Experimental study on the microstructural and anti-corrosion behaviour of Co-deposition Ni-Co-SiO2composite coating on mild steel
2018-03-12AtunyEkweghiririOele
C.U.Atuny,D.I.Ekweghiriri,C.M.Oele
aDepartment of Metallurgical and Materials Engineering,Nnamdi Azikiwe University,Awka,Anambra State,Nigeria
bDepartment of Polymer and Textile Engineering,Nnamdi Azikiwe University,Awka,Anambra State,Nigeria
1.Introduction
Electrodeposition of zinc is a widely used industrial process to coat on steel materials to enhance its service life.Zinc,being more active than iron,protects sacrificially by forming white rust.Zinc has been the most widely used electrodeposited metal for protecting steel fro mcorrosion because of economic usage and cost[1].Its corrosion resistance,however,is limited.During the last two decades,efforts to improve the corrosion stability of pure zinc coatings have been directed towards alloying it with more noble metals such as nickel,cobalt,iron,and cadmium[2,3].As a result,alloys such as Zn-Ni,Zn-Co,Zn-Fe,and Zn-Cd find wild spread use in industries as replacement for direct electroplating of zinc[4].
Recently,much effort has been devoted to fabricating Ni-Co composite coatings You et al.[5]studied electrodeposition of Ni-Co alloys from a deep eutectic solvent.The effects of saccharin and cobalt concentration in electrolytic solution on microhardness of nanocrystalline Ni-Co alloys was reported by Li et al.[6].Yuxinet al[7]reported on Ni-Co-TiO2nano-composite coatings on mild steel by adding transparent TiO2sol(0-50 mL/L)intothe Ni-Co plating solution.It was found that after adding an optimum sol concentration into the plating bath,a good dispersion of TiO2nanoparticles can be achieved in the Ni-Co coating matrix,resulting in a significant improvement in coating mechanical properties.
Tolumoye et al.[8]reported on composite electrodeposits of Zn-Ni/SiO2produced electrolytically using direct current.The linear polarisation resistance of coatings containing SiO2tends to increase with increasing particle content up to 5.5wt%SiO2and then decreases.Popoola et al.[9]reported on Surface characterization,mechanical properties and corrosion behaviour of ternary based Zn-ZnO-SiO2composite coating.The results showed that average hardness value of 142.5 and 251.2HV and corrosion rate of 0.13088 and 0.00122 mm/yr were obtained for the 0 and 16wt%SiO2in Zn-ZnO.
Eliaz et al.[10]reported on Zn-Ni,Zn-Co and Zn-Ni-Co coatings on mild steel from an acidic chloride bath containing paminobenzenesulphonic acid(SA)and gelatin.The effect of gelatin was more pronounced than that of SA.The Faradaic efficiency was higher than 90%.As the current density was increased or the bath temperature was decreased,the concentration of the nobler metal in the coating increased.Tamilselvi et al.[11]reported on the development of nano SiO2incorporated nano zinc phosphate coatings on mild steel at low temperature for better corrosion protection.The results showed that,the nano SiO2in the phosphating solution changed the initial potential of the interface between mild steel substrate and phosphating solution and reduce the activation energy of the phosphating process,increase the nucleation sites and yielded zinc phosphate coatings of higher coating weight,greater surface coverage and enhanced corrosion resistance.Better corrosion resistance was observed for coatings derived from phosphating bath containing 1.5 g/L nano SiO2.The new formulation reported in the present study was free from Ni or Mn salts and had very low concentration of sodium nitrite(0.4 g/L)as accelerator.
Development of a multilayer-modified coating that enhanced the surface characteristics of mild steel against chemical and mechanical deterioration with a ternary Zn-Ni-Al2O3composition induced with monoethylanine and triethylanine as surfactant using electrolytic chemical deposition was successfully done by Fayomi et al.[12].The deposition of admixed Zn-Ni-Al2O3ternary compositeparticles in the presence of bath-additive surfactanton tothe substrate was impressively enhanced.There exist an improvement in the structural modification and better interfacial adhesion of coatings on the substrate upon addition of triethylanine and monoethylanine as surfactants during the deposition process along with the electro-deposition variables considered.The corrosion resistance improvement of the coated surface was observed.Ghaziof and Gao[13]reported on Zn-Ni-Al2O3nanocomposite coatings on mild steel using a novel sol enhanced electroplating method.They found out,that Zn-Ni-Al2O3nanocomposite coatings produced more uniform and compact deposits,with fine grained microstructure when compared to Zn-Ni coatings.The corrosion resistance of Zn-Ni coatings improved significantly by incorporation of alumina nanoparticles into the coatings.In order to increases research in this novel area of co-deposition of mild steel pipeline,this present work study look at the development of a multilayer-modified coating that will enhance the surface characteristics of mild steel against chemical and mechanical deterioration with a ternary Ni-Co-SiO2composites surfactant using electrolytic chemical deposition.
2.Materials and method
2.1.Materials
Mild steel of dimension 200 mm×200 mm was used for the substrate.Table 1 shows the chemical composition of mild steel used.Pure nickel anode,nickel chloride,potassium chloride,boric acid,thiourea,2 dimethylaminoethanol,cobalt and silicon dioxide,cobalt and silicon oxide powders were sourced from Sunc International Limited and TLS Technik.
2.2.Method
Sectioning of the mild steel was done using automatic struers high precision cut-off machine.The mild steel was sectioned in plates of dimensions of 20 mm by 20 mm.After sectioning the steel plates,they were ground and polished with grit paper and diamond paste.The degreasing of the polished samples was done using ethanol.The composition and condition of the electro-bath to produce Ni-Co and Ni-Co-SiO2composites coating is shown in Tables 2 and 3.
The pH of the bath was adjusted to 4.5 by the addition of HCl and NaOH.Before co-deposition,the SiO2particulates of a mean diameter 55 nm were distributed in the bath electrolyte in the presence of other additives.The deposition setup test was achieved on a set up vessel connected to a laboratory rectifier.The bath was stirred by a magnetic stirrer consistently with about 200 rpm at 40°C.The morphologies of the composite coatings were observed with the help of atomic force microscope,scanning electron microscope and x-ray diffractometer.The electrochemical studies were performed with Autolab PGSTAT 101 Metrohm potentiostat using a three-electrode cell assembly in a 3.65%NaCl static solution at 40°C.The developed composite was the working electrode,platinum electrode was used as counter electrode and Ag/AgCl was used as reference electrode.The anodic and cathodic polarization curves were recorded by a constant scan rate of 0.012 V/s which was fixed from±1.5 mV.From the Tafel corrosion analysis,the corrosion rate,potential and linear polarization resistance was obtained.

Table 1Chemical composition of mild steel.
3.Results and discussion
3.1.XRD analysis
The XRD of the mild steel is shown in Fig.1.From Fig.1 it can be seen that the XRD spectrum has phases of Fe3C(cementite)anda-Fe(ferrite).It was observed that the steel hasa-Fe(ferrite)phase with 100 relative intensity.This supported the analysis of chemical composition of the steel in Table 1.This is par with the XRD pattern of Fayomi et al.[12].
Fig.2 shows the XRD pattern of the NiCo deposited mild steel.The XRD pattern revealed that hard phases of NiO,Co5Ni,Co2Ni3and NiCo5,covered the steel surface after deposition while Fig.3 shows the Ni-Co-SiO2deposited mild steel.Fig.3 revealed the presence of harder phases of NiOSiO2,CoNi7Si2and Co5Ni2Si3.These phases formed from the Ni-Co-SiO2deposited sample are harder than that of Ni-Co deposited sample.This is attributed to the presence of SiO2and Si in Fig.3 which help to strengthen the materials.Similar observation was obtained in the work of Yuxin Wang et al.[7]of deposition of Ni-Co-TiO2nano-composite coatings on mild steel.

Table 2Bath Composition of Ni-Co-SiO2Alloy co-deposition Matrix.

Table 3Formulated Designed bath composition of Ni-Co-SiO2.
3.2.Scanning electron microscopy analysis
Fig.4 shows the SEM/EDS of the uncoated sample.From Fig.4 it can be observed that the there is no coating at the surface of the mild steel.The surface has lines which corresponded to the cutting surface.Figs.5-6 shows the SEM/EDS of the mild steel after coating,it can be seen clearly that there is a morphological change of the microstructure after coating and that the surface of the mild steel is covered with hard layer of Ni-Co-SiO2(compare Fig.4 with Figs.5-6).The surface of Fig.6 is more uniform distributed than that in Fig.5.The uniform distribution of Fig.6 may be attributed to fine SiO2nanoparticle used in the deposition which helped to increase the average surface area of particle during deposition.From the EDS of Figs.5-6 it can be seen that there is evidence of Ni,Co,Fe,C(Fig.5)and Ni,Co,Fe,O,Si(Fig.6),why Fig.4 have Fe and C as the dominate element.
3.3.Atomic force Microscopy(AFM)analysis
The AFM analysis of the topography of the surface revealed that the surfaces are free from defects and porosity(Figs.7-8).When SiO2added coating exhibit a finer texture in which bigger particles formed by nanocrystals agglomerate that are more effectively packed,giving denser structures.It reveals that all films have a smooth surface morphology,and the cracks and pinholes were absent.Generally,Ni-Co-25SiO2uniform,compact and smoother coating were obtained.This is in good agreement with the SEM results.It can be seen that the average particles of the deposited samples are 14.6 nm(Fig.6)and 1.8 nm(Fig.7).This means that the Ni-Co-25SiO2deposited sample has smaller particle size than that of Ni-10Co coating.This supported the earlier observation that the presence of SiO2nanoparticles aided grain refinement that led to smaller size.
3.4.Coating thickness/weight gain
Fig.9 shows the coating thickness obtained after deposition,from Fig.9 it can be observed that the coating thickness of 110.7μm was obtained for Ni-10Co coating,while coating thickness of 135.7,157.7,165.0μm were obtained at Ni-10Co-xSiO2(x=10,15,25wt%)respectively.The weight gain after deposition is shown in Fig.10,from Fig.10 it can be seen clearly that the weight gain increased in the order:0.0299 g for Ni-10Co coating and 0.0310,0.0321,0.0350 g for Ni-10Co-xSiO2(x=10,15,25wt%)respectively.This means that the addition of SiO2to Ni-10Co formulation is beneficial to the increase in weight gain and coating thickness.This is attributed to the hard phases formed which helped in covering/coating the mild steel surface.
3.5.Corrosion rates
Fig.11 and Table 4 show the corrosion rate of the composites coating.It can be seen that the corrosion rate of the composites coating is better than that of the mild steel for all coated samples.The samples have potential,corrosion rate and current density of:-0.67123,-0.6345,-0.62903,-0.59512,-0.51836V,4.132E-4,8.07E-5,5.06E-6,4.14E-6,4.06E-6 mm/year and 1.84E-05,2.09E-7,1.31E-8,1.07E-08,1.46E-097 A/cm2for mild steel,Ni-10Co and Ni-10Co-xSiO2(x=10,15,25wt%)respectively.It can be seen from Fig.11 that the mild steel has lower potential and that after coating the potential shifted towards the right.The mean of 99.02%corrosion resistance was achieved at Ni-Co-25SiO2.This improvement in corrosion resistance after coating may be attributed to the hard and fine structure obtained after coating which helped in strengthening the composite coating.The improvement was attributed to a finer grain size which corresponds to lower porosity in the deposit.Moreover.Reducing the grain size may be advantageous to the overall corrosion performance because the deposit with fine grains can avoid the detrimental corrosion reactions which preferably occur along the grain boundaries[6,7].The better corrosion resistance obtained when SiO2was added to Ni-Co coating is attributed to the fine structure obtained in Fig.6.
The comparison of the microstructural characteristics and the corrosion behavior of the coatings strongly indicates that grain sizes is one of the most important microstructural responsible for corrosion resistance.The present study demonstrates that corrosion resistance is quite sensitive to the coating grain sizes.The result obtained is consistent with the observation made on zinc coatings which had a better corrosion resistance with fine grain sizes[4,5].
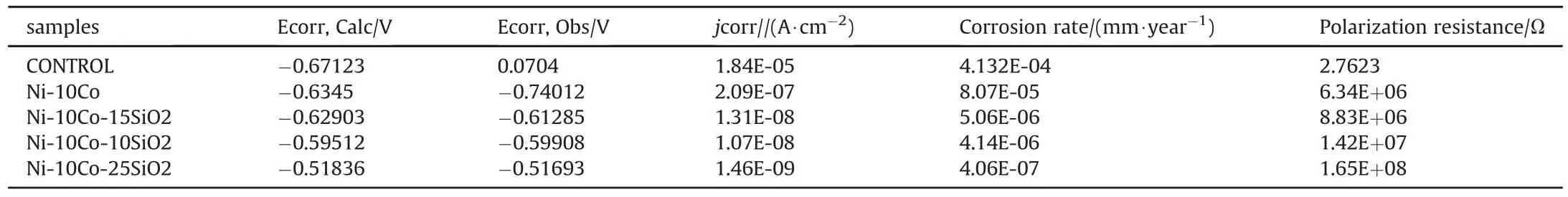
Table 4Electrochemical parameters of the corrosion rate.
4.Conclusions
From the results and discussion above the following conclusions are made:
1)The addition of SiO2nanoparticle in the coating of Ni-Co bath led to uniform microstructure.
2)XRD pattern of the NiCo deposited mild steel revealed the presence of hard phases of NiO,Co5Ni,Co2Ni3,NiCo5and that of Ni-Co-SiO2deposited mild steel revealed the presence of harder phases of NiOSiO2,CoNi7Si2,Co5Ni2Si3.
3)AFM revealed that average particles of the Ni-10Co coating deposited sample is 14.6 nm and that of Ni-Co-25SiO2is 1.8 nm.This mean that the Ni-Co-25SiO2deposited sample has smaller particle size than that of Ni-10Co coating.
4)Coating thickness of 110.7μm was obtained for Ni-10Co coating,while coating thickness of 135.7,157.7,165.0μm were obtained at Ni-10Co-xSiO2(x=10,15,25wt%)
5)99.90%corrosion resistance was achieved at Ni-Co-25SiO2.This improvement in corrosion resistance after composites coating could be attributed to the hard and fine structure obtained after coating
[1]Blejan D,Muresan LM.Corrosion behaviour of Zn-Ni-Al2O3 nanocomposite coatings obtained by electrodeposition from alkaline electrolytes.Mater Corros 2012;63:9999.
[2]Aperador W,Delgado A,Duque J.Corrosion resistance of the[TiN/CrN]n coatings deposited on steel AISI 4140.Int J Electrochem Sci 2013;8:10711-9.
[3]Hammami O,Dhouibi L,Bercot P,Rezrazi E,Triki E.Study of Zn-Ni alloy coatingsmodified by nano-SiO2particles incorporation.IntJCorros 2011;2012:8.
[4]Praveen BM,Venkatesha TV.Electrodeposition and properties of Zn-Ni-CNT composite coatings.Alloys Compd 2009;482:53-7.
[5]You YH,Gu CD,Wang XL,Tu JP.Electrodeposition of Ni-Co alloys from a deep eutectic solvent.Surf Coat Technol 2012;206:3632-8.
[6]Li YD,Jiang H,Wang D,Ge HY.Effects of saccharin and cobalt concentration in electrolytic solution on microhardness of nanocrystalline Ni-Co alloys.Surf Coat Technol 2008;202:4952-6.
[7]Wang Yuxin,Tay See Leng,Wei Shanghai,Xiong Chao,Gao Wei,Shakoor RA,Kahraman Ramazan.Microstructure and properties of sol-enhanced Ni-Co-TiO2nano-composite coatings on mild steel.J Alloys Compd 2015;649:222-8.
[8]Tuaweri Tolumoye J,Jombo Pressy P,Okpala Alexander N.Corrosion resistance characteristics of Zn-Ni/SiO2composite coatings.Int J Adv Mater Sci Eng(IJAMSE)2014;3(No.2).
[9]Popoola API,Aigbodion VS,Fayomi OSI.Surface characterization,mechanical properties and corrosion behaviour of ternary based Zn-ZnO-SiO2composite coating of mild steel.J Alloys Comp 2016;654:561-6.
[10]Eliaz N,Venkatakrishna KA,Chitharanjan Hegde.Electroplating and characterization of Zn-Ni,Zn-Co and Zn-Ni-Co alloys.Surf Coating Technol 2010;205:1969-78.
[11]Tamilselvi M,Kamaraj P,Arthanareeswari M,Devikalab S,ArockiaSelvi J.Development of nano SiO2 incorporated nano zinc phosphate coatings on mild steel.Appl Surf Sci 2015;332:12-21.
[12]Fayomi OSI,Abdulwahab M,Popoola API.Properties evaluation of ternary surfactant-induced Zn-Ni-Al2O3films on mild steel by electrolytic chemical deposition.J Ovonic Res 2013;9:123-32.
[13]Ghaziof S,Gao W.Mechanical and chemical properties of Zn-Ni-Al2O3nanocomposite coatings.Int J Chem Mol Nucl Mater Metall Eng 2015;9(No:8):945-9.
杂志排行
Defence Technology的其它文章
- Experimental study of the effect of wear parameters on the wear behavior of A356 alloy/cow horn particulate composites
- Chemical stability,thermal behavior,and shelf life assessment of extruded modified double-base propellants
- Modification of RDX and HMX crystals in procedure of solvent/anti-solvent by statistical methods of Taguchi analysis design and MLR technique
- Microstructure,properties and hot workability of M300 grade maraging steel
- Numerical and experimental study of wave shaper effects on detonation wave front
- Cold metal transfer(CMT)technology-An overview
