The effects of two free- floating plants(Eichhornia crassipes and Pistia stratiotes)on the burrow morphology and water quality characteristics of pond loach(Misgurnus anguillicaudatus)habitat
2018-03-07JinqingWngGuihuFuWeiyueLiYingShiJiciPngQingWngWeigungChngeLiuJinshengLiu
Jinqing Wng,Guihu Fu,Weiyue Li,Ying Shi,Jici Png,Qing Wng,Weigung Lü,Chnge Liu,Jinsheng Liu,**
aDepartment of Animal Nutrition and Fisheries,Shandong Vocational Animal Science and Veterinary College,Weifang 261061,China
bEco-environmental Protection Institute of Shanghai Academy of Agricultural Sciences,Shanghai 201403,China
cYangzhou City Yishan Agriculture Technology Co.,Ltd,Yangzhou 225624,China
1.Introduction
The pond loach Misgurnus anguillicaudatus,well known for its nutritional value,tender meat,and palatability,is considered“ginseng of the water”in China(Lei&Wang,1990;Wang et al.,2010).There has been growing interest in culturing and breeding loach in China because it is an excellent freshwater aquaculture species.In recent years,the market demand and price of loach have increased steadily,and as has the need to improve culturing techniques(Hu,Chu,&Wang,2012;Wang,Hu,Wang,&Cao,2009).
Many loach species possess intensive mud-drilling habits and burrowing activities which provide protection(Natuhara,2013).Loach mainly inhabit shallow water with a depth less than 1 m.These species are benthic fishes inponds and exhibit high tolerance to harsh environmental conditions,such as elevated ammonia and nitrate,hypoxia,and dehydration and a broad range of temperatures,from 0 to 38°C(Keller&Lake,2007;Koetsier&Urquhart,2012;Silva,Coimbra,Steffensen,&Wilson,2008).Their adaption to such conditions is attributed to the buffering function of their burrows in the mud.
Burrows provide ideal locations for fish mating,spawning,egg incubation,and escape from predators(Atkinson&Taylor,1991).Resin casting is currently the most common method used to study burrow morphology(Dinh,Qin,Dittmann,&Tran,2014;Frey,Basan,&Scott,1973;Wang,Bertness,Li,Chen,&Lü,2015)and was used in the present study.Burrow casting can reveal fish behavior patterns,adaptation characteristics and properties of the bottom sediment(Lee&Koh,1994).Burrow casting is also an important approach to understanding the physiological demands experienced by loach(Seike&Nara,2007;Wang et al.,2015).Studies on loach burrow morphology are very important to clarify their behavioral ecologyand to increase our understanding of loach growth,feeding,and reproduction.Burrow morphology also reflects the utilization and preference of loach with respect to bottom sediment.Several studies have examined burrows of goby and mudskipper in mudflats(Dinh et al.,2014),but little is known about loach burrows and the factors influencing their morphology.
Free- floating plants are important primary producers and structural elements in freshwater that greatly increase habitat complexity and heterogeneity,elevate fish biodiversity,and improve habitat conditions that maintain ecosystem health(Bond&Lake,2003;Kadye,Magadza,Moyo,&Kativu,2008;Marchetti&Moyle,2001).Free- floating plants are frequently cultivated on the surface of culture water to purify the water,serve as livestock feed,and enhance fish survival and growth rates,thereby enhancing multiple aspects of water utilization efficiency.
The water hyacinth Eichhornia crassipes and the water lettuce Pistia stratiotes are widely distributed species in tropical regions and are used extensively in the shallow water of loach culture ponds(Akinbile&Yusoff,2012;Mbati&Neuenschwander,2005).These two floating plant species attract fish into plant clusters on the water surface and thus facilitate the harvest of marketable loach(Huang&Huang,2011).They also have well-developed roots that directly affect pond sediment(Padial,Thomaz,&Agostinho,2009;deNeiff&Carignan,1997).The flourishing above-ground structure of floating macrophytes,e.g.,stem and leaves,forms a microhabitat of crown closure on the water surface.The welldeveloped roots directly contact pond sediment,with potentially strong effects on the sediment properties.These impacts likely change the burrowing behavior and inhabiting characteristics of loach,changes that may affect other aspects of loach culture.However,the interactive effects of floating plants and loach burrowing remain largely unexplored,in part due to the difficulty of casting loach burrows in the soft mud of shallow ponds.By analyzing the effects of the two floating plants on loach burrows,we explore the role of plants in loach reproduction and growth and further optimize the technologies for high-efficiency integrated aquaculture with approaches that combine planting and breeding.
Our study aimed to determine the primary morphological characteristics of loach burrows and analyze the differences in root distribution,stem and leaf status,and burrow morphology among the three treatments.These analyses illustrate the effects of planting the floating macrophytes E.crassipes and P.stratiotes on the morphology of loach burrows.This study may provide useful data and new insights for the high-efficiency culture of loaches and application of aquatic plants.
2.Materials and methods
2.1.Study methods
This study was conducted in a greenhouse in the Zhuanghang Experimental Station of Shanghai Academy of Agricultural Sciences,Zhuanghang Town,Fengxian District,Shanghai,China(30°53′25′′N,121°23′20′′E),from July to August 2013.Plastic rectangular tanks(size:57 cm L×37 cm W×35 cm H)were used as experimental units.Dried and crushed soil was placed into tanks at 20 cm depth.This soil was soaked for one week to give the soil homogeneous texture and moderate viscosity for facilitating loach burrowing and casting,after which the culture water was added to a 20 cm depth for stocking loach.
Floating plants were introduced into tanks to establish three treatments:E.crassipes,P.stratiotes,and a control.Each treatment was replicated four times.Ten E.crassipes individuals,with a total weight of 372.9 g,and seven P.stratiotes individuals,with a total weight of 359.5 g,were placed in each tank at approximately natural densities.Fresh plants were weighed and the root length,height and density were recorded.Water hyacinth(family Pontederiaceae,genus Eichhornia)has smooth and oval-shaped leaf blades with a 4-12 cm width.The perennial herb water lettuce(family Araceae,genus Pistia)has longer roots and greater plant weight and leaf number,but lower plant height above the water than water hyacinth(Table 1,Fig.1).Water hyacinth has more interstitial space and therefore allows greater light in filtration than water lettuce.
The loach used in this experiment was obtained from a local aquaculture market.The fish were sterilized with 5%NaCl solution for 10 min and used to stock the pool.The weights and lengths of loach were recorded.Three males and three females were placed into each tank.The mean wet weights for the male and female fish were 11.8±0.9 g and 19.5±1.5 g,respectively.Their mean body lengths were 128.5±3.0 mm and 155.5±5.38 mm,respectively.The weights and body lengths have no significant difference among the three treatments(P>0.05).During the experimental period(July to August),the water temperature was relatively high in all treatment in the greenhouse(31.9-33.2°C).The atmospheric pressure was lower in the experimental environment(984.1-996.5 kpa).Loach exhibited active burrowing behavior in tanks.
Water was sampled from the tanks at 09:00 at 10 day intervals and monitored in situ for pH,temperature,electrical conductivity(EC),dissolved oxygen(DO),and turbidity using a portable multiparameter water analyzer(HI9828,Hanna,Italy).Water total nitrogen(TN),total phosphorus(TP),ammonia nitrogen(NH4-N),and nitrate nitrogen(NO3-N)were measured using the alkaline potassium persiflage digestion-UV spectrophotometric method(GB 11894-89),the alkalifusion Mo-Sb anti-spectrophotometric method,Nessler's reagent colorimetric method,and the phenol disulfonic acid spectrophotometric method,respectively.CODMnwas determined according to permanganate index methods.Totalorganic carbon(TOC)was determined by combustion in a TOC analyzer(Apollo 9000,Teledyne Tekmar,USA).
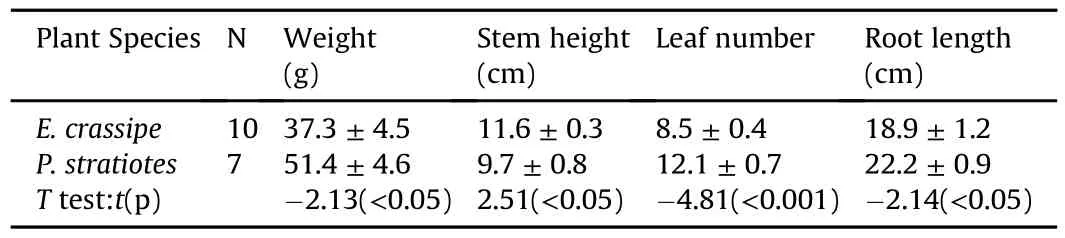
Table 1 Plant characteristics of water hyacinth and water lettuce.

Fig.1.The appearance of water hyacinth Eichhornia crassipes(A),water lettuce Pistia stratiotes(B).
After the experiment ran for one month,the upper water was pumped out of the tanks and the topography of the bottom and pit sizes(width and depth)were measured.After allowing the tanks to dry drying surface water in tanks for 3 days,loach burrow molds were made with resin casting methods as described by Wang et al.(2015).The burrow casts required approximately 3 h to harden after polyester resinwas poured into the burrows. The casts were then extracted using a small shovel and carefully cleaned.Ten burrow parameters were measured::volume,curved burrow length,straight burrow length,depth,width,opening number,branch number,opening diameter,neck diameter,mean diameter,aspect ratio of a cross-section,and sinuosity(i.e.,the curved-straight length ratio).The definition and measurement methods of each burrow parameter were those described by Wang et al.(2015).Multiple burrow casts in each tank were regarded as replicates(n=4-6).
2.2.Data analysis
The results are expressed as the mean±standard error of the mean.A main-effects ANOVA was used to test the effects of habitat type and sampling time on water quality and nutrient content,followed by Tukey's post hoc test.The effects of floating plants on burrow morphology were examined using a One-Way ANOVA.Student's t-test was used to examine the biological characteristics of E.crassipes and P.stratiotes.All analyses were run using the Statistica(Version 7.0,StatSoft)statistical package.
3.Results
3.1.Characteristics of the three treatment habitats
3.1.1.Physical and chemical properties of water
For the duration of the tank experiment,the presence loaches increased the agitation of water and sediment.Together with loach excretion and the effects of increasing temperature,pH(F2,4= 249.4,P<0.01;Fig.2A)and dissolved oxygen(F2,4=3.41,P=0.14;Fig.2B)significantly decreased,while electrical conductivity(F2,4=170.9,P<0.01;Fig.2C)and water turbidity(F2,4=24.40,P<0.01;Fig.2D)gradually increased.
The cultivation of floating plants in tanks affected water quality.After one month,the introduction of water hyacinth and water lettuce slightly decreased the pH,with the greatest effect in the control without loach and the least in water lettuce(F2,4=1.77,P=0.28;Fig.2A).DO changed little in the control treatment,and decreased in tanks with water hyacinth and water lettuce(F2,4=1.57,P=0.31;Fig.2B).The EC value of plant treatments was significantly higher than in the control(F2,4=9.0,P<0.05;Fig.2C),with the highest value in water hyacinth on July 15 and water lettuce on July 25.The placement of macrophytes on the water surface effectively inhibited the increase in water turbidity and the lowest turbidity was observed in tanks with water hyacinth and the highest in the control(F2,4=2.17,P=0.23;Fig.2D).
3.1.2.Water nutrient characteristics
The introduction of loaches resulted in various degrees of increase in the water nutrient content due to the effects of burrowing activities and excretion(TN:F2,4=23.69,P<0.01;TP:F2,4=2.42,P=0.20;NO3-N:F2,4=13.63,P<0.05;CODMn:F2,4=4.51,P=0.09;TOC:F2,4=89.67,P<0.01).No consistent trends for NH4-N were found in any of the three treatments(F2,4=4.62,P=0.09).The addition of aquatic plants decreased the concentrations of most nutrients.The TN concentration in water hyacinth tanks was the lowest,whereas that of the control was the highest(F2,4=1.88,P=0.27;Fig.3A).At the beginning of the experiment,the TP of the control was the highest.As time passed,the TP in water lettuce tanks become the lowest and that of water hyacinth tanks greatly increased and eventually exceeded that of the control(F2,4=1.98,P=0.25;Fig.3B).The NH4-N of the control treatment first increased and then decreased with time,and that of the plant treatment gradually decreased(Fig.3C).At the conclusion,the treatments ranked in the descending order of control,water lettuce,and water hyacinth.The water hyacinth tanks also had the lowest values for NO3-N(F2,4=1.58,P=0.31;Fig.3D)and CODMn(F2,4=0.22,P=0.81;Fig.3E).Interestingly,adding plants also increased the water TOC content.This effect was highest for water hyacinth(F2,4=24.35,P<0.01;Fig.3F).This finding suggests that plant roots provide a substantial contribution to water TOC.
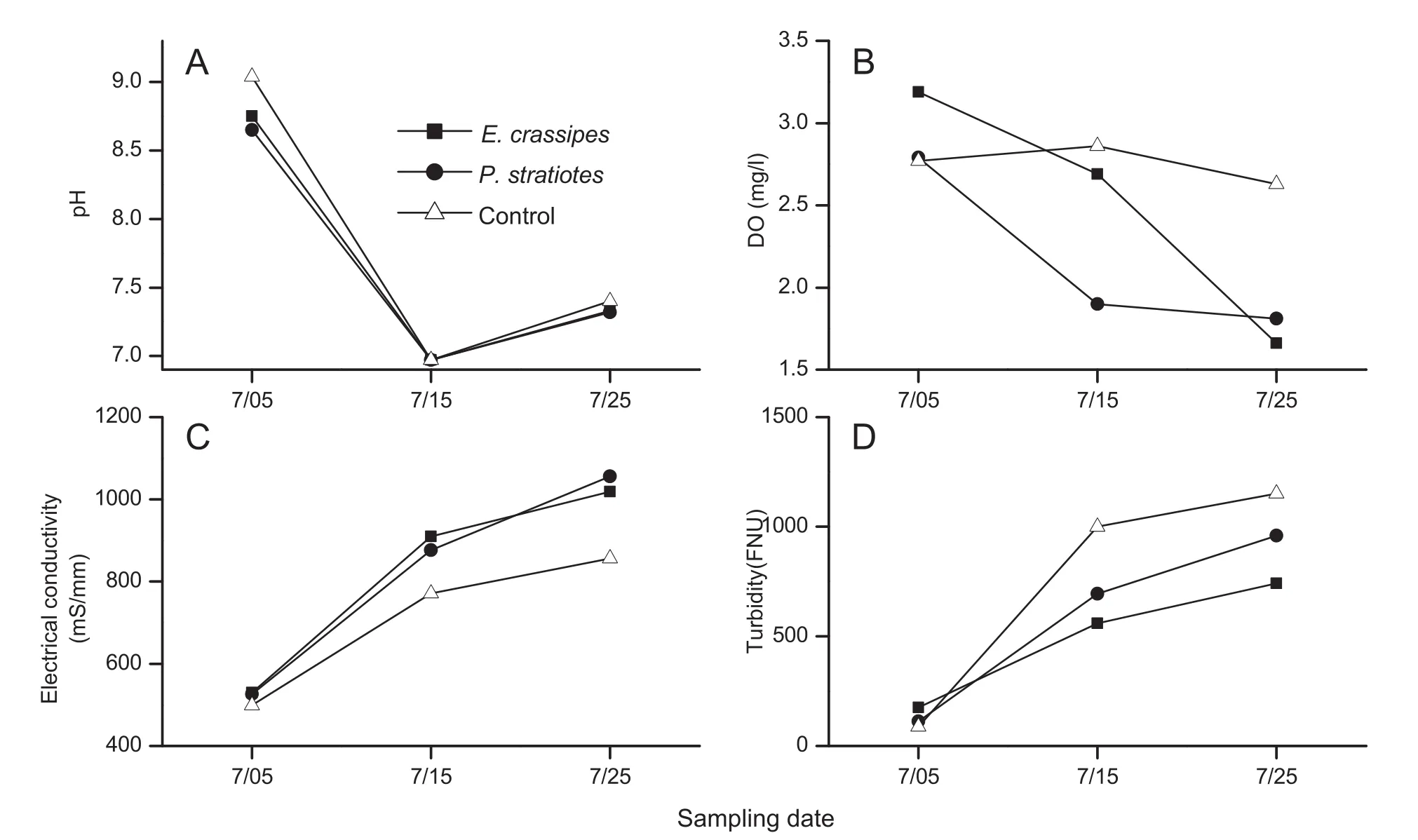
Fig.2.Comparisons of physical and chemical properties of water in the three treatments.

Fig.3.Comparisons of water nutrient concentrations in the three treatments.
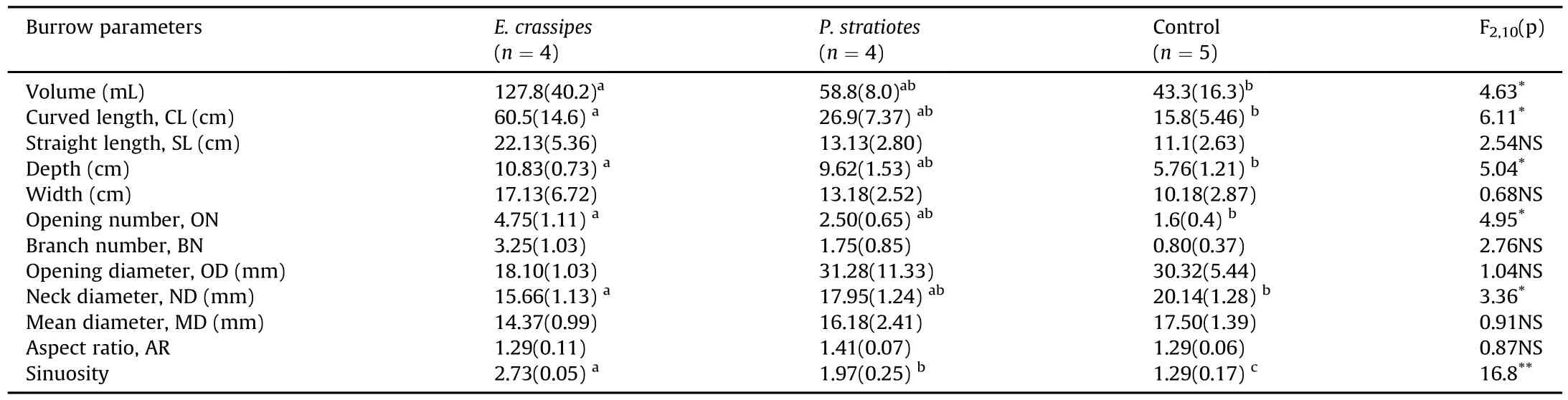
Table 2 Loach Misgurnus anguillicaudatus burrow morphology in tanks with Eichhornia crassipes,with Pistia stratiotes,and without plants.Shown are the mean values with standard error in parentheses.Different lowercase letters indicate the significant differences of parameters among the three treatments examined by a Tukey test(P<0.05).
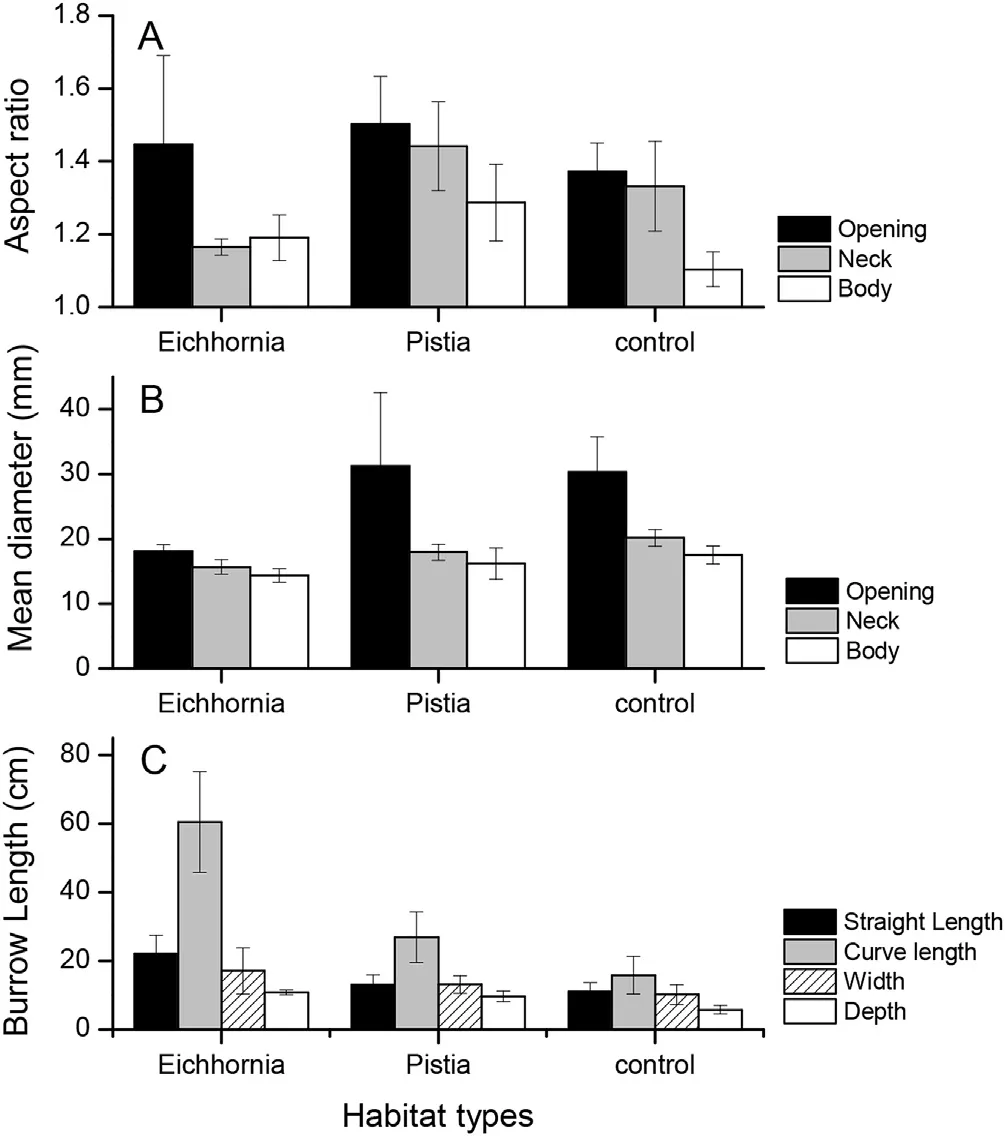
Fig.5.Comparisons of aspect ratios(A),mean diameter(B)and burrow length(C)among the three treatments.
3.2.Burrow morphology
The pits in the surface of the culture sediment were the biogenic structure produced during loach inhabiting.Their number,size,and depth are a proxy for loach behavior,such as the frequency of drilling and residence time on the bottom.The massive gray roots of water hyacinth provided enough structural support for burrows to form and maintain their structure in the sediment.Eight pits were created due to the long-term residence of loach on the bottom,with a total area of 385.2 cm2and depth of 1.38±0.24 cm in water hyacinth tanks.Six pits were found in water lettuce with total area of 581.9 cm2and intermediate depths(1.71±0.31 cm).The pits were mostly clustered on the tank margins.The sediment surface was least disturbed in the control treatment,which had 4 pits clustered in the tank corner with an area of 506.9 cm2and a depth of 1.50±0.20 cm.
A total of 13 loach burrows were casted during this study.The burrows included interconnected parts with 1-8 openings and 0-6 side branches.The loach burrow morphology in aquatic plant treatments was more diverse than in the control treatment.The volume,curved length,depth,neck diameter,opening numbers and sinuosity of the loach burrows were all significantly different among the three treatments;they were greatest in the water hyacinth tanks,followed by those of water lettuce and the control(Table 2).
Multiple burrows were interconnected with each other in the water hyacinth tanks,which formed branched network systems(Fig.4A).The burrow diameters under water hyacinth were the smallest due to the coverage of dense root mats,which made digging burrows more difficult.The shapes of burrow openings under water lettuce were the greatest and narrowest.These burrows exhibited V shapes and were dug by loach from two directions(Fig.4B).Loach in the control tank created a Y-shaped or inverted L-shaped burrows with larger necks and mean diameters(Fig.4C).
Fig.5 shows that the aspect ratio and diameter of the opening sections were the highest,followed by those of the neck and body sections(aspect ratio:F2,23=2.63,P=0.09,Fig.5A;diameter:F2,30=5.23,P<0.05;Fig.5B).Four burrow length indices ranked in descending order of curved length,straight length,depth,width(F3,40=12.49,P<0.01;Fig.5C).Burrow volume(y)was positively correlated with curved length(x)(y=2.14x+3.44,R2=0.91,P<0.01).Similarly,the neck diameter(y)was negatively correlated with the sinuosity(x)(y=-3.12x+24.16,R2=0.52,P<0.01).
4.Discussion
4.1.Burrowing characteristics of loach
Pond loach exhibit intensive drilling-mud behavior and frequently burrow to seek protection.The burrow volume of loach(18-246 mL)is smaller than Odontamblyopus lacepedii(4971 mL)(Gonzales,Katoh,&Ishimatsu,2008),but similar to Parapocryptes serperaster(373 ml)(Dinh et al.,2014).The burrow depth of loach(3.1-12.8 cm)is slightly lower than those of Taenioides cirratus(25 cm)(Itani&Uchino,2003),Parapocryptes serperaster(22.3 cm)(Dinh et al.,2014)and Odontamblyopus lacepedii(94 cm)(Gonzales et al.,2008).The loach burrows had fewer openings(2.8±0.5 ope.)and branches(1.8±0.5 ope.)than those of Odontamblyopus lacepedii(Gonzales et al.,2008),but approximately the same number as those of Parapocryptes serperaster(2.4±0.6 ope.)and Pseudapocryptes elongates(2.1±0.7 ope.)(Dinh et al.,2014).The burrow structure of loach was much simpler than that of Odontamblyopus lacepedii.Odontamblyopus lacepedii,Parapocryptes serperaster,and loach inhabited estuaries and burrowed in mud flats.They all had similar cylindrical body type and behavior ecology.As Dinh et al(2014)suggested, fish burrow morphology exhibits obvious species specificity,which may be closely associated with fish body size.
Burrows frequently collapse in softer mud at the bottom of shallow water ponds,and the fish body is in close contact with mud during loach burrowing.Consequently,burrow size is strongly correlated with loach size,as was found in Parapocryptes serperaster(Dinh et al.,2014)and Odontamblyopus lacepedii(Gonzales et al.,2008).
Loach created wavy tunnels with even diameters using a twisting undulation in this study,which also resulted in a great sinuosity of the burrows.The burrow opening was elliptical,with a greater diameter than the neck and body segments of the burrow,which can be explained by the intensive drilling of the loach on the mud surface at the site of initiation.Through continual drilling and excavation by loach,burrows were developed into diverse structures from simple escape pits in the mud surface.
The burrow structures of loach were V-shaped,Y-shaped,inverted L-shaped, or were complicated dendritic networks composed of multiple V shapes.These burrows were different from the U-and W-shaped Parapocryptes serperaster burrows(Dinh,2008),but similar to the Y-shaped burrows of Pseudapocryptes elongates(Dinh,2008).According to the diverse architecture of burrow casts,loach may use various integrated behaviors to burrow,including body twisting,head drilling,and pectoral pushing.This behavior is similar to that exhibited by Parapocryptes serperaster(Dinh et al.,2014),Periophthalmodon septemradiatus(Bhatt,Patel,Patel,&Patel,2009),and Valenciennea longipinnis(Takegaki&Nakazono,1999).
As suggested by Koetsier and Urquhart(2012),drought and hyperthermia can sharply increase burrowing behavior.Although loach normallyexhibit weak burrowing activity in submerged mud,the drying process after pumping out the upper water,which we used to facilitate burrow casting,may have increased the loach burrowing intensity in the greenhouse.Loach burrows in this study were obtained from indoor tanks and may have differed somewhat from field burrow morphology.The influences of specific environmental factors and their mechanisms should be explored in detail in subsequent studies.
4.2.Effects of floating plants
Since loach normally inhabit shallow water with a depth of less than 1 m(see Lorenz,Stoll,Sundermann,&Haase,2013),external environments,including vegetation,water depth,predation,grain size and their interaction,had large effects on the burrowing behavior and burrow configuration of loach.We found that introducing loach into tanks produced significant effects on the physical and chemical properties of the water.There was an obvious reduction in water pH and DO and an increase in electrical conductivity,turbidity,N and P.The physiological processes of the loach,i.e.,feeding,breathing,excretion and metabolism,might explain the change in water quality.Drilling by loach led to nutrient release from the bottom mud and oxygen depletion,which,in turn,affected loach behavior.The presence of the two floating species in shallow water significantly changed the dimension,branches and sinuosity of loach burrows.Burrow morphology varied greatly below floating plants,the reasons for which are described the following paragraphs.
Several studies have described the important role of aquatic plants in structuring aquaculture ecosystems by increasing structural diversity and complexity(Nash,Hendry,&Cragg-Hine,1999).The high structural heterogeneity and complexity provided by plant roots protect aquatic macroinvertebrates against predation(Padial et al.,2009)and provide suitable places for fish spawning and reproduction(Meerhoff,Mazzeo,Moss,&Rodríguez-Gallego,2003).The shading effects of plant mats create mosaic habitats with various environmental conditions,including temperature,light,pH and oxygen,enhancing habitat suitability.Floating plant communities normally attract zooplankton and periphyton,offering abundant food resources for fish,causing active movement and likely intensive burrowing of benthic fish below plants(Fontanarrosa,Chaparro,de Tezanos Pinto,Rodriguez,&O’Farrell,2010;Meerhoff et al.,2003).
As suggested by Reddy and Debusk(1984),the leaf shape of water hyacinth and water lettuce obviously differ,with the former oval-shaped,whereas the latter are wedge-shaped.Water hyacinth communities with intermediate biomass provided sufficient interstices to allow abundant light penetration and hence improved the photosynthesis and oxygen conditions,causing loach to be more active and to create abundant pits.In contrast,water lettuce can rapidly grow in warm water and easily expand to almost full horizontal coverage on water surface.As in Attionu,(1976),dense mats of water lettuce induce water stratification and oxygen deficiency because they shade the water from sunlight,leading to root decay and oxygen depletion.In general,the habitat suitability of water lettuce for loach was inferior to that of water hyacinth in this study.Finally,loach displayed weak burrowing performance in the control treatment.The habitat was less suitable because the loach were vulnerable to frequent external disturbance.
Previous studies have confirmed the capacity of the two plant species to purify culture water by removing excess N and P as plant nutrients(Zimmels et al.,2006).We found that water hyacinth effectively removed water TN,COD,NO3-N,and NH4-N and that water lettuce removed water TP and NH4-N.The additions of both water hyacinth and water lettuce markedly reduced water turbidity,which was explained by the high suspended sediment retention of roots(Poi De Neiff&Carignan,1997;Meerhoff et al.,2003).However,TOC elevation and DO depletion in plant treatments may be caused by root decay and respiration.Oxygen depletion and acidification due to root decay have been reported both in water hyacinth(Poi De Neiff&Carignan,1997)and water lettuce(Attionu,1976).Nevertheless,Jedicke,Furch,Saint-Paul,and Schlüter(1989)also reported that the roots of Eichhornia crassipes(Pontederiaceae)and Pistia stratiotes(Araceae)increase oxygen transport in the water.This finding suggested that root age and growth status together determine the alternative effects of roots on DO concentration with less algae in the water.Compared to water lettuce and the control,water hyacinth had a greater ability to purify water,providing suitable habitat for loach feeding,living,and burrowing.
Eichhornia crassipes and Pistia stratiotes in shallow water habitats had well-developed roots that extended into bottom mud or adhered to the mud surface,aggregating mud particles and thus increasing the soil stability and adhesion,serve as important component of effectively stabilizing the burrow structure.Similarly,in salt marshes,Spartina alterniflora roots offer structural support and soil associations for crab burrows(Wang,Gao,&Wang,2014,2015).Padial et al.(2009)reported that the root lengths of water hyacinth ranged from 5 cm to 1 m,longer than found in the present study(19 cm),and occupied the entire shallow water column.Abundant root interstices and interconnected burrows served as inhabiting places for loach,in additions to greater movement and drilling activity below water hyacinth.Additionally,the physical barriers produced by the roots of floating plants prevent fish from entering(Padial et al.,2009)and mayalso impede loach burrowing,thereby increasing burrow sinuosity.The pliable roots of water hyacinth are outstanding in organic particle retention(Zimmels et al.,2006)and provide a substrate for attaching invertebrates(Poi De Neiff&Carignan,1997;Montoya,2003;Rocha-Ramirez,Ramirez-Rojas,Chavez-Lopez,&Javier,2007;Kouame et al.,2010).The roots of water lettuce were stiffer than those of water hyacinth and reached lengths similar to those that have been previously reported(20 cm)(Rao&Reddy,1984),likely having a weak effect on loach burrowing.
In summary,the water hyacinth treatment was characterized by the greatest burrow volume,length,depth,and structural complexity,but the opening sizes were reduced by the presence of dense root mats;while the water lettuce treatment was characterized by intermediate volume,length,branches,and sinuosity but large openings and pit size;and the control treatment was characterized by a flat bottom with small and short Y-shaped or inverted L-shaped burrows.By altering floating plant species composition,density and distribution,we can regulate cultured populations of loach,eventually increasing fish growth rates and economic benefits.
Author contributions
F.G.H.,L.W.Y.and S.Y.performed experiments on breeding loach;P.J.C.and W.Q.analyzed water quality indices;L.W.G.and L.C.E.performed experiments on aquatic plants growth;W.J.Q.and L.J.S.designed the study and wrote the manuscript.
This study was financially supported by the Natural Science Foundation of the Science and Technology Commission of Shanghai Municipality(Grant No.13ZR1427300),Spark Program of the state ministry of science and technology of China(2015GA680005).
deNeiff,A.P.,&Carignan,R.(1997).Macroinvertebrates on Eichhornia crassipes roots in two lakes of the Parana River floodplain.Hydrobiologia,345,185-196.
Akinbile,C.O.,&Yusoff,M.S.(2012).Assessing water hyacinth(Eichhornia crassopes)and lettuce(Pistia stratiotes)effectiveness in aquaculture wastewater treatment.International Journal of Phytoremediation,14,201-211.
Atkinson,R.J.A.,&Taylor,A.C.(1991).Burrows and burrowing behavior of fish.In P.S.Meadows,&A.Meadows(Eds.),The environmental impact of burrowing animals and animal burrows(pp.133-155).London:Clarendon Press.
Attionu,R.H.(1976).Some effects of water lettuce on its habitat.Hydrobiologia,50,245-254.
Bhatt,N.Y.,Patel,S.J.,Patel,D.A.,&Patel,H.P.(2009).Burrowing activities of goby fish in the recent intertidal mud flats along the Navinal coast,Kachchh,Western India.Journal of the Geological Society of India,74,515-530.
Bond,N.R.,&Lake,P.S.(2003).Characterizing fish-habitat associations in streams as the first step in ecological restoration.Austral Ecology,28,611-621.
Dinh,T.D.(2008).Some aspects of biology and population dynamics of the goby Pseudapocryptes elongatus(cuvier,1816)in the mekong delta.Malaysia:University of Malaysia Terengganu.Ph.D.Dissertation.
Dinh,Q.M.,Qin,J.G.,Dittmann,S.,&Tran,D.D.(2014).Burrow morphology and utilization of the goby(Parapocryptes serperaster)in the Mekong Delta.Vietnam.Ichthyological Research,61,332-340.
Fontanarrosa,M.S.,Chaparro,G.,de Tezanos Pinto,P.,Rodriguez,P.,&O'Farrell,I.(2010).Zooplankton response to shading effects of free- floating plants in shallow warm temperate lakes:A field mesocosm experiment.Hydrobiologia,646,231-242.
Frey,R.W.,Basan,P.B.,&Scott,R.M.(1973).Techniques for sampling salt marsh benthos and burrows.American Midland Naturalist,89,228-234.
Gonzales,T.T.,Katoh,M.,&Ishimatsu,A.(2008).Intertidal burrows of the airbreathing eel goby,Odontamblyopus lacepedii(Gobiidae:Amblyopinae).Ichthyological Research,55,303-306.
Huang,B.,&Huang,X.H.(2011).Effects of the two artificial fish nests on culturing loach under static water condition without bottom mud.Journal of Aquaculture,5,1-3(in Chinese).
Hu,T.J.,Chu,Z.J.,&Wang,Y.C.(2012).Studies on the artificial large-scale propagation of loach(Misgurnus arguillicaudatus).Journal of Zhejiang Ocean University(Natural Science),31,12-17(in Chinese).
Itani,G.,&Uchino,T.(2003).Burrow morphology of the goby Taenioides cirratus.Journal of the Marine Biological Association of the United Kingdom UK,83,881-882.
Jedicke,A.,Furch,B.,Saint-Paul,U.,&Schlüter,U.B.(1989).Increase in the oxygen concentration in Amazon waters resulting from the root exudation of two notorious water plants,Eichhonria crassipes(Pontederiaceae)and Pistia stratiotes(Araceae).Amazoniana,11,53-69.
Kadye,W.T.,Magadza,C.H.D.,Moyo,N.A.G.,&Kativu,S.(2008).Stream fish assemblages in relation to environmental factors on a montane plateau(Nyika Plateau,Malawi).Environmental Biology of Fishes,83,417-428.
Keller,R.P.,&Lake,P.S.(2007).Potential impacts of a recent and rapidly spreading coloniser of Australian freshwaters:Oriental weatherloach(Misgurnus anguillicaudatus).Ecology of Freshwater Fish,16,124-132.
Koetsier,P.,&Urquhart,A.N.(2012).Desiccation tolerance in a wild population of the invasive oriental weather fish Misgurnus anguillicaudatus in Idaho,USA.Transactions of the American Fisheries Society,141,365-369.
Kouame,M.K.,Dietoa,M.Y.,Da Costa,S.K.,Edia,E.O.,Ouattara,A.,&Gourene,G.(2010).Aquatic macroinvertebrate assemblages associated with root masses of water hyacinths,Eichhornia crassipes(Mart.)solms-laubach,1883(Commelinales:Pontederiaceae)in taabo lake,ivory coast.Journal of Natural History,44,257-278.
Lee,Y.H.,&Koh,C.H.(1994).Biogenic sedimentary structures on a Korean mud flat:Spring-neap variations.Netherlands Journal of Sea Research,32,81-90.
Lei,F.Y.,&Wang,B.X.(1990).Studies on reproduction and growth of loach.Acta Hydrobiologica Sinica,14,60-67(in Chinese).
Lorenz,A.W.,Stoll,S.,Sundermann,A.,&Haase,P.(2013).Do adult and YOY fish benefit from river restoration measures?Ecological Engineering,61,174-181.
Marchetti,M.P.,&Moyle,P.B.(2001).Effects of flow regime on fish assemblages in a regulated California stream.Ecological Applications,11,530-539.
Mbati,G.,&Neuenschwander,P.(2005).Biological control of three floating water weeds,Eichhornia crassipes,Pistia stratiotes,and Salvinia molesta in the Republic of Congo.Biocontrol,50,635-645.
Meerhoff,M.,Mazzeo,N.,Moss,B.,&Rodríguez-Gallego,L.(2003).The structuring role of free- floating versus submerged plants in a subtropical shallow lake.Aquatic Ecology,37,377-391.
Montoya,J.V.(2003).Freshwater shrimps of the genus Macrobrachium associated with roots of Eichhornia crassipes(water hyacinth)in the Orinoco Delta(Venezuela).Caribbean Journal of Science,39,155-159.
Nash,K.T.,Hendry,K.,&Cragg-Hine,D.(1999).The use of brushwood bundles as fish spawning media.Fisheries Management and Ecology Ecol,6,349-355.
Natuhara,Y.(2013).Ecosystem services by paddy fields as substitutes of natural wetlands in Japan.Ecological Engineering,56,97-106.
Padial,A.A.,Thomaz,S.M.,&Agostinho,A.A.(2009).Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae.Hydrobiologia,624,161-170.
Poi De Neiff,A.,&Carignan,R.(1997).Macroinvertebrates on Eichhornia crassipes roots in two lakes of the Paran'a River fl oodplain.Hydrobiologia,345,185-196.
Rao,P.N.,&Reddy,A.S.(1984).Studies on the population biology of water lettuce:Pistia stratiotes L.Hydrobiologia,119,15-19.
Reddy,K.R.,&Debusk,W.F.(1984).Growth characteristics of aquatic macrophytes cultured in nutrient enriched water:I,water hyacinth,water lettuce and pennywort.Economic Botany,38,229-239.
Rocha-Ramirez,A.,Ramirez-Rojas,A.,Chavez-Lopez,R.,&Javier,A.(2007).Invertebrate assemblages associated with root masses of Eichhornia crassipes(Mart.)solms-laubach 1883 in the alvarado lagoonal system,veracruz,Mexico.Aquatic Ecology,41,319-333.
Seike,K.,&Nara,M.(2007).Occurrence of bioglyphs on Ocypode crab burrows in a modem sandy beach and its palaeoenvironmental implications.Palaeogeography Palaeoclimatology Palaeoecology,252,458-463.
Silva,J.M.,Coimbra,J.,Steffensen,J.F.,&Wilson,J.(2008).Effect of ammonia on weatherloach(Misgurnus anguillicaudatus)metabolism under aquatic and aerial conditions.Comparative Biochemistry and Physiology a-Molecular&Integrative Physiology,150.S120.
Takegaki,T.,&Nakazono,A.(1999).Division of labor in the monogamous goby,Valenciennea longipinnis,in relation to burrowing behavior.Ichthyological Research,46,125-129.
Wang,J.Q.,Bertness,M.D.,Li,B.,Chen,J.K.,&Lü,W.G.(2015).Plant effects on burrowing crab morphology in a Chinese salt marsh:Native vs.exotic plants.Ecological Engineering,74,376-384.
Wang,M.,Gao,X.Q.,&Wang,W.Q.(2014).Differences in burrow morphology of crabs between Spartina alterniflora marsh and mangrove habitats.Ecological Engineering,69,213-219.
Wang,Y.J.,Hu,M.H.,Wang,W.M.,&Cao,L.(2009).Effects on growth and survival of loach(Misgurnus anguillicaudatus)larvae when co-fed on live and microparticle diets.Aquaculture Research,40,385-394.
Wang,Y.J.,Hu,M.H.,Wang,W.M.,Cheung,S.G.,Shin,P.K.S.,&Cao,L.(2010).Effects of the timing of initial feeding on growth and survival of loach(Misgurnus anguillicaudatus)larvae.Aquaculture International,18,135-148.
Zimmels,Y.,Kirzhner,F.,&Malkovskaja,A.(2006).Application of Eichhornia crassipes and Pistia stratiotes for treatment of urban sewage in Israel.Journal of Environmental Management,81,420-428.
杂志排行
Aquaculture and Fisheries的其它文章
- Molecular characterization and expression of the feminization-1c(fem-1c)in the freshwater mussel(Hyriopsis cumingii)
- Molecular basis of pathogenesis of emerging viruses infecting aquatic animals
- Fish red blood cells express immune genes and responses
- Sensory and chemical assessment of silver pomfret(Pampus argenteus)treated with Ginkgo biloba leaf extract treatment during storage in ice
- Social-ecological dynamics of the small scale fisheries in Sundarban Mangrove Forest,Bangladesh
