A new combined criterion to better predict malignant lesions in patients with pancreatic cystic neoplasms
2018-03-08ChungenLanXinLiXiuchaoWangJihuiHaoHeRen
Chungen Lan, Xin Li, Xiuchao Wang, Jihui Hao, He Ren
Department of Pancreatic Cancer, Tianjin Medical University Cancer Institute and Hospital; National Clinical Research Center for Cancer; Key Laboratory of Cancer Prevention and Therapy, Tianjin; Tianjin’s Clinical Research Center for Cancer, Tianjin 300060, China
Introduction
The detection rate of pancreatic cysts has increased dramatically owing to the universal use of cross-sectional imaging modalities1,2. Pancreatic cystic neoplasms (PCNs)account for approximately 10%–15% of all pancreatic cystic lesions1,3and approximately 1% of all pancreatic neoplasms3.PCNs include three distinct common tumor types: serous cystic neoplasm (SCN), intraductal papillary mucinous neoplasm (IPMN), and mucinous cystic neoplasm (MCN)4,5.IPMN and MCN are believed to potentially lead to pancreatic ductal adenocarcinoma, whereas SCN is almost always benign4,6,7.
Some PCNs undergo malignant transformation, and detection thus provides an opportunity to surgically cure or prevent pancreatic adenocarcinoma8. In current clinical practice, evaluation of the benign or malignant status of PCNs remains a major challenge. Surgery is recommended for all suitable patients with suspected PCNs9,10. However, it is important to consider that pancreatic surgery is risky, and the possibility of major postsurgical complications (as well as of long-term impairment of pancreatic function) is nonnegligible11,12. The risk of over-treatment (unnecessary pancreatectomy) should be balanced carefully with the risk of under-treatment (missing the opportunity to cure a potentially curable malignant or premalignant lesion)4. The differentiation of a potentially malignant cystic neoplasm from other benign neoplasms prior to surgery plays an important role in treatment planning13,14. Use of a combined diagnostic method is recommended not only for preoperative diagnosis, to reduce the performance of unnecessary surgery in patients with observable progress, but also to prevent surgical delay when appropriate9.
Unfortunately, based on current guidelines, diagnosis and management of PCNs is a clinical challenge15. Preoperative diagnosis of pancreatic cysts is largely reliant on radiographic pathologists. Tumors were classified as benign or malignant according to the American Joint Committee on Cancer(AJCC), 7th edition.
Development of the combined criterion
The receiver operating characteristic (ROC) curve and area under the ROC curve were applied to determine the best cutoff values for baseline NLR. We defined the presence of any two or more of the three predictive factors, including abnormal serum CA19-9 level, NLR>cut-off value, and enhanced solid component on CT imaging, as meeting the criterion for malignancy.
Statistical analysis
For continuous variables, descriptive statistics were used and were reported as mean ± standard deviation (SD) or as median with range. Categorical variables were described using frequency distributions. An independent sample t-test was calculated to detect differences in the means of continuous variables. Chi-square and Spearman rank correlation coefficient testing were used for qualitative variables, and one-way analysis of variance (ANOVA) was applied to detect differences among several groups. All variables with statistically significant malignant predictive value in univariate analysis were selected for further investigation in the multivariate analyses. All statistical analysis was performed using the SPSS software (version 18.0; SPSS Inc., Armonk, NY, USA). P values were two-tailed and regarded as significant when less than 0.05.
Results
Characteristics of study subjects
Table 1 shows the clinical characteristics of 165 patients with PCNs. Among these patients, 65 were men and 100 were women, with a mean age of 56.59 years (range, 20–81).Regarding tumor types, 29 patients had pancreatic IPMN, 67 had SCN, and 69 had MCN. While 124 cysts were identified as benign, 41 were malignant. Sixty-nine patients were asymptomatic, and 96 patients experienced one or more symptoms including abdominal pain/discomfort, vomiting,jaundice, weight loss, or abdominal mass. The mean diameter of PCNs was 4.60±2.65 cm (range, 1.2–16 cm). Regarding location, 51.5% (85) of PCNs were located in the head or neck of the pancreas, and 48.5% (80) were located in the body or tail. The mean NLR in all patients with PCN was 2.10±1.34. Serum CA19-9, CEA, and CA242 were measured in all patients. The ratios of normal to abnormal were 127/38,112/53, and 144/21 for CA19-9, CEA, and CA242,respectively. An enhanced solid component inside the cyst was observed in 77 patients (46.7%) on CT imaging.
Correlation between clinical characteristics and PCN malignancy
According to pathology results, the patients were divided into benign and malignant groups. Sex could not be considered a predictor of malignancy in the patients with PCNs. Tumor location between body or tail of the pancreas and older age were significantly associated with malignancy (P=0.005,P=0.002, respectively) (Table 1). Symptoms at admission were recorded more often in the malignant group (31/41;75.6%) (P=0.009). Preoperative serum CA19-9, CA242, and CEA levels were closely associated with PCN malignancy(P<0.001, P<0.001, and P=0.010, respectively) (Table 1).
Representative pathology and images of both benign and malignant lesions are shown in Figure 1. Patients with malignant PCNs had a higher probability of exhibiting enhanced solid components on CT images (P<0.001)(Table 1). It is unsurprising that the presence of an enhanced solid component is strongly associated with malignant PCNs.
To determine whether NLR prior to surgery was predictive of malignant potential, we compared the preoperative NLR in 124 patients with benign PCNs with that in 41 patients with malignant PCNs. The NLR of patients with benign PCNs (1.87±0.84) was significantly lower than that of patients with malignant PCNs (2.81±2.14, P=0.009).Furthermore, we compared peripheral blood NLR in patients with benign PCNs, malignant PCNs, PNET, PDAC, and IgG4-related sclerosing pancreatitis, with that in healthy controls (Figure 2). The NLR in patients with malignant PCNs was similar to that in patients with PDAC (P=0.640),and was significantly higher than that in patients diagnosed with PNET (1.90 ± 0.69, P=0.013) or in healthy donors (1.40 ±0.48; P<0.001). NLR in patients with malignant PCNs and PDAC was higher than that in healthy volunteers, indicating that the increase in NLR might be caused by tumor microenvironment.
Independent factors predicting malignancy in PCNs
The optimal cut-off value of NLR for predicting malignant PCNs was 1.976 (AUC=0.673, Figure 3). Patients were divided into a low NLR group (NLR≤1.976; n=96) and a highNLR group (NLR >1.976; n=69). To evaluate the utility of NLR prior to surgery in identifying patients with malignancy,logistic regression analysis was performed with clinical parameters including NLR (Table 2). Univariate analysis showed that high NLR (>1.976), older age (>56 years), high CA19-9 (≥39 U/mL), high CEA (≥5 ng/dL), high CA242 (≥12 U/mL), presence of enhanced solid component, and tumor location in the body or tail of pancreas were all significantly associated with malignancy. Multivariate analysis revealed that high CA19-9 (≥39 U/mL), presence of enhanced solid component, and high NLR were independent predictors of PCN malignancy.

Table 1 Background characteristics of patients with PCNs
Clinical utility of NLR and the new combined criterion

Figure 2 Distribution of NLR in 603 patients with various types of pancreatic disease is shown. NLR values are compared among these groups. Healthy volunteers (n=330), PDAC: pancreatic ductal adenocarcinoma (n=49), PNET: pancreatic neuroendocrine tumor(n=44), IgG4: IgG4-related sclerosing pancreatitis (n=15), benign PCNs in the study (n=124), malignant PCNs in the study (n=41).*P<0.05, **P<0.01.
Next, we aimed to identify whether NLR could be a supportive index to predict malignancy of PCNs when combined with other conventional indicators. The distribution of NLR value, serum CA19-9 level, presence of enhanced solid component, and the presence of PCN malignancy is shown in Figure 4. The sensitivity, specificity,positive predictive value (PPV), and negative predictive value(NPV) for predicting PCN malignancy were evaluated with the combined criterion of NLR>1.976, abnormal serum CA19-9 level, and presence of enhanced solid component.The sensitivity, specificity, PPV, NPV, and accuracy of the new diagnostic criterion for predicting PCN malignancy are shown in Table 3, in comparison with each conventional indicator of PCN malignancy. Furthermore, the sensitivity,specificity, PPV, and NPV predicted by the new criterion were 68.8%, 93.2%, 80.5%, and 87.9%, respectively. The new criterion demonstrated both a high PPV of 80.5% and a high NPV of 87.9%. Thus, a higher accuracy (86.1%) in identifying malignancy could be obtained with the new diagnostic criterion.

Table 2 Univariate and multivariate analysis for predicting malignancy in PCNs
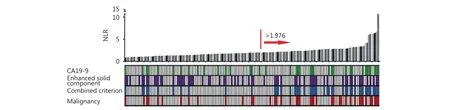
Figure 4 High NLR level is associated with the presence of malignant PCNs. Distributions of NLR level and serum CA19-9 level, presence of enhanced solid component, and presence of malignant lesions in patients with PCNs (n=165) are shown. Each bar chart shows the patient's NLR value, and lower heat map shows if patient meets the criterion. Red arrow shows the high NLR group (>1.976).
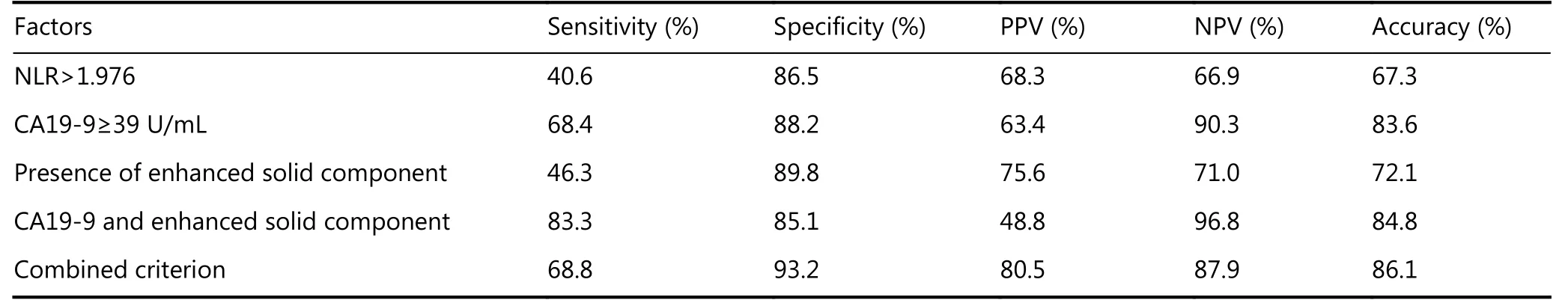
Table 3 The sensitivity, specificity, positive predictive value (PPV), negative predictive values (NPV) and accuracy for predicting PCNs malignancy of NLR, CA19-9, presence of enhanced solid component and combined criterion are all shown
Discussion
PCNs are now more frequently discovered with the increased use of abdominal CT imaging33. Some of these neoplasms undergo malignant transformation8,34. Therefore,considering the current efficacy of pancreatic resection, it is not surprising that the number of operations for PCNs has increased. Pancreatic resection can remove symptomatic,malignant, or potentially malignant lesions. However, some cystic lesions are benign or slow growing, and their potential for malignant transformation remains unclear33. Recent studies have shown that only one-fifth of the resected asymptomatic pancreatic cysts are malignant35. Surgeons require a rapid and accurate assessment of the risk benefit ratio of observation versus resection of these neoplasms in individual patients.
Serous cystadenomas, which are often located in the body or tail of the pancreas, occur most commonly in middle-aged women. In contrast, IPMN occurs more frequently in the pancreatic head in elderly male patients, and mucinous cystadenomas occur mostly in elderly females34,36. In our results, sex could not be considered a predictor of malignant PCN. Patients with benign MCN have a median age of 50 years, whereas patients with mucinous cyst adenocarcinoma have a median age of 65 years3. Other studies reported that patients with malignant cysts were older than patients with benign cysts, with statistical significance16,37. This observation is consistent with our findings that age is correlated with malignant cystic pancreatic neoplasms.
Medical consensus suggests 3 cm as the cut-off value for resection of asymptomatic lesions8. On the other hand, large numbers of studies have reported that cyst size had little correlation with pathological malignant traits35. Sarr et al.38reported no difference in the mean size between benign mucinous cystadenoma and mucinous cystadenocarcinoma.Lee et al.39found that 19% (31/166) of cystic pancreatic tumors <3 cm proved to be malignant. In the present study,lesion size was also not predictive of malignant cysts.However, we found that tumor location was a predictive factor of malignant PCNs. Lesions located in the body or tail of the pancreas were more likely to be malignant.
Owing to the morphological overlap in imaging, and poor inter-observer consensus in relation to the standards between benign and malignant PCNs, CT images allow correct characterization of only 25%–60% of cystic pancreatic masses40. Numerous researchers have reported that the presence of solid component inside the cyst on imaging is a significant predictor of malignancy40. In the present study also, the most significant radiological finding correlated with malignancy was the presence of enhanced solid component.Moreover, the presence of enhanced solid component was found to be an independent predictor in multivariate analysis.
In clinical practice, CA19-9 and CEA are two of the important biomarkers for patients with pancreatic malignant diseases41. Serum CA19-9 level is elevated in more than three quarters of patients with pancreatic ductal adenocarcinoma42.These biomarkers were found to be correlated with the presence of malignancy. An increased level of CA19-9 was found to be specifically correlated with malignant PCNs.Thus, CA19-9 is considered to be a vital test for all patients, a finding consistent with the results of other series8. However,although the specificity of serum CA19-9 is high, its sensitivity has been shown to be very low42. Some researchers have suggested that endoscopic ultrasound with fine-needle aspiration (EUS-FNA) and cystic fluid analysis hold promise in identifying lesions with malignant potential43. EUS is not always accessible, however, particularly in rural areas where the equipment for distinguishing malignant lesions from benign PCNs is not available44. Limitations to the access of EUS indicate that clinicians must rely on other noninvasive techniques to assess the risk of PCN.
Various investigations of NLR have been performed in various types of cancer, considering its role in the prediction of cancer development29,45. A strong connection between neutrophil infiltration and malignant progression has been described by inflammatory mediators released by peripheral blood cells, which play a vital role in the intersection between neoplastic and inflammatory cells46. Recently, it was reported that tumorigenesis in the pancreas correlates with distinct intra- and peri-tumoral inflammation47. Thus, despite its non-specificity, increased NLR might be indicative of increased inflammatory activation in PCN-derived malignancies. The present study demonstrated that NLR is significantly higher in patients diagnosed with malignant PCNs than in those with benign lesions. As there was a remarkable difference in NLR value between healthy volunteers and patients with malignant PCNs, NLR might be important for monitoring malignant progression of PCNs.Intriguingly, in our study, we found that the cut-off value of NLR >1.976 was an independent predictor for the malignant potential of PCNs. Other studies selected different cut-off values of NLR48,49, but these findings all indicate that high NLR is a supportive predictor of malignancy in PCNs.
The conventional guidelines for the diagnosis of malignancy of PCNs were unsatisfactory and complex, owing to the variety of predictors and their ambiguity. Preoperative prediction of malignancy often depends on the surgeon’s experience or other important factors that are difficult to clearly ascertain and have low PPV and NPV50. The new combined criterion is a quantitative standard that is more accurate, practical, and convenient for all surgeons.Therefore, we recommend the diagnosis of malignancy of PCNs on the basis of CA19-9, NLR, and CT imaging.
In conclusion, we defined a new combined criterion to predict malignancy in patients with PCNs. The combined accuracy for predicting malignant PCNs was superior to that of any one of the abovementioned three standards. This criterion is superior to current practice in the identification of patients with malignant lesions in PCNs. It is important to distinguish benign from malignant lesions to select individuals who would benefit from early surgery.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Garcea G, Ong SL, Rajesh A, Neal CP, Pollard CA, Berry DP, et al.Cystic lesions of the pancreas. A diagnostic and management dilemma. Pancreatology. 2008; 8: 236-51.
2.Jang DK, Song BJ, Ryu JK, Chung KH, Lee BS, Park JK, et al.Preoperative diagnosis of pancreatic cystic lesions: The accuracy of endoscopic ultrasound and cross-sectional imaging. Pancreas. 2015;44: 1329-33.
3.Atef E, El Nakeeb A, El Hanafy E, El Hemaly M, Hamdy E, El-Geidie A. Pancreatic cystic neoplasms: Predictors of malignant behavior and management. Saudi J Gastroenterol. 2013; 19: 45-53.
4.Del Chiaro M, Verbeke C. Cystic tumors of the pancreas:Opportunities and risks. World J Gastrointest Pathophysiol. 2015;6: 29-32.
5.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C,Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med.2004; 351: 1218-26.
6.Del Chiaro M, Verbeke C, Salvia R, Kloppel G, Werner J, McKay C,et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013; 45: 703-11.
7.Plichta JK, Brosius JA, Pappas SG, Abood GJ, Aranha GV. The changing spectrum of surgically treated cystic neoplasms of the pancreas. HPB Surg. 2015; 2015: 791704
8.Jones NB, Hatzaras I, George N, Muscarella P, Ellison EC, Melvin WS, et al. Clinical factors predictive of malignant and premalignant cystic neoplasms of the pancreas: A single institution experience.HPB. 2009; 11: 664-70.
9.Bai XL, Zhang Q, Masood N, Masood W, Zhang Y, Liang TB.Pancreatic cystic neoplasms: A review of preoperative diagnosis and management. J Zhejiang Univ Sci B. 2013; 14: 185-94.
10.Horvath KD, Chabot JA. An aggressive resectional approach to cystic neoplasms of the pancreas. Am J Surg. 1999; 178: 269-74.
11.Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR,Wargo JA, Warshaw AL, et al. 851 resected cystic tumors of the pancreas: A 33-year experience at the massachusetts general hospital. Surgery. 2012; 152: S4-12.
12.Permuth JB, Choi JW, Chen DT, Jiang K, DeNicola G, Li JN, et al.A pilot study of radiologic measures of abdominal adiposity:Weighty contributors to early pancreatic carcinogenesis worth evaluating? Cancer Biol Med. 2017; 14: 66-73.
13.Postlewait LM, Ethun CG, McInnis MR, Merchant N, Parikh A,Idrees K, et al. Association of preoperative risk factors with malignancy in pancreatic mucinous cystic neoplasms: A multicenter study. JAMA Surg. 2017; 152: 19-25.
14.Gasslander T, Arnelo U, Albiin N, Permert J. Cystic tumors of the pancreas. Dig Dis. 2001; 19: 57-62.
15.Kwon RS. Advances in the diagnosis of cystic neoplasms of the pancreas. Curr Opin Gastroenterol. 2012; 28: 494-500.
16.Farrell JJ. Prevalence, diagnosis and management of pancreatic cystic neoplasms: Current status and future directions. Gut Liver.2015; 9: 571-89.
17.de la Santa LG, Retortillo JAP, Miguel AC, Klein LM. Radiology of pancreatic neoplasms: An update. World J Gastrointest Oncol.2014; 6: 330-43.
18.Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C,Brugge WR, Hahn PF. Cystic pancreatic lesions: A simple imagingbased classification system for guiding management.Radiographics. 2005; 25: 1471-84.
19.Sethi V, Giri B, Saluja A, Dudeja V. Insights into the pathogenesis of pancreatic cystic neoplasms. Dig Dis Sci. 2017; 62: 1778-86.
20.de Pretis N, Mukewar S, Aryal-Khanal A, Bi Y, Takahashi N, Chari S. Pancreatic cysts: Diagnostic accuracy and risk of inappropriate resections. Pancreatology. 2017; 17: 267-72.
21.Lee HJ, Kim MJ, Choi JY, Hong HS, Kim KA. Relative accuracy of ct and mri in the differentiation of benign from malignant pancreatic cystic lesions. Clin Radiol. 2011; 66: 315-21.
22.Fan ZH, Yan K, Wang YJ, Qiu JX, Wu W, Yang L, et al. Application of contrast-enhanced ultrasound in cystic pancreatic lesions using a simplified classification diagnostic criterion. BioMed Res Int. 2015;2015: 974621
23.Vasile TA, Feier D, Socaciu M, Anton OM, Seicean A, Iancu C,et al. Contrast enhanced ultrasound and computer tomography diagnosis of solid and mixed pancreatic tumors - analysis of confounders. J Gastrointest Liver Dis. 2012; 21: 285-92.
24.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M,Jang JY, et al. International consensus guidelines 2012 for the management of ipmn and mcn of the pancreas. Pancreatology.2012; 12: 183-97.
25.Xu MM, Yin S, Siddiqui AA, Salem RR, Schrope B, Sethi A, et al.Comparison of the diagnostic accuracy of three current guidelines for the evaluation of asymptomatic pancreatic cystic neoplasms.Medicine. 2017; 96: e7900
26.Bai XL, Ye LY, Zhang Q, Prasoon P, Wang J, Liang TB. Surgical resection and outcome of pancreatic cystic neoplasms in china:Analysis of a 16-year experience from a single high-volume academic institution. World J Surg Oncol. 2014; 12: 228
27.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420: 860-7.
28.Rizzo A, Cengel KA. Anti-inflammatory therapy for pancreatic cancer: A sorely needed advance in therapeutics? Cancer Biol Ther.2008; 7: 1051-2.
29.Zhang X, Zhang W, Feng LJ. Prognostic significance of neutrophil lymphocyte ratio in patients with gastric cancer: A meta-analysis.PLoS One. 2014; 9: e111906
30.Arima K, Okabe H, Hashimoto D, Chikamoto A, Tsuji A,Yamamura K, et al. The diagnostic role of the neutrophil-tolymphocyte ratio in predicting pancreatic ductal adenocarcinoma in patients with pancreatic diseases. Int J Clin Oncol. 2016;21: 940-5.
31.Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35: 868-72.
32.Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Okamura Y.Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of survival after gastroenterostomy in patients with advanced pancreatic adenocarcinoma. Ann Surg Oncol. 2013;20: 4330-7.
33.Fisher WE, Hodges SE, Yagnik V, Morón FE, Wu MF, Hilsenbeck SG, et al. Accuracy of ct in predicting malignant potential of cystic pancreatic neoplasms. HPB. 2008; 10: 483-90.
34.Kern SE, Hruban RH, Hidalgo M, Yeo CJ. An introduction to pancreatic adenocarcinoma genetics, pathology and therapy.Cancer Biol Ther. 2002; 1: 607-13.
35.Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A,Komorowski RA, et al. Cystic pancreatic neoplasms - observe or operate. Ann Surg. 2004; 239: 651-7.
36.Nougaret S, Mannelli L, Pierredon MA, Schembri V, Guiu B. Cystic pancreatic lesions: From increased diagnosis rate to new dilemmas.Diagn Interv Imaging. 2016; 97: 1275-85.
37.Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A,Komorowski RA, et al. Cystic pancreatic neoplasms: Observe or operate. Ann Surg. 2004; 239: 651-9.
38.Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms?Ann Surg. 2000; 231: 205-12.
39.Lee CJ, Scheiman J, Anderson MA, Hines OJ, Reber HA, Farrell J,et al. Risk of malignancy in resected cystic tumors of the pancreas< or =3 cm in size: Is it safe to observe asymptomatic patients? A multi-institutional report J Gastrointest Surg. 2008; 12: 234-42.
40.Bassi C, Salvia R, Molinari E, Biasutti C, Falconi M, Pederzoli P.Management of 100 consecutive cases of pancreatic serous cystadenoma: Wait for symptoms and see at imaging or vice versa?World J Surg. 2003; 27: 319-23.
41.Zheng L, Wolfgang CL. Which patients with resectable pancreatic cancer truly benefit from oncological resection: Is it destiny or biology? Cancer Biol Ther. 2015; 16: 360-2.
42.Moris D, Damaskos C, Spartalis E, Papalampros A, Vernadakis S,Dimitroulis D, et al. Updates and critical evaluation on novel biomarkers for the malignant progression of intraductal papillary mucinous neoplasms of the pancreas. Anticancer Res. 2017; 37:2185-94.
43.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA,Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology.2004; 126: 1330-6.
44.Ahmad NA, Kochman ML, Ginsberg GG. Practice patterns and attitudes toward the role of endoscopic ultrasound in staging of gastrointestinal malignancies: A survey of physicians and surgeons.Am J Gastroenterol. 2005; 100: 2662-8.
45.Luo GP, Guo M, Liu ZQ, Xiao ZW, Jin KZ, Long J, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015; 22: 670-6.
46.Gregory AD, Houghton AM. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res. 2011; 71: 2411-6.
47.Steele CW, Jamieson NB, Evans TRJ, McKay CJ, Sansom OJ,Morton JP, et al. Exploiting inflammation for therapeutic gain in pancreatic cancer. Br J Cancer. 2013; 108: 997-1003.
48.Arima K, Okabe H, Hashimoto D, Chikamoto A, Kuroki H, Taki K,et al. The neutrophil-to-lymphocyte ratio predicts malignant potential in intraductal papillary mucinous neoplasms. J Gastrointest Surg. 2015; 19: 2171-7.
49.Goh BKP, Tan DMY, Chan CY, Lee SY, Lee VTW, Thng CH, et al.Are preoperative blood neutrophil-to-lymphocyte and platelet-tolymphocyte ratios useful in predicting malignancy in surgicallytreated mucin-producing pancreatic cystic neoplasms? J Surg Oncol. 2015; 112: 366-71.
50.Cho CS, Russ AJ, Loeffler AG, Rettammel RJ, Oudheusden G,Winslow ER, et al. Preoperative classification of pancreatic cystic neoplasms: The clinical significance of diagnostic inaccuracy. Ann Surg Oncol. 2013; 20: 3112-9.
杂志排行
Cancer Biology & Medicine的其它文章
- Biology, staging, and treatment of breast cancer during pregnancy: reassessing the evidences
- Post-irradiation pericardial malignant mesothelioma with deletion of p16: a case report
- Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable non-small cell lung cancer
- Modified Blumgart anastomosis without pancreatic ductto-jejunum mucosa anastomosis for pancreatoduodenectomy:a feasible and safe novel technique
- Parkin protein expression and its impact on survival of patients with advanced colorectal cancer
- Calcium channel α2δ1 subunit as a novel biomarker for diagnosis of hepatocellular carcinoma
