γ-Ray radiolysis of acetohydroxamic acid in HNO3and its radiolytic product
2018-03-07JinHuaWangChaoLiQianLiMingHongWuWeiFangZhengHuiHe
Jin-Hua Wang•Chao Li•Qian Li•Ming-Hong Wu•Wei-Fang Zheng•Hui He
1 Introduction
In order to realize a closed nuclear fuel cycle,the spent fuel discharged from nuclear power reactors must be reprocessed for isolating and recycling the unburned U and Pu generated.In the partition cycle of the improved Purex process used for the reprocessing of spent fuel,both Pu and Np are expected to be separated from U.On the other hand,U puri fication also involves the removal of Pu and Np.Two methods have been proposed for the separation of Pu and Np from U:one is the reduction of Pu(IV)and Np(VI)to Pu(III)and Np(V),which are unextractable by tri-butylphosphate(TBP),the other is to complex Pu(IV)and Np(IV),while U(VI)is unaffected.In the partition cycle,the concentrations of Pu and Np are relative higher,and hence,an appropriate reductant or complexant can effectively separate these nuclides from U.In the U puri fication cycle,however,the Pu and Np contents are very low;thus,only a complexant would be effective for the decontamination of U from Pu and Np[1,2].A series of organic ligands,namely carboxymethylamine,formic acid,acetic acid,butyric acid,pyruvic acid,glycolic acid,formohydroxamic acid,acetohydroxamic acid(AHA),and n-propionyl-hydroxamic acid,were proposed and tested.The results showed that AHA is the best[2–6].Many researchers studied the complexation of AHA with Pu(IV),Np(IV),and U(VI)[2–4,7–11]and found that the stability constants for AHA-Pu(IV)and AHA-Np(IV)are much higher than that for AHA-U(VI).Therefore,AHA can preferentially strip Pu(IV)and Np(IV)from the TBP phase into the aqueous phase,while U(VI)is unaffected.In addition,AHA can rapidly reduce Np(VI)to Np(V);U(VI)is not reduced,but Pu(IV),after initial complex formation,is slowly reduced to Pu(III)[3,12].As AHA-Pu(IV),AHA-Np(IV),Np(V),and Pu(III)are unextractable by TBP,Pu and Np can be well separated from U by AHA[5,13–18].AHA is composed of C,H,O,and N,and it can be completely incinerated to NO2and CO2.Since no solid waste is generated by the addition of AHA[16],it is expected to be useful for the reprocessing of spent fuel.However,AHA might suffer from radiation damage during its use,which would affect its ef ficiency,and the resulting radiolytic products may hinder effective separation.Karraker[19]studied the radiolysis of AHA in HNO3at doses up to 11 kGy,which was estimated from the spent fuel to be reprocessed,and found that radiation decomposed a minor fraction of AHA compared to the loss by hydrolysis and that AHA radiolysis showed weak dependence on both the HNO3concentration and the absorbed dose.In his study,the residual AHA content was analyzed by the light absorption of an Fe(III)-AHA complex.However,he compared the effect of radiation damage on AHA based on the light absorption value of the diluted samples and not the residual concentration of AHA.As the irradiated samples were diluted 100–400 times,even a slight change in absorption may cause a signi ficant difference in the AHA concentration,and the absorbance value obtained will not be representative of the AHA concentration;hence,these results are not reliable.In this study,the radiolysis of AHA is monitored based on the change in the AHA concentration,and the radiolytic product of AHA is also reported.These results are expected to serve as an important reference for the application of AHA in the reprocessing of spent fuel.
2 Experiment
2.1 Reagent
AHA was supplied by Aldrich Chemical Company,and its purity was 98%.Two kinds of standard gas mixtures were supplied by Shanghai Institute of Measurement and Testing Technology:one was composed of 2.50%hydrogen,1.00%carbon monoxide,1.00%carbon dioxide,0.30%methane,0.01%ethane,0.01%ethene,and 95.18%nitrogen;the other contained 3.00%nitrous oxide and 97.00%nitrogen.
2.2 Main equipment and accessories
A 3.7 × 1015Bq60Co-γ ray irradiator was supplied by Shanghai Institute of Applied Physics,Chinese Academy of Sciences.A T90 UV–VIS spectrophotometer was supplied by Beijing Purkinje General Instrument Co.Ltd.An ion chromatograph(research-style)was obtained from Switzerland Metrohm Co.Ltd.A GC900A gas chromatograph and a packed column with 5 A˚molsieve(2 m×3 mm)were supplied by Shanghai Ke Chuang Chromatograph Instruments Co.Ltd.A capillary column with aluminum oxide(50 m×0.53 mm)was supplied by Lanzhou Institute of Chemistry and Physics,Chinese Academy of Sciences.
2.3 Sample preparation,irradiation,pretreatment,and analysis
0.2 mol L-1AHA solutions containing different concentrations of HNO3were prepared as follows.A certain amount of AHA was put in a beaker,which was then placed in an ice-waterbath.A certain amountof 0.5 mol L-1HNO3was slowly dropped into the beaker with stirring,and then,a certain amount of slightly diluted HNO3was added.The solution was transferred to a 100-mL measuring flask to a constant volume.Thus,solutions in which the AHA concentration was 0.2 mol L-1and the HNO3concentration was 0.2,0.5,1.0,and 2.0 mol L-1were prepared.Four milliliters of each solution was placed in 7-mL glass vials,which were sealed with a sealing machine.The samples were irradiated by60Co γ-ray to 5,10,15,20,and 25 kGy,and the absorbed doses were monitored by dichromate dosimeters.Control samples were used for comparison of the radiation effect on the loss of AHA by hydrolysis as well as on the amount of radiolytic product.The gases evolved from the irradiated AHA solutions were tested by gas chromatography for the analysis of H2,N2O,CH4,and C2H6.The irradiated AHA solutions and control samples were neutralized by KOH solution and then diluted to the suitable concentrations.Finally,these pretreated samples were tested by ultraviolet–visible spectrophotometry and ion chromatography for the analysis of AHA,HCOOH,and HNO2.
2.4 Analysis of AHA and its radiolytic products
Quantitative analysis of AHA was performed by ultraviolet–visible spectrophotometry.2.7,5.3,8.0,and 10.7 m mol L-1standard AHA solutions were prepared using high-purity water.1.00 mL of each solution was placed in a 10-mL measuring flask,to which 3.00 mL FeCl3solution(10 wt%)and 4.00 mL CCl3COOH solution(2.5 wt%)were separately added.Then,water was added to constant volume,and the solutions were shaken well.The reference solution was prepared in the same manner as the standard AHA solutions but without AHA.These standard AHA solutions were tested at 502 nm by using an ultraviolet–visible spectrophotometer.The response curve for AHA was obtained from the AHA concentrations and the corresponding absorbance.The irradiated AHA solutions and control samples were neutralized by KOH solutions and diluted to suitable concentrations.These pretreated samples were tested by using the ultraviolet–visible spectrophotometer.From the response curve for AHA and the absorbance of the diluted samples,the residual AHA concentrations in the samples were calculated.H2,N2O,CH4,and C2H6were analyzed by gas chromatography.For H2analysis,a 5 A˚molsieve packed column was used,and the column temperature was 80°C;a thermal conductivity detector(TCD)was employed,and its temperature was 110°C.The carrier gas was Ar,with a flow rate of 10 mL min-1.N2O analysis was conducted using a packed shincarbon T column and a TCD detector.H2was used as the carrier gas,and its flow rate was 25 mL min-1.The column temperature was programmed as follows:initial temperature,100°C;initial isothermal period,7 min;programmed heating rate,35°C min-1;final temperature,220°C;and final isothermal period,1 min.The TCD temperature was 120°C.CH4and C2H6were analyzed using a PLOT Al2O3column and a flame ionization detector(FID).The carrier gas was N2,and its flow rate was 19 mL min-1.The column temperature was 40 °C,and the FID temperature was 110 °C.CH3COOH and HNO2were analyzed by ion chromatography with an electric conductivity detector,using a METROSEP A SUPP 5–250 column.The eluent was a solution containing 3.2 mmol L-1Na2CO3and 1.0 mmol L-1NaHCO3,and its flow rate was 0.7 mL min-1.
2.5 Formulae used in thepaper
The hydrolysis rate of AHA is de fined as follows:hydrolysis rate=(C0-Cc)/C0×100%,where C0is the original AHA concentration and Ccis the AHA concentration in the control sample.The radiolysis rate of AHA is de fined by the equation:radiolysis rate=(Cc-Ci)/Cc×100%,where Ciis the AHA concentration in the irradiated sample.The volume fraction of the gas product is calculated as follows:if the response curve equation of a component is y=ax+b,the X axis is the injected volume of the standard gas mixture,and the Y axis is the corresponding peak area of the component.Then,the volume fraction of the component is calculated by the following formula:volume fraction=(A-b)c/ae,where A is the component peak area in the gas chromatogram of the gas sample,c is the volume fraction of the component in the standard gas mixture,and e is the injected volume of sample gas.
3 Results and discussion
3.1 Radiolysis of AHA in HNO3solution
As AHA can complex with Fe(III)in an acidic solution to yield a colored product,it can be analyzed by ultraviolet–visible spectrophotometry[15].The response curve for AHA is y=114.38x+0.0142(concentration range 2.70–10.7 m mol L-1),and the correlation coef ficient(R2)is 0.9994.TheAHA concentrationincontrolsamples decreases drastically with increasing HNO3concentration.When the HNO3concentration is 0,0.2,0.5,1.0,and 2.0 mol L-1,the hydrolysis rate of AHA is 8.20,23.1,41.2,66.2,and 85.9%,respectively,indicating that the rate increases with the HNO3concentration.This result is consistent with that reported by Taylor[20]and Tkac[10].In an acidic solution,AHA hydrolyzes to acetic acid and hydroxylamine:
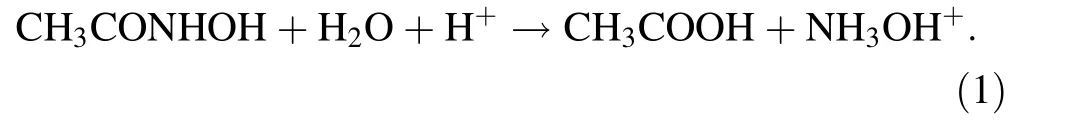
As the time lag among sample preparation,irradiation,and neutralization is very long(about 9 h),this observation cannot be used to elucidate the hydrolysis of AHA employed in the advanced Purex process.In the separation of Pu and Np from U by countercurrent liquid–liquid extraction,the residence time is about 30 min;if the separation occurs in the centrifugal contactor,the residence time is much shorter.We have studied the hydrolysis of AHA in HNO3at different times[21].At 0.2 mol L-1AHA,with 0.2,0.5,1.0,and 2.0 mol L-1HNO3,the hydrolysis rate of AHA at 0.5 h is 2.40,5.30,7.60,and 8.40,respectively;thus,thehydrolysisofAHA in 0.2–2.0 mol L-1HNO3would not be drastic.In addition,fast complexation of AHA with a metal leads to slower hydrolysis and stabilization of AHA in solution[10],so the hydrolysis of AHA will not affect its application in the separation of Pu and Np from U.Figure 1 illustrates the radiolysis rate of AHA as a function of dose.
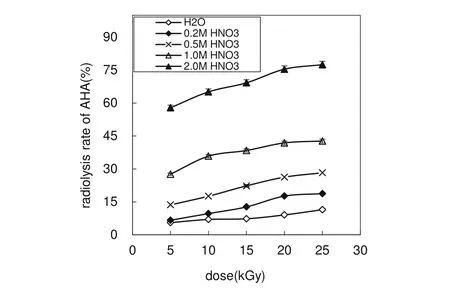
Fig.1 Radiolysis rate of AHA as a function of dose
Figure 1 shows that the radiolysis rate of AHA is 6.63–77.5%in AHA solution irradiated to 5–25 kGy,and it increases with the dose and HNO3concentration.Karraker[19]reported that AHA radiolysis shows weak dependence on both HNO3concentration and absorbed dose,and this differs from our experimental results.When the dose is increased fivefold,from 5 to 25 kGy,the radiolysis rate increases 1.3–2.8 times.When the HNO3concentration is increased fivefold, from 0.2 to 1.0 mol L-1,the radiolysis rates increases 2.3–4.2 times.Thus,the effect of HNO3concentration on the radiolysis of AHA is stronger than that of the dose.Water radiolyzes to produce·OH,·H,and so on:

·OH may react with HNO3by H abstraction to form·NO3[22]:

When using 0.2–2.0 mol L-1HNO3,most of the acid dissociates to form NO3-.γ-Ray may react directly with HNO3and NO3-to produce·NO3[23]:

·OH and ·NO3are oxidative radicals,and they may react with AHA as follows[24]:

Since the concentrations of ·OH and ·NO3increase with dose(Eqs.2,4–5*)and the ·NO3concentration increases with HNO3concentration(Eqs.3–5*),the radiolysis rate increases with both dose and HNO3concentration.
3.2 Radiolytic product of AHA
HNO3ionizes to produce H+,andcan react with H+to form H:

H·may react with AHA to form H2:


Two·NO2radicals may react with each other to form N2O4,which can react with water to produce HNO2:

The C–C bond in the excited AHA molecule may be broken as follows[25]:

·CH3may react with AHA by H abstraction to form CH4,or two·CH3radicals may also react with each other to produce C2H6:

Two CH3CONHO·radicals may react with each other to produce CH3CON=O[26]:

CH3CON=O may hydrolyze to form acetic acid and nitroxyl[27,28]:

Two nitroxyls can react with each other to generate N2O:
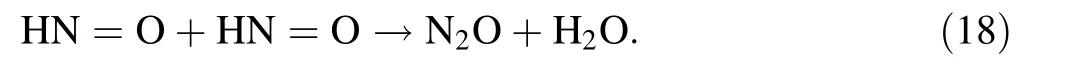
Thus,the main radiolytic product of AHA in HNO3may be H2,N2O,CH4,C2H6,CH3COOH,and HNO2.
3.2.1 H2generated by the radiolysis of AHA in HNO3
H2was analyzed by gas chromatography using a packed 5molsieve column and a TCD detector[29].The response curve of H2is y=2297.8x+48.87(volume range 0.100–1.20 mL),and R2is 1.000.The volume fraction of H2as a function of dose is shown in Fig.2.
As shown in Fig.2,the volume fraction of H2evolved from the AHA solutions irradiated with a dose of 5–25 kGy is(1.30–11.8)× 10-3.The H2volume fraction increases with the dose but decreases with increased HNO3concentration.H2is produced by the reaction of H·and AHA(Eq.9).H·is generated from the radiolysis of H2O(Eq.2),and it may also be produced by the reaction of H+and(Eq.8).As the concentrations of H·andincrease with the dose,the H2volume fraction also increases with increasing dose.Upon the addition of HNO3,NO3-can react with·H and(Eqs.10–11),so that the ·H concentration is reduced;hence,the volume fraction of H2decreases with increased HNO3concentration.
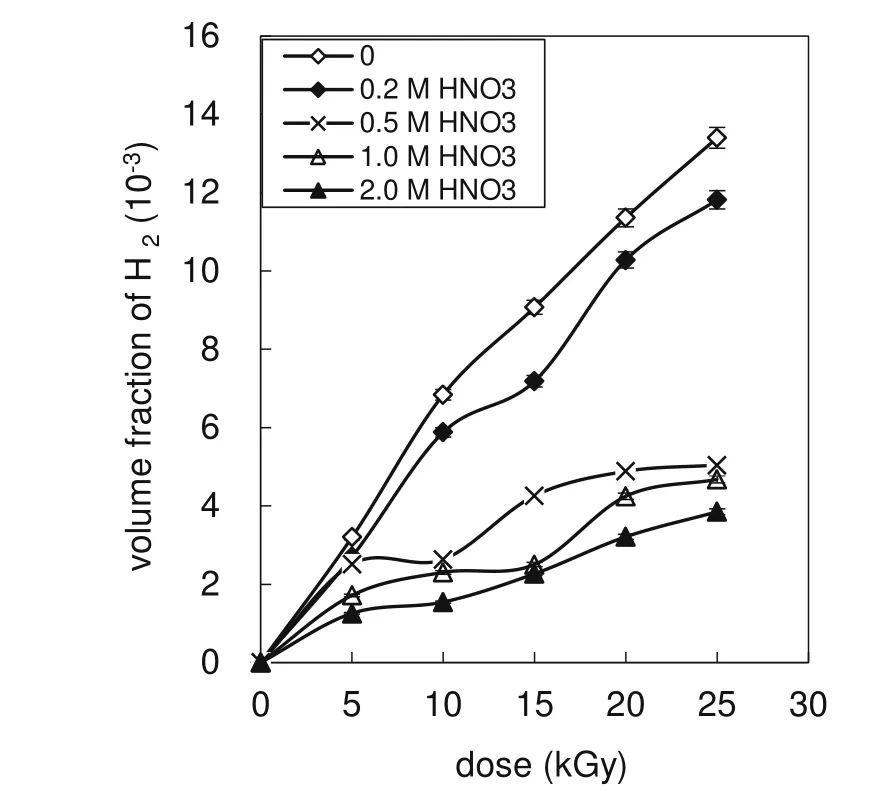
Fig.2 Volume fraction of H2as a function of dose
3.2.2 N2O generated from the radiolysis of AHA in HNO3
We used gas chromatography with a packed shincarbon T column and a TCD detector to analyze N2O.The response curve of N2O is y=220.83x-17.67(volume range 0.020–3.00 mL),and R2is 0.9933.The volume fraction of N2O as a function of dose is shown in Fig.3.
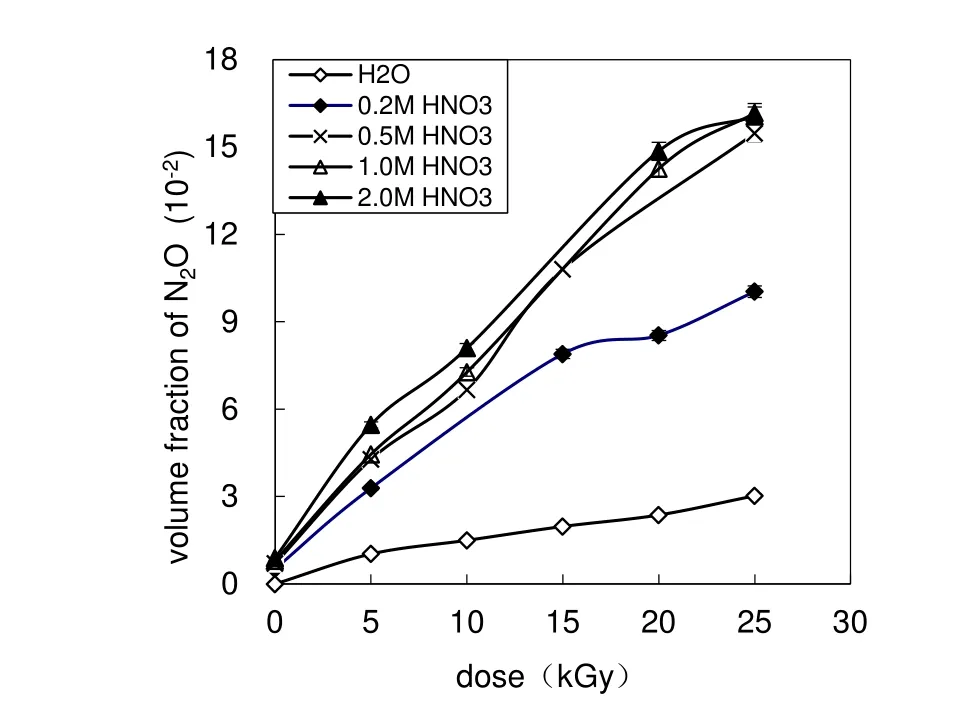
Fig.3 Volume fraction of N2O as a function of dose
Figure 3 reveals that the volume fraction of N2O produced from AHA solutions irradiated up to a dose of 5–25 kGy is(3.29 –16.2)× 10-2.The N2O volume fraction increases with the dose and with the HNO3concentration when the acid concentration is≤0.5 mol L-1.The dependence of the N2O volume fraction on the HNO3concentration is not obvious when the acid concentration is higher than 0.5 mol L-1. ·OH, ·NO3, ·H,and ·CH3react with AHA to form CH3CONHO·(Eqs.6–7,9,14),and two CH3CONHO·radicalsreacttoproduceCH3CON=O(Eq.16).After hydrolysis,the HNO produced generates N2O(Eqs.17–18).Since the concentrations of·OH,·NO3,·H,and ·CH3increase with the dose(Eqs.2–5,13),the volume fraction of N2O also increases with the dose.On the other hand,the higher the HNO3concentration,the higher is the concentration of ·NO3,CH3CONHO·,CH3-CON=O,and HNO(Eqs.3–5,7,16,17).N2O is produced by the reaction between two moieties of HNO(Eq.18),and thus,the volume fraction of N2O increases with HNO3concentration.However,as discussed above,the radiolysis rate of AHA increases with HNO3concentration,and the reduction of AHA concentration causes a decrease in the N2O content(Eqs.7,16,17).At higher HNO3concentration,The increase in N2O content resulting from the increased HNO3concentration is balanced by the decrease in N2O content due to the reduced AHA concentration;consequently,the volume fraction of N2O changes very slightly at high HNO3concentrations.
3.2.3 CH4and C2H6generated from radiolysis of AHA in HNO3
CH4and C2H6were analyzed by gas chromatography with a PLOT Al2O3column and a FID detector[21].The response curves of CH4and C2H6are y=242.7x+193.8 and y=25.56x-171.3,respectively.The volume range is 2.0–200.0 μL,and the corresponding R2values are 0.9998 and 0.9956.The volume fractions of CH4and C2H6evolved from AHA solutions irradiated up to 5–25 kGy are(1.90–47.4)× 10-5and (0.200–1.00)× 10-5,respectively;the volume fraction of CH4is much higher than that of C2H6.CH4is produced by the reaction of·CH3and AHA(Eq.14),while C2H6is generated by the reaction of two·CH3radicals(Eq.15).As the concentration of AHA exceeds that of·CH3,the volume fraction of CH4is much higher than that of C2H6.
Figure 4 shows that the volume fraction of CH4increases with the dose and with the HNO3concentration at HNO3≤1.0 mol L-1.When the HNO3concentration increases from 1.0 to 2.0 mol L-1,there is no obvious change in the CH4volume fraction.CH4is generated by the reaction of ·CH3and AHA(Eq.14).The ·CH3concentration increases with the dose,so the volume fraction of CH4also increases with the dose.·CH3may react with HNO3as below:


Fig.4 Volume fraction of CH4as a function of dose
Thus,the volume fraction of CH4increases with the HNO3concentration.However,the radiolysis rate of AHA also increases with the HNO3concentration,as shown above,and the reduction of AHA concentration leads to a decrease in the CH4content(Eq.14).At higher HNO3concentration,the reduction in CH4content caused by the decrease in AHA concentration is balanced by the increase in CH4content resulting from the increase in HNO3concentration;hence,the volume fraction of CH4changes slightly at high HNO3concentrations.The volume fractions of N2O,H2,CH4,and C2H6have the following relationship:N2O≈10 H2≈150 CH4≈7500 C2H6.
3.2.4 CH3COOH and HNO2generated by radiolysis of AHA in HNO3
Under alkaline conditions,CH3COOH and HNO2can be converted into CH3COO-and NO2-,which can be analyzed by ion chromatography.The response curves of CH3COOH and HNO2are y=872.97x and y=3766.1x,respectively.The concentration range for CH3COOH is 0.169–1.70 m mol L-1and that for HNO2is 0.0217–0.435 m mol L-1.The R2values for the response curves of CH3COOH and HNO2are 0.9998 and 0.9984,respectively.
As shown in Fig.5,the CH3COOH concentration is(4.64–19.7)× 10-2mol L-1inAHA solutions irradiatedup to 5–25 kGy.The CH3COOH concentration increases markedly with HNO3concentration and slightly with the dose.·OH, ·NO3, ·H,and ·CH3react with AHA to form CH3-CONHO·(Eqs.6–7,9,14);these radicals react with each other to produce CH3CON=O(Eq.16),which in turn hydrolyzes to produce CH3COOH(Eq.17).Since the concentrationof·OH,·NO3,·H,and ·CH3increaseswiththedose(Eqs.2–5,13),the CH3COOH concentration also increases with the dose.When the HNO3concentration is high,the concentration of ·NO3,CH3CONHO·,and CH3CON=O(Eqs.3–5,7,16)also increases,as does the concentration of CH3COOH(Eq.17).For this reason,the CH3COOH concentration increases with HNO3concentration.

Fig.5 CH3COOH concentration as a function of dose
Figure 6 revealsthatthe HNO2concentration is(0.321–4.80)× 10-3mol L-1in AHA solutions irradiated at a dose of 5–25 kGy;the concentration of HNO2increases with increasing concentration of HNO3.When the HNO3concentration is≤1.0 mol L-1,the dependence of the HNO2concentration on the dose is not obvious.At a HNO3concentration of 2.0 mol L-1,the HNO2concentration increases with the dose at lower dose levels,reaching the maximum value at 15 kGy,and then decreases with a further increase in the dose but changes only slightly beyond 20 kGy.Some papers[27,29,30]reported that AHA can react with HNO2and can scavenge HNO2and stabilize Pu(III)and Np(V);thus,an additional holding reductant is unnecessary[17].The above-mentioned results show that a signi ficant concentration of HNO2exists in the irradiated AHA-HNO3solution;this means that AHA cannot effectively destroy HNO2,so a holding reductant should be used when AHA is applied for the separation of Pu and Np from U.
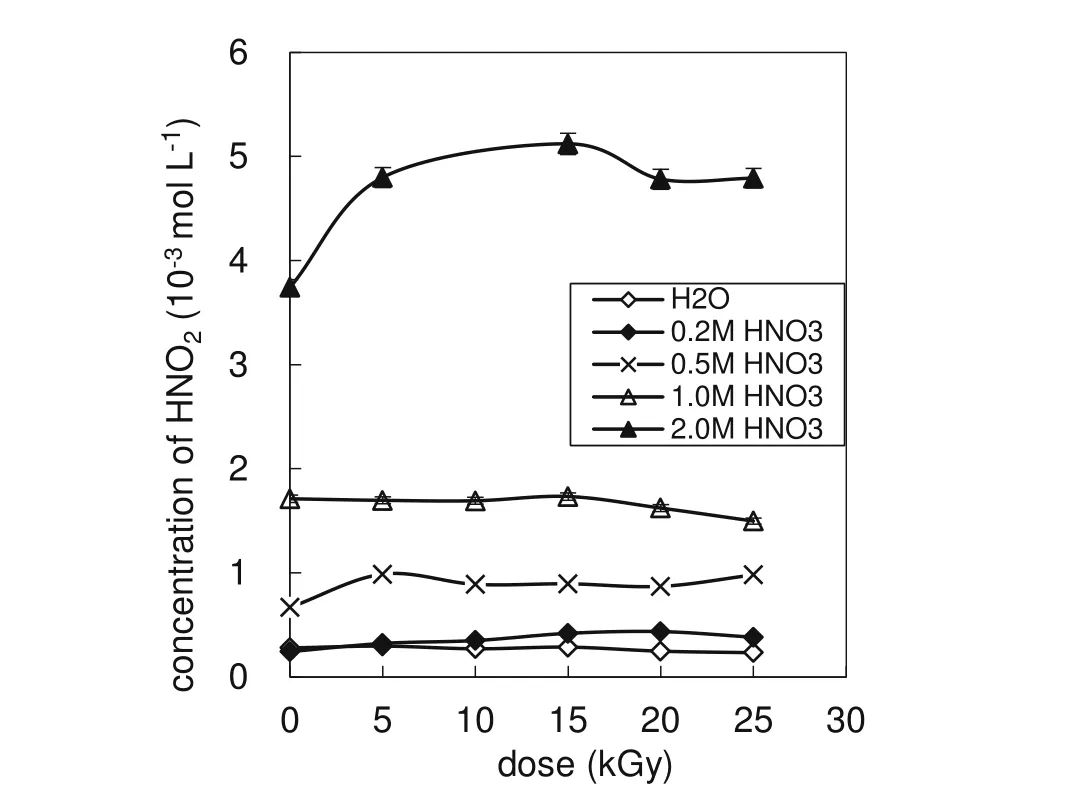
Fig.6 HNO2concentration as a function of dose
4 Conclusion
AHA is a potential complexant and reductant used in the separation of Pu and Np from U in the advanced Purex process for the reprocessing of spent fuel.The radiation stability of AHA in HNO3depends on the absorbed dose and HNO3concentration,but the effect of the latter is greater than that of the former.At a dose of 5–25 kGy,0.2 mol L-1AHA is recommended for use in HNO3with concentrations lower than 0.5 mol L-1,where the radiolytic rate of AHA is lower than 29%.The use of AHA with 2.0 mol L-1HNO3should be avoided,as the radiolytic rate of AHA is higher than 57%.The main gaseous products are N2O and H2,and the volume fraction of N2O is higher than that of H2.The main liquid products are CH3COOH and HNO2,and the concentration of the former is much higher than that of the latter.As HNO2will affect the stability of Pu(III)and Np(V),a holding reductant should be applied to scavenge HNO2when AHA is used for the separation Pu and Np from U.The effect of the holding reductant on the destruction of HNO2,as well as on the stabilization of AHA and the radiolytic product of AHA in HNO3,will be studied in the near future.
1.G.A.Ye,Review on the study and application of organic salt-free reagent in Purex process.At.Energy Sci.Technol.38,152–158(2004)
2.W.F.Zheng,L.M.Liu,Z.Chang,Improvement of separation of Pu from U of U-cycle in Purex process by acetohydroxamic acid.At.Energy Sci.Technol.34,110–115(2000)
3.R.J.Taylor,I.May,The reduction of actinide ions by hydroxamic acid.Czechoslovak J.Phys.49(Suppl.S1),617–621(1999).https://doi.org/10.1007/s10582-999-1041-0
4.I.May,R.J.Taylor,G.Brown,The formation of hydrophilic Np(IV)complexes and their potential application in nuclear fuel reprocessing.J.Alloys Compd.271–273,650–653(1998).https://doi.org/10.1016/S0925-8388(98)00179-0
5.H.Jiang,Z.Chang,Y.J.Pan et al.,Effect of hydroxamic acid ligands on the extraction of plutonium by TBP.J.Nucl.Radiochem.22,1–7(2000)
6.W.F.Zheng,H.Jiang,J.M.Zhu et al.,Complexation,reduction and stripping of Pu(IV)by simple hydroxamic acid.J.Nucl.Radiochem.25,65–68(2003)
7.M.J.Carrott,O.D.Fox,C.J.Maher et al.,Solvent extraction behavior of plutonium(IV)ions in the presence of simple hydroxamic acids.Solvent Extr Ion Exch 25,723–745(2007).https://doi.org/10.1080/07366290701634560
8.S.Sukumar,P.K.Sharma,P.Govindan et al.,Puri fication of uranium product from plutonium contamination using acetohydroxamic acid based process.J.Radioanal.Nucl.Chem.295,191–196(2013).https://doi.org/10.1007/s10967-012-1855-2
9.R.J.Taylor,S.I.Sinkov,G.R.Choppin et al.,Solvent extraction behavior of neptunium(IV)ions between nitric acid and diluted 30%tri-butyl phosphate in the presence of simple hydroxamic acids.Solvent Extr.Ion Exch.26,41–61(2008).https://doi.org/10.1080/07366290701784159
10.P.Tkac,B.Matteson,J.Bruso et al.,Complexation of uranium(VI)with acetohydroxamic acid.J.Radioanal.Nucl.Chem.277,31–36(2008).https://doi.org/10.1007/s10967-008-0705-8
11.W.F.Zheng,Z.Chang,Synthesis of acetohydroxamic acid and determination of stability constants of its complexes with Pu(IV)and Np(IV).J.Nucl.Radiochem.23,2–6(2001)
12.C.Li,T.H.Yan,Ch.Zuo et al.,Kinetics of reductive stripping of Np(VI)by acetohydroxamic acid from 30%TBP/kerosene to nitrate medium using a high-speed stirred cell.Radiochim.Acta 103,627–634(2015).https://doi.org/10.1515/ract-2014-2375
13.P.Govindan,S.Sukumar,R.V.S.Rao,Partitioning of uranium and plutonium by acetohydroxamic acid.Desalination 232,166–171(2008).https://doi.org/10.1016/j.desal.2007.12.014
14.P.Tkac,A.Paulenova,G.F.Vandegrift et al.,Distribution and identi fication of Pu(IV)species in tri-n-butyl phosphate/HNO3extraction system containing acetohydroxamic acid.J.Radioanal.Nucl.Chem.280,339–342 (2009).https://doi.org/10.1007/s10967-009-0524-6
15.W.F.Zheng,Z.P.Zhang,Z.Lin et al.,Study on separation of Np from U by acetohydroxamic acid in Purex process.Nucl.Sci.Eng.21(4),369–374(2001)
16.YCh.Dong,H.L.Eil,The effect of acetohydroxamic acid on the distribution of Np(IV)and Np(VI)between 30%TBP and nitric acid.J.Alloys Compd.451,440–442(2008).https://doi.org/10.1016/j.jallcom.2007.04.238
17.R.J.Taylor,I.May,A.L.Wallwork et al.,The applications of formo-and aceto-hydroxamic acids in nuclear fuel reprocessing.J.Alloys Compd.271–273,534–537(1998).https://doi.org/10.1016/S0925-8388(98)00146-7
18.G.Bernier,M.Miguirditchian,E.Ameil et al.,Hot test in mixer settlers of alpha barrier with AHA in PUREX process.Procedia Chem.7,160–165(2012).https://doi.org/10.1016/j.proche.2012.10.027
19.D.G.Karraker,Radiation Chemistry of Acetohydroxamic Acid in the Urex Process,WSRC-TR-2002-00283(2002),pp.1–8
20.R.J.Taylor,I.May,The reduction of actinide ions by hydroxamic acids.Czech J.Phys.49,617–621(1999).https://doi.org/10.1007/s10582-999-1041-0
21.X.J.Cao,J.H.Wang,Q.Li et al.,Stability of acetohydroxamic acid in nitric acid.At.Energy Sci.Technol.48,1933–1937(2014).https://doi.org/10.7538/yzk.2014.48.11.1933
22.Y.Katsumura,P.Y.Jiang,R.Nagaishi,Pulse radiolysis study of aqueous nitric acid solutions.formation mechanism,yield and reactivity of NO3radical.J.Phys.Chem.93,4435–4439(1991).https://doi.org/10.1021/j100164a050
23.Y.Katsumura,N-centered Radicals(Wiley,New York,1998),pp.393–412
24.Y.Samuni,U.Samuni,S.Goldstein,The mechanism underlying nitroxyl and nitric oxide formation from hydroxamic acids.Biochim.Biophys.Acta 1820,1560–1566(2012).https://doi.org/10.1016/j.bbagen.2012.05.006
25.H.Y.Cheng,C.L.Xiang,Y.H.Chen,Radiation stability of extractant(I)—comparison of isomer radiolytic product of tributylphosphate.At.Energy Sci.Technol.7,767–773(1964)
26.A.Samuni,S.J.Goldstein,One-electron oxidation of acetohydroxamic acid:the intermediacy of nitroxyl and peroxynitrite.Phys.Chem.A 115(14),3022–3028(2011).https://doi.org/10.1021/jp201796q
27.F.N.Shirota,E.G.DeMaster,M.C.Lee et al.,Generation of nitric oxide and possibly nitroxylby nitrosation of sulfohydroxamic acids and hydroxamic acids.Biol.Chem.3,445–453(1999).https://doi.org/10.1006/niox.1999.0257
28.M.M.Gutierrez,A.E.Almaraz,E.Bari et al.,The HNO donor ability of hydroxamic acids upon oxidation with cyanoferrates(III).J.Coord.Chem.68,3236–3246(2015).https://doi.org/10.1080/00958972.2015.1068938
29.J.H.Wang,M.H.Wu,B.R.Bao et al.,Study on hydrogen and CO produced by radiation degradation of N,N-dimethyl hydroxylamine.J.Radioanal.Nucl.Chem.273(2),371–373(2007).https://doi.org/10.1007/s10967-007-6842-7
30.H.L.Yale,The acetohydroxamic acid.Chem.Rev.33,209–256(1943)
杂志排行
Nuclear Science and Techniques的其它文章
- B4C/NRL flexible films for thermal neutron shielding
- Wetting behaviors of methanol,ethanol,and propanol on hydroxylated SiO2substrate
- Design and implementation of power and phase feedback control system for ICRH on EAST
- Data decomposition method for full-core Monte Carlo transport–burnup calculation
- New design for multi-crystal data collection at SSRF
- Design of a control system with high stability for a streak camera using isolated ADC
