Dose-related effects of dexmedetomidine on immunomodulation and mortality to septic shock in rats
2018-03-17YanMaXiangyouYuYiWang
Yan Ma, Xiang-you Yu, Yi Wang
Department of Intensive Care Unit, the First Affiliated Hospital, Xinjiang Medical University, Urumqi, China
INTRODUCTION
Sepsis-induced septic shock and multiple organ dysfunction syndrome (MODS) result in a very high mortality rate in the intensive care unit (ICU).Current studies suggest that sepsis, from beginning to end, is a systemic inflammatory response of hyperthyroidism and acquired immune function disorder. In this process, inflammatory response-induced immunosuppression occurs. Immunosuppression leads to difficulty in controlling the infection; additionally,immunosuppression further aggravates the inflammatory response. Both events strengthen each other and promote the occurrence and development of sepsis; all these events occur throughout the whole course of the disease.
Dexmedetomidine, an α-2 adrenergic receptor agonist, has already been used in septic patients as a new sedative agent. However, few studies have examined its effects on immunomodulation, which may play an important role in perpetuating sepsis syndrome. We did not determine whether these beneficial effects were dose-dependent in the cecal ligation and puncture (CLP)model in rats. Therefore, the authors have designed a controlled experimental study to characterize the immunomodulation effects of dexmedetomidine in the cecal ligation and puncture (CLP) model in rats.
METHODS
Forty-eight male Wistar rats, aged 10±1 weeks,weighing 222.7±19.6 g that were bred and grown by the experimental animal department of the First Affiliated Hospital of Xinjiang Medical University [SYXK (Xin)2010-0003, AAALAC accredited number:001279] were provided by the Shanghai Southern Biomodel Research Center. They were fed in a specific-pathogen-free (SPF)feeding room. All the procedures related to the animal experiments were approved (IACUC-20130216-99)by the Animal Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University. The Wistar rats were housed in an animal facility in 12:12 hour light:dark cycles and were allowed free access to chow and water.
Animal preparation
The method of CLP model was adopted from our previous studies.[1,2]After receiving an intraperitoneal injection of sodium pentobarbital (40 mg·kg-1, Hypnol 3%),the left carotid artery was catheterized (polyethylene-24G catheter), allowing continuous mean arterial blood pressure(MAP) and heart rate (HR) monitoring, as well as blood sampling. The caudal vein was also catheterized for fluid infusion and drug injection. These catheters were filled with a heparinized saline solution (40 IU/mL). Experiments were performed on awake animals 5 h after the injection.
MAP, HR, and temperature monitoring
The mean arterial blood pressure was continuously monitored during the study period through the arterial catheter and a blood pressure transducer (MX9505T;Smiths Medical ASD, USA). The software automatically determined HR from the pressure trace and expressed it as beats per min. The body temperature of the rats was maintained with a heating pad placed near the animal and was controlled by a rectal thermistor.
Arterial blood gases and lactate analysis
Blood samples (0.25 mL) were withdrawn from the arterial catheter 3 h after the procedure and were immediately analyzed in a blood gas analyzer (CG4+cartridge, i-STAT System, Abbott Laboratories, Abbott Park, IL) for pH, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), bicarbonate level(HCO3), base excess (BE), total carbon dioxide content(TCO2-), arterial oxyhemoglobin saturation (SatO2), and arterial lactate concentrations (Lac).
HLA-DR and plasma cytokine measure
Arterial blood samples (0.8 mL) were drawn to measure plasma cytokine concentrations and HLA-DR levels at 1, 3, and 5 hours after the CLP procedure. A total amount of 2.65 mL of blood was drawn from each animal over the 24 hours observation period.
Experimental protocol
Animals were suitable for experiments if their baseline MAP, HR, and rectal temperature were within the normal range and if they showed no signs of inflammation or bleeding in the skinfold chamber.The included 48 rats were randomly allocated into four groups (n=12 per group).
Cecal ligation and puncture group (CLP group)
The abdomen was disinfected per standard methods.The center of the skin incision was 1.5 cm long. The abdominal cavity was opened for the appendix, carefully separating the mesentery between the distal cecum and the large intestine; care was taken to avoid bruising mesenteric vessels. A ligature was placed using sterile 4 silk at the orifice of the ileocecal valve. Taking care to avoid intestinal obstruction, a small amount of excrement and urine was squeezed out from the remote central place in the puncture using 18 G sterile needles in the cecum ligation. Then, the cecum was put back into the abdominal cavity, and the abdominal cavity was closed with two layers of suture. Postoperatively, the rats were cage fed.
Small-dose treatment group (group Dex2.5)
The operation was performed as in the CLP group.Dexmedetomidine was intravenously administered(infusion, 2.5 μg·kg-1·h-1) immediately after the CLP procedure.
Medium-dose treatment group (group Dex5.0)
The operation was performed as in CLP group.Dexmedetomidine was administered intravenously(infusion, 5 μg·kg-1·h-1) immediately after the CLP procedure.
Large-dose treatment group (group Dex10.0)
The operation was induced as in group CLP.Dexmedetomidine was administered intravenously(infusion, 10 μg·kg-1·h-1) immediately after the procedure of the CLP.
All animals were sacrificed by an intravenous overdose of sodium pentobarbital (>200 mg·kg-1; Hypnol 3%; Sigma, USA).
Statistical analysis
The data are presented as the means±SDs. Differences between groups at baseline were analyzed using Student’s t-test. Arterial blood gases, lactate, HLA-DR and cytokine changes during the study were analyzed using ANOVA followed by a post-hoc test (Bonferroni’s method). Hemodynamic changes during the study were analyzed using repeated measures ANOVA.The mortality rates of the groups were compared using Kaplan-Meier analysis. Statistical significance was defined asP<0.05. All statistical analyses were performed using SAS JMP PRO.
RESULTS
The average body weight of rats was 222.7±19.6 g, with no significant differences among groups. All animals survived the entire experimental protocol,leaving no missing data for the statistical analysis.
Evaluation of HLA-DR
We evaluated whether DEX affects immunomodulation mediator production in rat whole blood after a CLP operation. No significant differences were observed at any time point during the experimental period. However,the HLA-DR in the Dex5.0 group was significantly lower than in the other groups; a major increase was observed in the CLP group, which led to a significant difference among the four groups (Pgroup=0.0202).
In addition, we evaluated whether immunomodulation mediator production functioned in a dose-dependent manner in DEX-treated animals. The HLA-DR decreased in DEX-treated animals and was lower in the Dex5.0 group (Figure 1). However, although these variables did not exhibit significant differences after the DEX administration, the HLA-DR values maintained a better concentration gradient in the Dex5.0 group than in the Dex10.0 group and the Dex2.5 group (Figure 2).
Plasma cytokine concentrations (pro- and anti-inflammatory)
We evaluated whether DEX was mediated by suppressing or promoting effects on serum blood proinflammatory or anti-inflammatory mediator in rats.There were no significant differences in IL-4, IL-10 and TNF-α levels among the experimental groups at any time point. However, a major increase was observed in IL-6 in the Dex5.0 and Dex10.0 groups when compared with the CLP group at 3 h (P=0.0113). However, no significant increase was observed in the dexmedetomidine-treated group when compared with the CLP group at 5 h. No other significant difference was found.
HR and MAP alterations
HR values were not significantly different in the CLP group at any time point. The systemic administration of dexmedetomidine elicited statistically similar reductions in HR levels in the dexmedetomidine treated groups (P<0.001); HR levels gradually decrease with the extension of infusion time (Figure 3). A major decrease was observed in the Dex5.0 group at 5 hours(127.32±53.39). Dexmedetomidine induced a significant mean arterial blood pressure (MAP) level initial (1 hour) decrease compared to the CLP group (P=0.0004),but MAP level was not significantly different with the extension of infusion time. In contrast, MAP levels gradually decreased with the extension of infusion time in the CLP group (Figure 3). No significant relationship was observed between HR and MAP changes in the four groups.
Arterial blood gases and lactate analysis
A significant improvement in arterial lactate concentration was observed in the dexmedetomidinetreated groups (P<0.0001). However, pH levels were significantly reduced in dexmedetomidine-treated groups(P=0.0002). In contrast, a marked improvement in BEecf and TCO2was observed in the dexmedetomidinetreated group, which led to a significant difference between the CLP and dexmedetomidine-treated animals(P<0.0001). PCO2increased after dexmedetomidine was intravenously administered (P<0.0001), but PO2was not significantly different between the study groups(P=0.2022) (Figure 4).

Figure 1. HLA-DR during the experimental period. Note that a significant difference was observed between four groups (Pgroup=0.0202).HLA-DR values (given as means±SD) were measured at 1 hour (1 h),3 hours (3 h) and 5 hours (5 h) of normal saline or dexmedetomidine infusion. CLP: cecal ligation and puncture, normal saline treated(n=12); DEX2.5: cecal ligation and puncture, dexmedetomidine treated, 2.5 mg·kg-1·h-1 infusion (n=12); DEX5.0: cecal ligation and puncture, dexmedetomidine treated, 5.0 mg·kg-1·h-1 infusion (n=12);DEX10.0: cecal ligation and puncture, dexmedetomidine treated, 10.0 mg·kg-1·h-1 infusion (n=12).
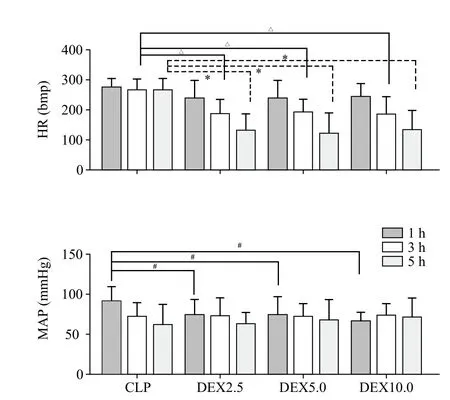
Figure 3. Heart rate (HR) and mean arterial blood pressure (MAP)evolution during the experimental period. Values (given as means±SD)were measured at 1 hour (1 h), 3 hours (3 h) and 5 hours (5 h) of normal saline or dexmedetomidine infusion. CLP: cecal ligation and puncture, normal saline treated (n=12); DEX2.5: cecal ligation and puncture, dexmedetomidine treated, 2.5 mg·kg-1·h-1 infusion (n=12);DEX5.0: cecal ligation and puncture, dexmedetomidine treated, 5.0 mg·kg-1·h-1 infusion (n=12); DEX10.0: cecal ligation and puncture,dexmedetomidine treated, 10.0 mg·kg-1·h-1 infusion (n=12). △P<0.001 as compared with 3h, *P<0.001 as compared with CLP groups in 5 h,#P<0.01 as compared in 1 h.
Mortality rate
The mortality rates were significantly different in the five groups 24 h after the operation; observed mortality rates were 91.7%, 66.7%, 25% and 18% for the CLP,Dex2.5, Dex5.0, Dex10.0 groups, respectively (Figure 5).The mortality rates for the groups that received Dex5.0 and Dex10.0 were significantly lower than in the other groups.
DISCUSSION
In our study, dexmedetomidine treatment decreased anti-HLA-DR levels and increased pro-inflammatory mediator (IL-6) level production, which was increased at 3 hours and then attenuated at 5 hours; additionally,dexmedetomidine treatment groups decreased heart rate while maintaining blood pressure and remarkably improving lactate acidosis. All of these factors led to a significant decrease in the mortality rate. Therefore,our key result was that dexmedetomidine treatment in the setting of experimental sepsis partially induced immunomodulation that was initiated within 5 hours and improving mortality rate dose-dependently.
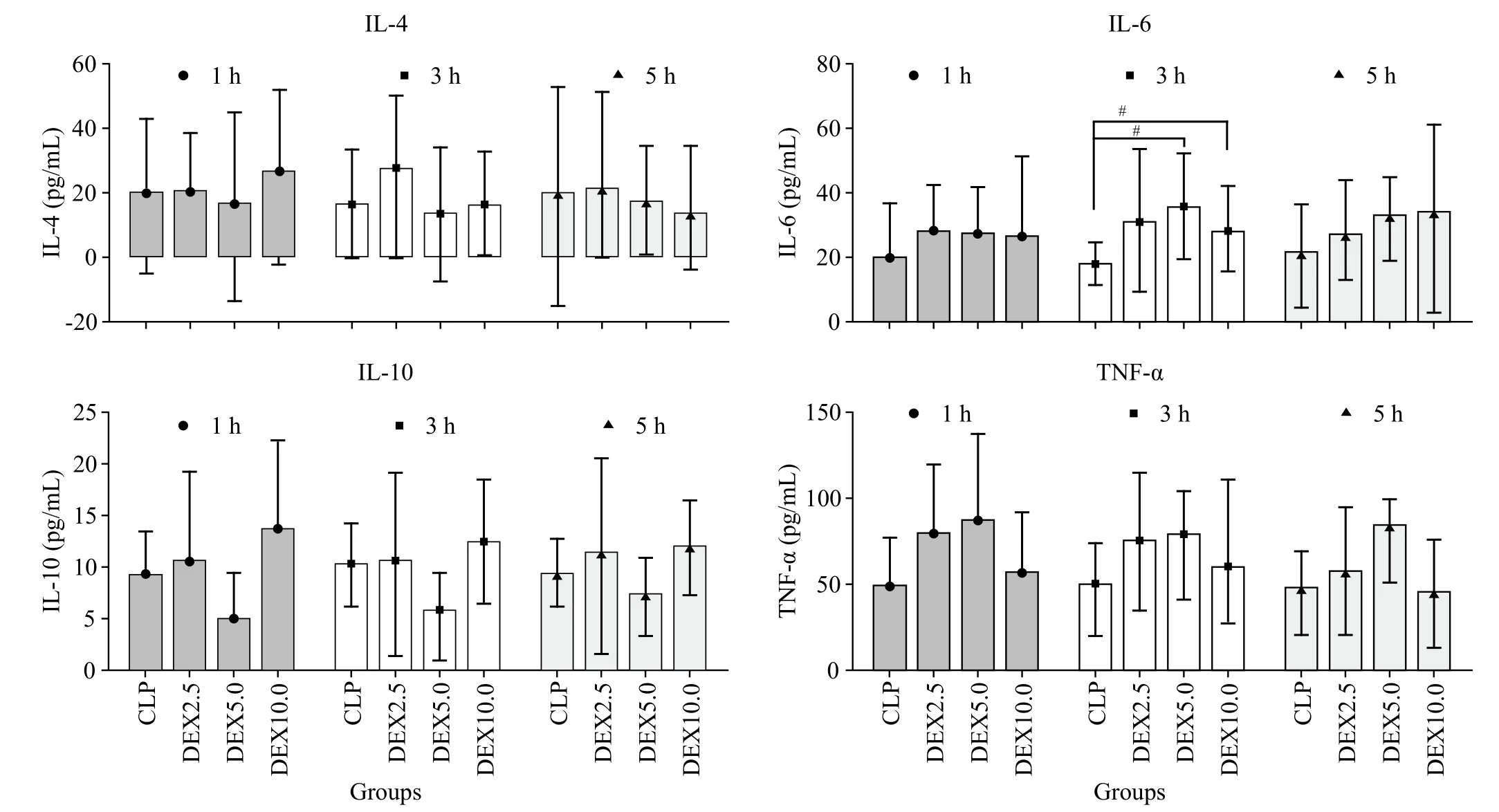
Figure 2. Levels of interleukin-4 (IL-4), interleukin-10 (IL-10), interleukin-6 (IL-6) and plasma tumor necrosis factor α (TNF-α) at diffrents time points (1 h, 3 h and 5 h) after normal saline or dexmedetomidine infusion (means±SD). CLP: cecal ligation and puncture, normal saline treated(n=12); DEX2.5: cecal ligation and puncture, dexmedetomidine treated, 2.5 mg·kg-1·h-1 infusion (n=12); DEX5.0: cecal ligation and puncture,dexmedetomidine treated, 5.0 mg·kg-1·h-1 infusion (n=12); DEX10.0: cecal ligation and puncture, dexmedetomidine treated, 10.0 mg·kg-1·h-1 infusion (n=12). #P<0.05 as compared with CLP groups at 3 h.

Figure 5. Survival curve for CLP, DEX2.5, DEX5.0, DEX10.0 groups at 24 hours. CLP: cecal ligation and puncture, normal saline treated(n=12); DEX2.5: cecal ligation and puncture, dexmedetomidine treated, 2.5 mg·kg-1·h-1 infusion (n=12); DEX5.0: cecal ligation and puncture, dexmedetomidine treated, 5.0 mg·kg-1·h-1 infusion (n=12);DEX10.0: cecal ligation and puncture, dexmedetomidine treated, 10.0 mg·kg-1·h-1 infusion (n=12).
No significant difference in HLA-DR was observed in either group at any time point, but a significant difference was observed between groups at the same time point. The HLA-DR levels were reduced by dexmedetomidine and were lower in the Dex5.0 group.Thus, we hypothesize that DEX may start immune regulation with the infusion beginning at approximately 1 hour. However, there was no different correlation with dose. An excessive immune stress response may cause organ damage. Our study showed that HLA-DR was obviously increased in the CLP group, accounting for a prompt immune stress response. In contrast, DEX inhibition of the immune stress response occurs within 3 hours, and then, immune regulation gradually occurs with the extension of time.
The anti-inflammatory cytokine including IL-4 and IL-10 had no significant differences among the experimental groups at any time point. However, for the pro-inflammatory factor, despite theoretical concerns regarding possible IL-6 and TNF-α levels decreasing with dexmedetomidine use in septic rats and human whole blood,[3,4]our studies have shown that the TNF-α level increased in dexmedetomidine-treated groups within 5 hours, but without significant differences. In the dexmedetomidine-treated groups, the IL-6 level did not obviously change in 1 hour, but along with the time extension, it increased at 3 hours and attenuated at 5 hours. Indeed, several studies have shown that dexmedetomidine reduces the plasma concentration of proinflammatory cytokines and mortality rates in septic animals and humans.[5-9]Thus, we hypothesize that α-2 adrenergic receptor agonists may even promote pro-inflammatory cytokine (IL-6 and TNF-α) in the beginning and then immunomodulated within 5 hours.The reason still needs further study.

Figure 4. Arterial blood gases and lactate analysis during the experimental period. Values (given as means±SD) were measured at 3 hours (3 h)and 5 hours (5 h) of normal saline or dexmedetomidine infusion. CLP: cecal ligation and puncture, normal saline treated (n=12); DEX2.5: cecal ligation and puncture, dexmedetomidine treated, 2.5 mg·kg-1·h-1 infusion (n=12); DEX5.0: cecal ligation and puncture, dexmedetomidine treated,5.0 mg·kg-1·h-1 infusion (n=12); DEX10.0: cecal ligation and puncture, dexmedetomidine treated, 10.0 mg·kg-1·h-1 infusion (n=12). *P<0.001 as compared with CLP groups, △P<0.0001 as compared with CLP groups.
Studies have shown that in critically ill patients,both cardiac output and blood pressure are satisfactorily maintained during dexmedetomidine infusion, regardless of the decrease in HR.[10-14]In our result, although HR values were not significantly changed in the CLP group at any time point, dexmedetomidine induced a gradual decrease in HR values significantly with the extension of infusion time. On the contrary, dexmedetomidine induced a decrease in MAP levels, but no significant changes were observed with the extension of infusion time. These results indicate that dexmedetomidine decreased heart rate, while maintaining blood pressure. Therefore, these cardiovascular effects might limit the usefulness when used in a patient with cardiovascular disease.
Lower BE, TCO2and higher Lac values found in the CLP group are indicative of metabolic acidosis,most likely secondary to tissue hypoperfusion.However, lower pH and higher PCO2values were found in dexmedetomidine-treated groups, which account for transient respiratory depression. Therefore,these respiratory depression effects might limit the usefulness in patients without mechanical ventilation.Moreover, the improvement of metabolic acidosis in the dexmedetomidine-treated groups suggests better tissue perfusion compared with the CLP group.In two previous studies, Taniguchi et al[6,7]already observed an attenuation in the degree of acidosis in dexmedetomidine-treated endotoxemic rats when compared with endotoxemic controls. Accordingly,we consider the possibility that dexmedetomidine could effectively improve microcirculation in a shorttime treatment but induce the side-effect of transient respiratory depression. Marik et al[15]hypothesize that the short treatment period with dexmedetomidine has been insufficient for a decrease in lactate concentrations in the lipopolysaccharide–dexmedetomidine group.Miranda et al[16]suggest that dexmedetomidine yields beneficial effects on the microcirculation of endotoxemic animals. Our study observed that the DEX infusion could obviously improve the blood lactic acid level and BE level within 5 hours.
It is noteworthy that mortality decreases obviously in dexmedetomidine-treated groups, although hemodynamic changes distinctly occur with the extension of infusion time. Interestingly, our study showed a positive correlation between survival rate and dose of dexmedetomidine. However, the dose of dexmedetomidine that we used was relatively high compared with the doses required to produce anesthesia in humans. Furthermore, the dexmedetomidine dose(5.0 μg·kg−1·h−1) was chosen based on previously published experimental studies that showed that this dose was able to reduce cytokine levels in endotoxemic rodents, increasing the survival rate.[6-8]The significance of this finding is the fact that a continuous infusion of dexmedetomidine could effectively improve microcirculation and accompany a positive correlation with dose. However, large doses of DEX infusion led to significant hemodynamic changes, such as significantly decreasing blood pressure and heart rate.This experiment data cannot explain why mortality had a positive correlation that was associated with dose.Further investigation is needed.
Limitations
Dexmedetomidine is commonly used in intensive care units. We reproduced the peritonitis process with animal models and observed the immunomodulation reaction, hemodynamic changes, environmental changes and mortality at three time points (1 hour, 3 hours, and 5 hours) through the process of DEX infusion.
Our study has some limitations. First, CLP rats may reproduce sepsis syndrome, it is in fact imitated the peritonitis process; thus, the results can't equate with human sepsis. Second, fluid resuscitation and norepinephrine infusion are commonly used in human sepsis, but in our study both treatments may influence the effects of dexmedetomidine on the microcirculation independently of fluid therapy effects. For a similar reason, our study did not intervene sepsis-induced changes in MAP.
Through the process of change in sepsis proinflammatory factor, Kawasaki et al[3]demonstrated the suppressing effect of DEX on inflammatory mediator production in human whole blood after 12 hours of LPS stimulation. Our studies observed sepsis proinflammatory factor changing within thefirstfive hours. This experiment showed that the TNF-α level increased in dexmedetomidinetreated groups within 5 hours but without significant differences. In the dexmedetomidine-treated groups, the IL-6 level did not obviously change in 1 hour, but along with the time extension, it increased at 3 hours and attenuated at 5 hours. The results need to be further confirmed.
Studies have concluded that a short time use of DEX may not improve microcirculation, but our observed results show that 5 hours of DEX infusion obviously improved blood lactic acid level and BE level; however,the reason for the obvious improvement of the acidosis level still needs further elucidation. The experimental results indicate that DEX obviously reduces mortality rate and is positively related with dose, but large doses of DEX infusion have significant hemodynamic changes,such as decreased blood pressure, significantly decreased heart rate. This experimental data cannot explain the reason for the clear reduction in mortality that is associated with DEX dose and needs further research.
CONCLUSION
In our study, dexmedetomidine treatment decreased anti-HLA-DR levels and increased pro-inflammatory mediator (IL-6) level production at 3 hours, which was then attenuated at 5 hours. Statistically similar reductions in HR levels were observed with the extension of infusion time while inducing an initial decrease in significant mean arterial blood pressure (MAP) levels (1 hour). Then, the HR levels were maintained at a steady state with the extension of infusion time, remarkably improving metabolic acidosis. All of these factors led to a significant decrease in the mortality rate accompanying a positive correlation with dose. Therefore, our key result was that dexmedetomidine treatment in the setting of experimental sepsis partially induced immunomodulation that was initiated within 5 hours, decreased heart rate,maintained blood pressure, remarkably improved lactate acidosis and improved mortality rates dose-dependently.Further studies in experimental models closer to human sepsis are required to confirm this benefit.
The authors thank Dong Guo Lv (Clinical Medical Research Institute in the First Affiliated Hospital of Xinjiang Medical University) for their help with laboratory experimental research and Chun Zhang (Section of Laboratory Animal Research in the First Affiliated Hospital of Xinjiang Medical University) for their help with animal care.
This study was supported by grants from NSFC (National Natural Science Foundation of China, grant number 81160232),CMA (Chinese Medical Association Intensive Scientific Research Fund project, grant number 13091520537) and the First Affiliated Hospital of Xinjiang Medical University Natural Science Fund project (grant number 2013ZRQN11). The funders had no role in study design, data collection and analysis, or preparation of the article.
Funding:This study was supported by grants from NSFC(National Natural Science Foundation of China, grant number 81160232), CMA (Chinese Medical Association Intensive Scientific Research Fund project, grant number 13091520537)and the First Affiliated Hospital of Xinjiang Medical University Natural Science Fund project (grant number 2013ZRQN11).
Ethical approval:This study protocol was approved by the experimental animal department of the First Affiliated Hospital of Xinjiang Medical University [SYXK (Xin) 2010-0003, AAALAC accredited number:001279].
Conflicts of interests:The authors declare that they have no competing interests. The funders had no role in the design, conduct,analysis, or interpretation of data or in writing the manuscript.
Contributors:Study design and conception, YM; literature research, YM; experimental animal model, YW; quality evaluation,YM and XYY; data analysis or interpretation, YM; manuscript draft: YM; and manuscript approval, all authors.
1 Ma Y, Yu XY. Dose-related effects of dexmedetomidine on immunomodulation and mortality to septic shock in rats. Chin J Emerg Med. 2016;25(9):1149-50.
2 Ma Y, Yu XY. Wang Y. Dose-related effects of dexmedetomidine on hemodynamics and mortality in sepsis shock: experiment with rats. Chin J Emerg Resusc Disaster Med. 2017;12(9):838-41.
3 Kawasaki T, Kawasaki C, Ueki M, Hamada K, Habe K, Sata T. Dexmedetomidine suppresses proinflammatory mediator production in human whole blood in vitro. J Trauma Acute Care Surg. 2013;74(5):1370-5.
4 Taniguchi T, Kurita A, Kobayashi K, Yamamoto K, Inaba H. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22(3):221-8.
5 Memiş D, Hekimoğlu S, Vatan I, Yandim T, Yüksel M, Süt N.Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth. 2007;98(4):550-2.
6 Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K.Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med.2004;32(6):1322-6.
7 Ge Y, Huang M, Ma YF. The effects of microRNA-34a regulating Notch-1/NF-κB signaling pathway on lipopolysaccharide-induced human umbilical vein endothelial cells. World J Emerg Med.2017;8(4): 292-6.
8 Qiao H, Sanders RD, Ma D, Wu X, Maze M. Sedation improves early outcome in severely septic Sprague Dawley rats. Crit Care. 2009;13(4):R136.
9 Hofer S, Steppan J, Wagner T, Funke B, Lichtenstern C,Martin E, et al. Central sympatholytics prolong survival in experimental sepsis. Crit Care. 2009;13(1):R11.
10 Pandharipande PP, Sanders RD, Girard TD, McGrane S,Thompson JL, Shintani AK, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: An a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14(2):R38.
11 Venn M, Newman J, Grounds M. A phase II study to evaluate the efficacy of dexmedetomidine for sedation in the medical intensive care unit. Intensive Care Med. 2003;29(2):201-7.Epub 2002 Nov 22.
12 Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA.2009;301(5):489-99.
13 Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD,Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA.2007;298(22):2644-53.
14 Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: A meta-analysis.Intensive Care Med. 2010 ;36(6):926-39.
15 Marik PE, Baram M. Noninvasive hemodynamic monitoring in the intensive care unit. Crit Care Clin. 2007;23(3):383-400.
16 Miranda ML, Balarini MM, Bouskela E. Dexmedetomidine attenuates the microcirculatory derangements evoked by experimental sepsis. Anesthesiology. 2015;122(3):619-30.
杂志排行
World journal of emergency medicine的其它文章
- Perceptions of emergency medicine residents on the quality of residency training in the United States and Saudi Arabia
- Intravenous fluid selection rationales in acute clinical management
- Association between the elderly frequent attender to the emergency department and 30-day mortality: A retrospective study over 10 years
- Differential diagnoses of magnetic resonance imaging for suspected acute appendicitis in pregnant patients
- Ultrasound curriculum taught byfirst-year medical students: A four-year experience in Tanzania
- Falls from height: A retrospective analysis
