe)。以上数据与文献报道的化合物基本一致[12],故鉴定其结构为cissampentine B,为首次从该植物中获得。
化合物10:白色粉末;ESI-MS m/z595[M+H]+;1H-NMR(CDCl3,400 MHz)δ:3.79(1H,dd,J=13.8,9.4 Hz,H-1),3.44(1H,ddd,J=13.0,5.2,4.6 Hz,H-3a),2.93(1H,m,H-3b),3.04(1H,m,H-4a),2.59(1H,dd,J=12.0,4.6 Hz,H-4b),6.68(1H,d,J=8.2 Hz,H-5),6.45(1H,dd,J=8.2,1.7 Hz,H-6),6.69(1H,d,J=1.7 Hz,H-8),6.46(1H,d, J=1.8 Hz,H-10),6.79(1H,d,J=8.3 Hz,H-13),7.07(1H,dd,J=8.3,1.8 Hz,H-14),2.89(1H,dd,J=13.8,9.4 Hz,H-15a),2.73(1H,dd,J=13.8,9.4 Hz,H-15b),2.84(1H,dd,J=10.0,2.1 Hz,H-1′),3.52(1H,m,H-3′a),3.09(1H,m,H-3′b),3.09(1H,m,H-4′a),3.01(1H,m,H-4′b),6.92(1H, s, H-5′),5.78(1H, s,H-8′),7.22(1H,d,J=7.5 Hz,H-12′),6.68(1H,d,J=7.5 Hz,H-13′),3.26(1H,dd,J=10.0,2.1 Hz,H-15′a),2.74(1H,dd,J=10.0,2.1 Hz,H-15′b),2.33(3H,s,2-NMe),2.69(3H,s,2′-NMe),3.91(3H,s,6′-OMe),3.92(3H,s,12-OMe);13C-NMR(CDCl3,100 MHz)δ:60.3(C-1),43.8(C-3),21.5(C-4),124.1(C-4a),108.2(C-5),131.8(C-6),156.2(C-7),114.9(C-8),123.1(C-8a),132.8(C-9),121.3(C-10),142.1(C-11),146.9(C-12),115.2(C-13),125.5(C-14),39.3(C-15),64.9(C-1′),45.2(C-3′),23.8(C-4′),126.3(C-4′a),112.7(C-5′), 149.5 (C-6′), 144.4 (C-7′), 117.8(C-8′),126.7(C-8′a),131.1(C-9′),138.9(C-10′),148.2(C-11′),129.3(C-12′),113.7(C-13′),137.9(C-14′), 38.1(C-15′),40.3(2-NMe),40.4(2′-NMe),55.4(6′-OMe),55.4(12-OMe)。以上数据与文献报道基本一致[13],故确定其结构为(-)-pseudocurine,为首次从该植物中获得。
4 活性测试
大鼠巨噬细胞在RPMI1640培养基中培养,于37℃、5%CO2下生长。将其接种到48孔板中培养24 h,然后加入不同浓度的待测生物碱类化合物(5.0,10.0,20.0,40.0,80.0μmol·L-1,每个浓度3个平行孔)及阳性对照地塞米松(10-6mol·L-1),1 h后加入LPS(1μg·mL-1)培养24 h。取细胞培养基上清液100μL,加入等体积的Griess试剂,室温静置20 min,用酶标仪测定570 nm处吸光度,计算所测样品中亚硝酸盐NO2-的浓度[14-15]。研究结果表明,化合物6,8,9和10对LPS诱导的大鼠巨噬细胞释放NO具有较好的抑制活性,其IC50值分别为6.0、12.0、8.5、9.7μmol·L-1(见图1、表 1)。
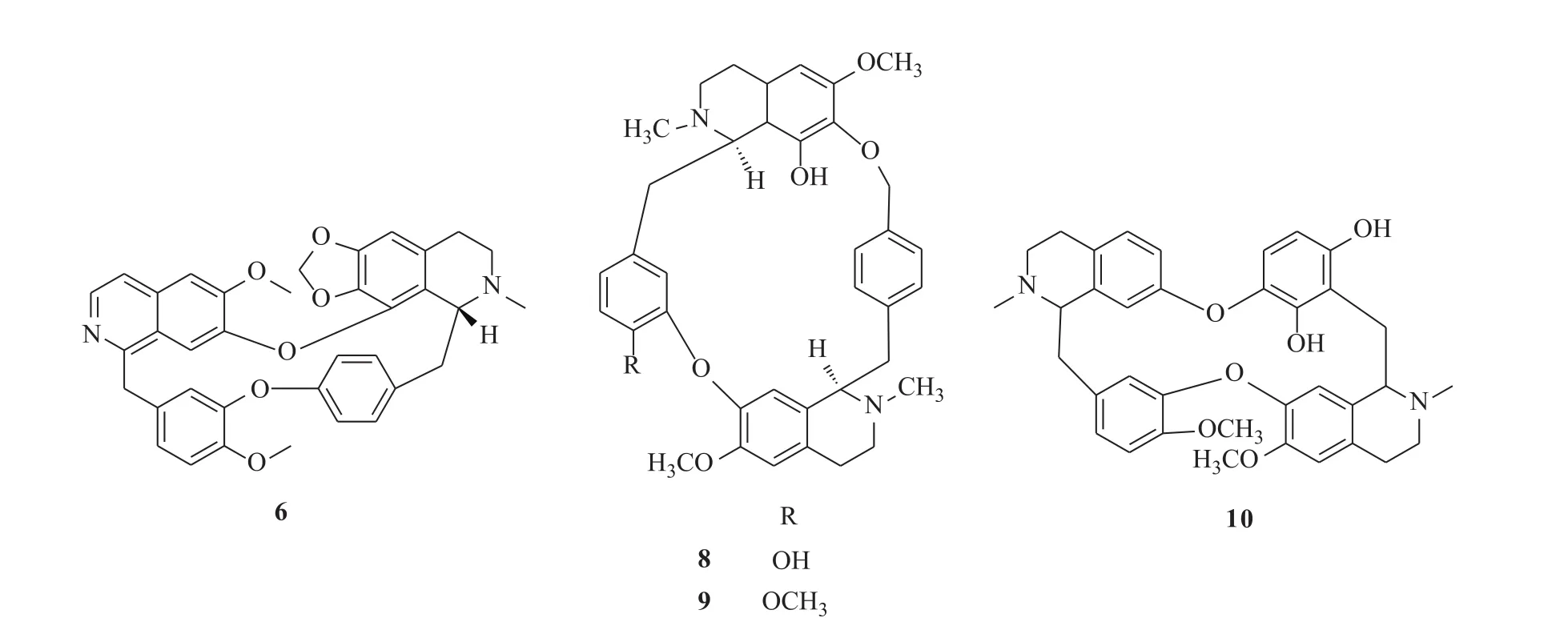
图1 北豆根中具有抗炎活性的化合物结构
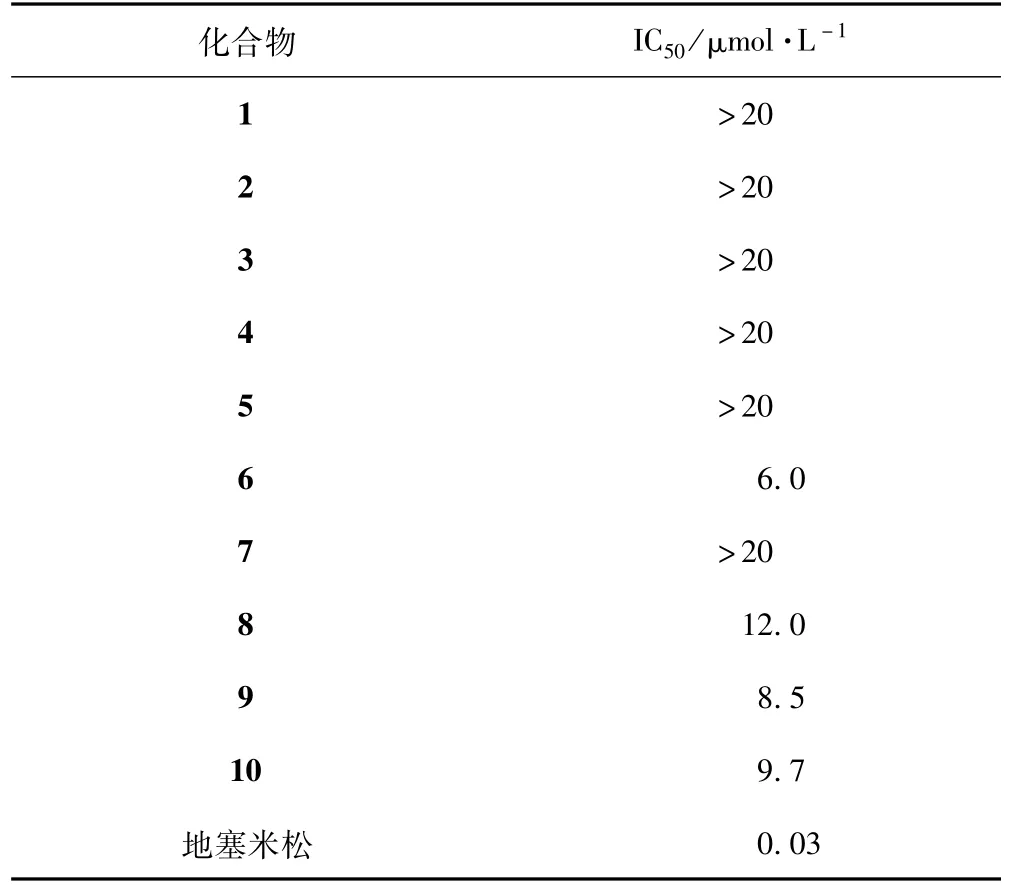
表1 化合物1~10对LPS诱导的大鼠巨噬细胞释放NO的抑制活性
[1] 高学敏.中药学[M].北京:人民卫生出版社,2000:532-533.
[2] 国家药典委员会.中华人民共和国药典:一部[S].北京:中国医药科技出版社,2015:99-100.
[3] 张艳,彭玉勃,陈效忠,等.北豆根根茎中双苄基异喹啉类生物碱成分研究[J].中国现代中药,2016,18(8):951-955.
[4] 曹令红,徐小阳.北豆根化学成分及药理作用研究综述[J].黑龙江医药,2009,22(1):68-69.
[5] 徐兵勇,富志军.北豆根的研究概况[J].海峡药学,2008,20(11):l-4.
[6] 陈淑娟,肖宙,潘锡平,等.RP-HPLC法对不同产地蝙蝠葛几种主要生物碱的测定[J].药物分析杂志,1999,19(2):79-81.
[7] Sugimoto Y,MatsuiM,Takikawa H,et aL.Dechlomdauricumime from cultured roots of Menispermum dauricum[J].Phytochemistry,2005,66(22):2627-2631.
[8] Lew K,Frederick G V,Alan JF,et al.Lakshminine,a new rare oxoisoaporphine alkaloid from Sciadotenia toxifera,and structural revisions of telazoline and teladiazoline,two related oxoaporphines from Telitoxicum peruvianum and T.glaziovii[J].JNat Prod,2003,66(1):115-118.
[9] Yu BW,Meng L H,Chen JY,et al.Cytotoxic oxoisoaporphine alkaloids from Menispermum dauricum[J].J Nat Prod,2001,64(7):968-970.
[10]Mukhtar M R,Hadi A H,Litaudon M,et al.Morphinandienone alkaloids from Dehaasia longipedicellata[J].Fitoterapia,2004,75(7):792-794.
[11]LYU JJ,Xu M,Wang D,et al.Cytotoxic bisbenzylisoquinoline alkaloids from Stephania epigaea[J].JNat Prod,2013,76(5):926-932.
[12]Wang JZ,Liao J,XuW L,etal.Bisbenzylisoquinoline alkaloids from the roots of Cyclea tonkinensis[J].Planta Med,2015,81(7):600-605.
[13]Omole R A,Gathirwa J,Akala H,et al.Bisbenzylisoquinoline and hasubanane alkaloids from Stephania abyssinica(Dillon&A.Rich)(Menispermeceae)[J].Phytochemistry,2014,103:123-128.
[14]Sacco R E,Waters W R,Rudolph K M,et al.Comparative nitric oxide production by LPS-stimulated monocyte-derived macrophages from Ovis canadensis and Ovis aries[J].Comp Immunol Microbiol Infect Dis,2006,29(1):1-11.
[15]García-Argáez A N,Ramírez Apan TO,Delgado H P,etal.Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model[J].Planta Med,2000,66(3):279-281.
