Regulation of neuronal survival by DNA methyltransferases
2017-12-15JuditSymmankGeraldineZimmer
Judit Symmank, Geraldine Zimmer
Institute of Human Genetics, University Hospital Jena, Jena, Germany
How to cite this article:Symmank J, Zimmer G (2017) Regulation of neuronal survival by DNA methyltransferases. Neural Regen Res 12(11):1768-1775.
Introduction
Due to the limited regenerative capacity of neurons in the adult brain the precise regulation of their survival and death is essential for proper brain function and longevity (Götz et al., 2016).Moreover, survival and cell death regulation plays a pivotal role during brain development to ensure the correct establishment of functional circuits that are composed of particular neuronal subsets. Thereby, neurons are generated to a greater extent than finally needed, which makes the regulated cell death an elementary feature of brain development (Miller, 1995; Bartolini et al.,2013). Hence, the generation, fine tuning and maintenance of correct numbers and subtypes of neurons destined for diverse brain regions requires tight orchestration during the distinct stages of development.
In general, diverse signaling molecules activating intrinsic signaling cascades contribute to neuronal survival regulation (Kristiansen and Ham, 2014). To integrate and process pro- survival and pro-apoptotic cues cell type specifically, particular downstream proteins are shown to regulate each other’s expression or functionality in an interconnected network deciding about survival or cell death inducing programs (Pfisterer and Khodosevich, 2017). Although alternative cell death pathways exist (Yuan et al., 2003), in developing as well as in matured brains the elimination of the majority of neurons occursviathe programmed cell death (Buss et al., 2006; Dekkers and Barde, 2013). In this regard, apoptotic processes are regulated by different protein families of specific biochemical signal transduction pathways(Dekkers et al., 2013).
Superordinate to genetic transcriptional networks epigenetic mechanisms like DNA methylation by DNA methyltransferases (DNMTs) as well as histone modifications contribute to the regulation of cell death through development, aging and disease(Fagiolini et al., 2009; Akbarian et al., 2013). In the developing but also in the adult brain the DNA methyltransferases DNMT1 and DNMT3A are expressed and perform promoter as well as gene body cytosine methylation often correlated with repression of transcription (Jang et al., 2017). Traditionally, DNMT1 was regarded as the maintenance methyltransferase copying methylation marks of hemimethylated DNA to the newly synthesized daughter strand during DNA replication, making the enzyme indispensable for dividing progenitor cells (Hermann et al.,2004; Hirasawa et al., 2008). This is supported by the finding that DNMT1 has a higher affinity to hemimethylated DNA (Bashtrykov et al., 2012) and that a total gene knockout ofDnmt1in the central nervous system is lethal in mice (Fan et al., 2001).Although,Dnmt1deletion in all dividing somatic cells is also lethal (Li et al., 1992; Fan et al., 2001; Jackson-Grusby et al., 2001;Trowbridge et al., 2009; Sen et al., 2010), mouse embryonic stem cells are viable, despite the resulting global loss of DNA methylation (Tsumura et al., 2006). Notably, human embryonic stem cells (ESCs) also displayed a global demethylation uponDnmt1deletion (Liao et al., 2015). However, in contrast to mice they undergo rapid cell death resulting in total lethality implicating distinct functions in embryonic stem cells of rodents and humans(Liao et al., 2015).
DNMTs Regulate Cellular Survival During Neuronal Development and Maturation
Besides its major role in retaining the methylation pattern in progenitor cells, different studies have already linked DNMT1 to post-mitotic developmental processes as well as to adult neuron functionality (Fagiolini et al., 2009; Akbarian et al., 2013; Lv et al., 2013). A surprisingly highDnmt1expression was detected in post-mitotic neurons of the central nervous system, suggesting functional implications in these cells (Chestnut et al., 2011;Kadriu et al., 2012). Indeed, decreasedDnmt1gene function in embryonic excitatory neurons of the neocortex caused progressive cell death and a subsequent reduction in cortical thickness as revealed by conditional deletion ofDnmt1inEmx1-expressing telencephalic precursors from embryonic day 13.5 on (Hutnick et al., 2009). In addition to neocortical excitatory cells, the survival and post-mitotic maturation of hippocampal neurons is also promoted by DNMT1 (Hutnick et al., 2009). For both,neocortical and hippocampal neurons, a prominent DNA hypomethylation was detected inDnmt1deficient cells during development and over life span coupled with a misexpression of genes involved in cell death and postnatal maturation, like layer-specification and ion channel functionality (Hutnick et al.,2009). In addition to its function in regulating cell survival and maturation of embryonic cortical neurons, DNMT1 further was reported to suppress precocious astroglial differentiation during neurogenesis in progenitors of the developing cerebral cortex(Fan et al., 2005). Thereby, DNMT1 was described to maintain the methylation states of astrocytic marker genes as well as genes related to crucial components of the gliogenic JAK-STAT pathway (Fan et al., 2005).
Besides regulating cell death associated gene expression in cortical and hippocampal projection neuron development, DNMT1 was also reported to promote the survival of photoreceptor cells and other types of neurons in the postnatal retina (Rhee et al.,2012) as well as of proliferative cells in the adult dentate gyrus(Noguchi et al., 2015). Interestingly, as soon as these newly generated hippocampal neurons in the adult brain accomplished their differentiation, DNMT1 expression was dispensable for further survival maintenance (Noguchi et al., 2015).
DNMT1 is A Key Regulator of the Survival of Inhibitory Cortical Interneurons
By shaping the responses of the excitatory glutamatergic principal neurons in complex interconnected neuronal circuits inhibitory gamma-aminobutyric acid (GABA)-expressing local interneurons contribute essentially to the neuronal information processing in the cerebral cortex, the seat of higher cognitive functions (Kepecs and Fishell, 2014). In contrast to the excitatory cortical neurons that arise from the cortical proliferative zones, inhibitory GABAergic interneurons originate in particular domains of the basal telencephalon, including the medial and caudal ganglionic eminence (MGE and CGE) as well as the pre-optic area (POA) (Gelman et al., 2011; Bandler et al., 2017).Post-mitotic immature cortical interneurons adopt a polarized migratory morphology with leading and trailing processes and perform long-range tangential migration through the basal telencephalon up to the cerebral cortex (Martini et al., 2009)directed by diverse guidance cues (Peyre et al., 2015).
Throughout the extended period of cortical interneuron development, including their progenitor state as well as their prolonged post-mitotic migration, cell survival has to be regulated and maintained. Misguided targeting or other defects in migration of immature cortical interneurons could induce cell death by activating intrinsic cascades (Uribe and Wix, 2012).Upon reaching their final position cortical interneurons lose their migratory morphology, form axons and dendrites as well as synaptic contacts (Wamsley and Fishell, 2017). At postnatal stages the fine-tuning of interneuron numbers occurs through intrinsically determined apoptotic events (Southwell et al.,2012), diminishing improperly or non-connected cells to ensure inhibitory network establishment and functionality.
In addition to its relevance during development, survival regulation of cortical interneurons is crucial during life time, due to their important function in cortical information processing(Kepecs and Fishell, 2014). Especially fast-firing interneuron subtypes show high energy consumption rates and are exposed to extensive oxidative stress (Laughlin et al., 1998; Sullivan and O’Donnell, 2012; Kann et al., 2014), which makes them vulnerable for damage and cell death (Kannan and Jain, 2000). As the generation of new neurons at adult stages is highly limited (Ming and Song, 2011), matured neurons employ multiple and often redundant strategies to prevent cell death and to promote survival(Kole et al., 2013).
In addition to excitatory cortical neurons, single cell transcriptomic profiling revealed thatDnmt1is expressed in progenitors as well as in post-mitotic embryonic GABA-expressing cells of the cortical interneuron generating domains, the POA and MGE (Pensold et al., 2016). This points to a potential function in the post-mitotic development and maturation of these immature cortical GABAergic interneurons.
Rhee et al. (2012) highlighted in their studies on postnatal eye development an increased mortality of retinal interneurons associated to a diminishedDnmt1expression. To approach whether DNMT1 promotes the survival and maturation of post-mitotic cortical interneurons,Dnmt1was conditionally deleted inHmx3-Cre-expressing POA cells in the mouse model (Pensold et al., 2016). This post-mitotic deletion caused diminished numbers of POA-derived interneurons in the adult cerebral cortex. Phenotypic analysis of embryonic stages ofHmx3-Cre/tdTomato/Dnmt1wild-type and knockout revealed that the reduced cell densities in the adult cortex were due to increased cell death of immature embryonic interneurons in basal parts of the telencephalon (Pensold et al., 2016).Dnmt1-deficient cells further displayed defective migration due to morphological abnormalities (Pensold et al., 2016). These were characterized by a loss of their polarized migratory shape and an adoption of a multipolar morphology indicative of precocious maturation. Thereby,Dnmt1-deficient cells showed prolonged side-processes, no prominent leading process and increased process branching.
To approach potential target genes of DNMT1 involved in the regulation of interneuron survival, correlative methylome and transcriptome analysis by MeDIP and RNA sequencing of embryonic FAC-sortedHmx3-Cre/tdTomato/Dnmt1wildtype and knockout cells was performed (Pensold et al., 2016).Consistent with the observed morphological abnormalities and increased cell death events, many cell death- and cytoskeleton-associated genes were changed in expression uponDnmt1deletion. Among themPak6coding for a member of the p21-activated kinases (Kumar et al., 2017), was up-regulated in expression inDnmt1-deficient cells (Pensold et al., 2016).
Potential Downstream Targets of DNMT1 Mediating the Regulation of Cortical Interneuron Survival
PAKs are known to be involved in cell survival regulation as well as cytoskeletal rearrangements (Kumar et al., 2017) and PAK6 was already shown to promote neurite complexity in excitatory cortical neurons (Civiero et al., 2015). Consistently, forced expression of PAK6 induced by aPak6-green fluorescent protein(GFP) expression construct caused a multipolar morphology of embryonic POA-derived cells comparable to theDnmt1-deficient migrating interneurons (Pensold et al., 2016). In contrast,siRNA-mediatedPak6depletion reduced neurite complexity.As several PAKs were reported to influence cell survival (Kumar et al., 2017) and as increased cell death was observed inDnmt1-deficient cells that display elevatedPak6expression levels, it was tested whether PAK6 also affects the survival of embryonic POA cells (Pensold et al., 2016). To this end, the number of TUNEL-positive cells afterPak6siRNA-mediated knockdown was determined. Consistent with the increased rate of cell death inDnmt1-deficient mice,Pak6depletion diminished the number of TUNEL-positive cells. Hence,Pak6represents a potential downstream target of DNMT1-dependent transcriptional repression involved in cell death and cytoskeleton regulation.
The relevance of PAK6 for proper brain function was already suggested byin silicostudies proposing PAK6 as a potential candidate for epileptic encephalopathy (Oliver et al.,2016) and gene deletion resulted in deficits in learning, memorizing and movement (Nekrasova et al., 2008; Furnari et al.,2013) underlying the physiological relevance of DNMT1-dependent regulation ofPak6expression.
In regard to cell death regulation, some members of the PAK family including PAK6 were already shown to regulate the function of proteins of the BCL2 family like BAD (Schürmann et al., 2000; Tang et al., 2000; Zhang et al., 2010; Ye et al., 2011)and BCL6 (Barros et al., 2009) by phosphorylation. Both factors,BAD and BCL6, regulate pro-apoptotic and pro-survival effects in various cell types including neurons (Hatok and Racay, 2016).Interestingly, we detected a significantly altered expression ofBcl6inDnmt1-deficient POA-derived interneurons (unpublished data), indicating that DNMT1 controls different factors of the survival-regulating network.
Furthermore, phosphorylation by PAKs was also shown to regulate the activity of mitogen-activated protein (MAP) kinases that in turn activate programmed cell death pathways (Déléris et al., 2011; De la Mota-Peynado et al., 2011; Qing et al., 2012).Some transcripts of MAP-kinases (MAPKs) we found up-regulated inDnmt1-deficient mice (unpublished data). This includesMapk4, encoding for the atypical MAP-kinase ERK3, which was already shown to be activated by PAK1-mediated phosphorylation and to activate downstream kinases (Déléris et al., 2011; De la Mota-Peynado et al., 2011). Furthermore, RNA-sequencing revealed an increased expression ofMap3k5inDnmt1knockout cells (unpublished data), coding for the MAPK-kinase-kinase apoptosis signal regulating kinase 1 (ASK1) (Pensold et al., 2016). In neurons ASK1 is associated with the regulation of JNK/p38/MAPK-mediated apoptotic processes (Tobiume et al.,2001; Nishitoh et al., 2002). However, whether PAK6 indeed activates specific MAPKs is not yet investigated, but it was already proposed by others (Lee et al., 2002; Déléris et al., 2011).
Taken together, the elevatedPak6expression inDnmt1-deficient immature cortical interneurons could lead to enhanced phosphorylation levels of BCL2 family members and MAPKs,which could be involved in mediating the increased cell mortality seen inDnmt1deficient cells. As mitogen activated protein kinases, as well as some BCL2 family members are phospho-regulated,further studies determining the phosphorylation levels of relevant BCL2 proteins and MAPKs as a function of altered PAK6 expression levels could shed light on these open questions. Alternatively,as it was also shown that PAK6 translocates into the nucleus and regulates gene expression through interaction with specific transcription factors (Lee et al., 2002), a potential direct transcriptional control ofBcl6or genes encoding for specific MAPKs by PAK6 is conceivable and under current investigation.
In addition toPak6, we also found increasedFoxo1expression as one of the cell death-associated transcripts inDnmt1deficient cells of the interneuron generatingHmx3-expressing cell population from the POA (unpublished data).Foxo1encodes for a “pioneer” transcription factor that is also able to bind to condensed chromatin regions and regulates gene transcription (Hatta and Cirillo, 2007; Zaret and Mango, 2016). Members of the Forkhead Rabdomyosarcoma (FoxO) transcription factor family can be activated by MAPK-mediated phosphorylation (Asada et al., 2007),but also by serine/threonine protein kinases as it was already shown for PAK1 (Mazumdar and Kumar, 2003; de la Torre-Ubieta et al., 2010). They contribute to the regulation of a variety of genes, including genes encoding for different pro-apoptotic factors,viathe dynamic modulation of chromatin states (Fu and Tindall, 2008). Recently it was also shown that FOXO1 together with the protein kinase MST1 triggers cell death in cerebellar granular neurons (Yuan et al., 2009). Thus, the repression ofFoxo1-mediated by DNMT1 might be relevant for the survival of embryonic POA-derived interneurons, possibly together with the repression ofPak6and therefore a PAK6 dependent phospho-activation. However, this requires further investigations to uncover potential interrelationships.
All together, these data emphasize the relevance of DNMT1 for regulating the expression of genes as part of a complex network involved in the regulation of cell death and survival in embryonic post-mitotic neurons, which is summarized in Figure 1.
Potential Crosstalk of DNMTs with Histone Modifications in Cell Death Regulation
In addition to their canonical function performing DNA methylation, DNMTs were described to influence transcription through interactions with histone modifications (Du et al.,2015). To explore, whether DNMT1 regulatesPak6expression through its DNA-methylating activity in post-mitotic interneurons, MeDIP-sequencing of FACS-enriched embryonicDnmt1-deficient and wild-type cells was applied (Pensold et al.,2016). In contrast to changes in gene expression, no differences in the methylation level between wild-type andDnmt1knockout samples were detected, neither inPak6gene locus, nor upor downstream (Pensold et al., 2016). Consistently, elevatedPak6expression levels in a neuroblastoma cell culture model(N2a cells) were only observed after siRNA-mediatedDnmt1depletion but not after inhibiting the DNA-methylating activity of DNMTs by RG108 (Pensold et al., 2016). This indicates that direct DNA methylation ofPak6is not the way of action by which DNMT1 regulates its transcription. Moreover, for all transcription factors with predicted binding sites in thePak6gene locus, no changes in DNA methylation were identified in post-mitotic interneurons that correlated with transcriptional alterations (Pensold et al., 2016). This implies that DNMT1 modifiesPak6gene expression in developing interneurons through non-canonical actions, potentiallyviacrosstalk with histone-modifying enzymes.
Interactions between DNA methylation and histone modifications were already shown to be partially regulated by methylcytosin binding proteins, recruiting histone deacetylases to methylated DNA sequences (Jones et al., 1998; Nan et al., 1998).Acetylations at histones H3 and H4 are associated with higher gene accessibility compared to non-acetylated histones (Eberharter and Becker, 2002). Furthermore, there is also evidence that DNA methylation inhibits permissive and supports repressive histone methylation to ensure gene silencing (Hashimshony et al., 2003; Lande-Diner et al., 2007). Recent studies revealed that there are direct interactions between DNA-methylating and histone-modifying enzymesviaspecific binding domains,possibly regulating the recruitment of proteins to complexes and mediating the catalytic activity of their binding partners (Viré et al., 2006; Smallwood et al., 2007; Clements et al., 2012).
For instance, DNMT1 was shown to interact with EZH2 (Viré et al., 2006; Ning et al., 2015; Purkait et al., 2016), the core enzyme of the polycomp repressor complex 2. PCR2 mediates repressive trimethylations on lysine 27 at the N terminal amino acid tail of histone 3 (H3K27me3) (Margueron and Reinberg,2011). It was reported that EZH2 is able to recruit DNMT1 to polycomp target genes (Viré et al., 2006; Ning et al., 2015), but does not alter the expression level of the DNA methyltransferase (Purkait et al., 2016). In contrast, manipulation of DNMT1 function or expression was found to be associated with changes in EZH2 expression levels as well as the occurrence of H3K27 trimethylation (So et al., 2011; Purkait et al., 2016). Inhibiting DNMT1 enzymatic function by 5-azacytidine or reducingDnmt1expression by target-specific siRNAs decrease the expression levels of PCR2 components (So et al., 2011). Additionally, an enhanced expression of miRNAs was detected, that target polycomp repressor complex proteins (So et al., 2011).Gain or loss of function experiments of PCR2 components like EZH2 as well as SUZ12 and EED already revealed an epigenetic control ofPak6expression by H3K27 trimethylation in hepatoma cells (Liu et al., 2015). In addition, further studies suggested an epigenetic control ofPak6transcript level by miRNAs (Cai et al., 2015), which were already shown to be regulated by DNA methylation activity (Han et al., 2007; Lujambio et al., 2008).
Taken together, in POA-derived interneurons DNMT1 could indirectly repressPak6expression by facilitating the assembly of H3K27 trimethylation inPak6promoter regions, either through modulatingEzh2expression and other PCR2 components or by promoting PCR2 function. To what extent thePak6expression is indeed regulatedviacrosstalk of DNMT1 with EZH2 in neurons modulating repressive histone lysine methylation is subject of current studies. Beyond that, a possible contribution of specific DNMT1-dependent miRNAs in regulatingPak6expression is also under investigation.
However, polycomb-induced repression by H3K27 trimethylation was shown to be easily reversible and PC-target genes are often marked bivalently by co-occurrence of H3K27me3 and H3K4me/me2/me3 to realize an optimal transition between repressive and active transcriptional states (Bernstein et al., 2006;Barski et al., 2007; Mikkelsen et al., 2007; Pan et al., 2007; Voigt et al., 2013). H3K4 mono-, di- and trimethylation are regulated by the MLL complex 1 and 2, consisting of the respective his-tone methyltransferases MLL1 and MLL2 (KMT2B) as well as other regulatory proteins like WDR5 and ASH2 that mediate complex stability, specificity and efficiency (Zhang et al., 2012;Carbonell et al., 2013; Jiang et al., 2013).
In support of this bivalent model of chromatin state, we detected increased transcript levels ofKmt2d(also known as MLL2) inDnmt1-deficient cells (unpublished data).Kmt2dencodes for a histone methyltransferase which is the core enzyme of the MLL2 complex regulating mono- and dimethylation of H3K4, which are associated with activated gene transcription (Froimchuk et al., 2017). Furthermore, reduced expression ofCul4binDnmt1-deficient cells points to a possible contribution of an altered H3K4 methylation status in DNA methylation-independent transcriptional changes in developingDnmt1-deficient interneurons (unpublished data).Cul4bencodes for a small scaffold protein in the E3 ubiquitin-protein ligase complex targeting WDR5,which is one part of the MLL complex that promotes permissive H3K4 trimethylation (Nakagawa and Xiong, 2011). Apart from that, reducedCul4bexpression was also shown to result in a decreased retention of the SIN3A/HDAC complex followed by increasing acetylation of histone 3 and 4 (Ji et al., 2014). Histone acetylation facilitates transcriptional expression of respective genes (Eberharter and Becker, 2002) and could contribute to an overall open chromatin state enabling abnormalPak6expression in migrating POA-deriving interneurons afterDnmt1deletion.Hence, DNMT1-dependent repression ofPak6gene transcription in immature cortical interneurons could be mediated by hampering the establishment of active histone marks like H3K4 methylation or histone acetylation. Together these studies emphasize the complexity of epigenetic transcriptional networks in regulating cell viability and highlight a pivotal role of DNMT1 in the regulation of survival genes in immature cortical interneurons. Further studies on DNMT1-dependent changes of histone H3 lysine 4 methylation as well as of acetylation at histone 3 and 4 could give us more insights into the methylation-independent activities of the DNA methyltransferase 1.
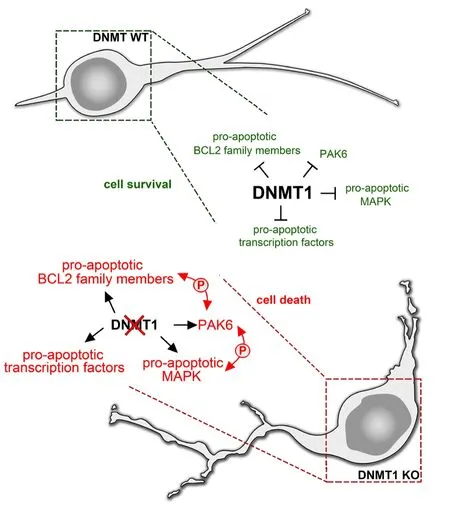
Figure 1 DNA methyltransferase 1 (DNMT1) promotes the survival of immature migrating inhibitory interneurons of the cerebral cortex.DNMT1 seems to inhibit the expression of pro-apoptotic genes of the PAK family like Pak6 as well as genes encoding for MAP-kinases(MAPK) and BCL2 family members to ensure the proper survival and typical migratory phenotype of immature interneurons with leading and trailing process (DNMT1 wild type (WT)). Furthermore, DNMT1 seems to repress transcription factors like FOXO1 with potential impact on cell death induction. Dnmt1 deletions abolish the repression of these pro-apoptotic genes resulting in increased expression in migrating interneurons during development (DNMT1 knockout (KO)). The subsequent activation of each other and downstream signaling pathways by phosphorylation events could facilitate morphological defects and results in increased mortality.
The Role of DNA Methylation Mediating Neuronal Survival in the Adult and Diseased Brain
In contrast to studies on immature developing neurons it was reported that in adult mice degeneration and apoptosis of motor neurons is caused by an aberrant high DNA methylation state through increased expression of DNMT1 and DNMT3a in a disease-relevant background (Chestnut et al., 2011). Although context-dependently different, alterations in DNA methylation seem to also play a fundamental role in the adult nervous system and in the etiology of neuropsychiatric disorders. While DNA demethylation of neuronal cell death-associated genes together with neuronal cell loss were related with major depression disorder (Duman, 2009; Xin et al., 2013; Chen et al., 2014), increasedDnmt1expression and subsequently elevated DNA methylation levels were observed in cortical interneurons of patients diagnosed with schizophrenia (Costa et al., 2003; Veldic et al., 2004;Ruzicka et al., 2007). Thereby, increased methylation was reported for genes likeRelnandGad1encoding for transcripts associated to GABAergic neurotransmission and interneuron function(Costa et al., 2003; Veldic et al., 2004; Ruzicka et al., 2007). The changed methylation patterns correlate with reduced expression of these genes pointing to impaired interneuron function (Costa et al., 2003; Veldic et al., 2004; Ruzicka et al., 2007). Disruption of GABAergic interneuron functionality has been associated with the pathophysiology of schizophrenia as well as of other psychological disorders including autism and epilepsy (Costa et al., 2003; Veldic et al., 2004; Levitt, 2005; Konradi et al., 2011a, b;Marín, 2012). In addition to the reported transcriptional changes caused by altered DNA methylation, a significant layer-specific loss of interneurons as well as of projection neurons was found in post-mortem studies of schizophrenia patients (Benes et al.,1991, 1998). These findings suggest that DNMT1 might indirectly influence interneuron survival possibly by impairing their functionality. Moreover, a death receptor pathway was recently shown to be implicated in the pathology of schizophrenia (Catts and Weickert, 2012). Whether the reported hypermethylation in schizophrenia patients (Veldic et al., 2004; Ruzicka et al., 2007;Catts and Weickert, 2012) also affects the transcriptional activity of gene promoters of respective death receptors, similar to how it was shown for cancer cells (van Noesel et al., 2002; Petak et al.,2003), needs to be investigated. The transcriptional regulation by DNA methylation in cortical interneurons in disease-relevant contexts reported so far mostly refers to genes relevant for brain development and physiology including neuronal activity (Costa et al., 2003; Veldic et al., 2004; Ruzicka et al., 2007). The modulation of signal transmission, synaptic plasticity and membrane excitability by DNMT1 was also reported in cortical excitatory neurons under normal conditions (Levenson et al., 2006; Feng et al., 2010; Meadows et al., 2016). That neuronal activity is closely linked to neuron survival has already been shown in various studies (Pfisterer and Khodosevich, 2017; Rozycka and Liguz-Lecznar, 2017). Hence, DNMT-dependent DNA methylation could regulate cell death in the healthy and diseased adult brain indirectly by affecting the expression of genes involved in synaptic neurotransmission.
ElevatedDnmt1expression in cortical interneurons is also related to the pathogenesis of mental impairments and psychosis due to neural injury and drug abuse (Veldic et al., 2005; Guidotti et al., 2007; Lewis et al., 2012; Moore et al., 2013). Hence,the modulation of DNMT1 expression and function seems to be important for proper neuronal network performance and the functionality of the adult brain with potential impact on neuronal survival.
DNA Methylation in the Aging Brain
In addition to the developing and adult brain, dynamic changes in DNA methylation landscape were observed during aging including a global hypomethylation (Bollati et al., 2009; Shimoda et al., 2014; Lardenoije et al., 2015; Moore et al., 2016)with site-specific hypermethylation predominantly in promoter regions (Xu et al., 2007; Numata et al., 2012). Dynamic changes in methylation, not restricted to neurons, even enable the use of methylation marks to predict the age of the organism very exactly (Horvath, 2013; Moore et al., 2016).
These data point to a relevance of DNA methylation and demethylation for the age-associated alterations of the brain involving decreased functionality and neurodegeneration of particular neuronal subsets. In the cerebral cortex, we (unpublished data) and others found reduced densities of cortical interneuron numbers (Pugliese et al., 2004; Hua et al., 2008). Due to their important function for cortical information processing, this loss of interneurons likely contributes to the cognitive decline and somato-motoric defects observed in elderly. Many of the genes that are hypermethylated in the aged brain are implicated in neurodevelopmental functions (Siegmund et al., 2007; Rakyan et al.,2010). Hypermethylation mostly correlates with gene silencing(Mo et al., 2015). Consistently, aging, especially of the cerebral cortex, is rather associated with transcriptional repression than induction (Xu et al., 2007). Thereby, the targets of age-associated gene silencing are implicated in synaptic function and plasticity (Jiang et al., 2001; Lu et al., 2004), which is closely linked to neuronal survival (Bito and Takemoto-Kimura, 2003; Segal,2010). As DNMT1 modulates synaptic plasticity often through regulation of the brain derived neurotrophic factor (Martinowich et al., 2003; Levenson et al., 2006; Feng et al., 2007), it may play indirectly a role in the long-term survival of neurons.
However, the underlying mechanisms of neuronal aging that can culminate in neuronal death or neurodegeneration seem manifold. They involve oxidative stress, disturbed calcium homeostasis, chromosomal instability, impaired DNA repair, and the accumulation of nuclear and mitochondrial DNA damage(Chouliaras et al., 2010) contributing either individually or combined to the age-associated cell death in the central nervous system. Although in cancer cells DNMT1 was already reported to function coordinately with the DNA damage repair machinery (Jin and Robertson, 2013), potential involvements in regulating neuronal aging-related cell death still remains elusive.
Another aspect is the age-associated decrease in enzymatic activity described for DNMT1 (Casillas et al., 2003), which is consistent with the global hypomethylation shown for the aging brain (Bollati et al., 2009; Shimoda et al., 2014; Lardenoije et al., 2015). Moreover, in patients diagnosed with Alzheimer´s disease, an age-related neurodegenerative disorder, 5-methylcytosine (5mC) immunoreactivity in neurons of post-mortem cortical tissue was found significantly reduced compared to agematched controls (Mastroeni et al., 2010). As the levels of 5mC inversely correlate with the markers of late-stage neurofibrillary tangles in the same neurons, a significant global loss of 5mC was suggested to take place in brains of Alzheimer´s patients(Mastroeni et al., 2010). As active ways of DNA demethylation have been described for differentiated cells (Bhutani et al., 2011;Chen and Riggs, 2011), the age-associated inability to re-establish the methylation pattern upon DNA demethylation could be involved in the regulation of long-term neuronal survival and age-associated neurodegeneration. Similar to studies in the developing brain, potential interactions of DNMTs with histone modifications could further contribute to the regulation of neuronal function and survival upon aging.
Conclusion
Taken together, these studies underline a dynamic and complex network of DNMT-dependent regulation of neuron survival during development, in adults, during aging and in related diseases. Thereby, the functions and mode of actions seem to differ at the distinct stages of life, emphasizing the relevance of context-specific epigenetic regulation. Hence, the development of therapeutic strategies necessitates the consideration of cell and stage-specific approaches, which are based on the detailed and context-specific analysis of the epigenetic transcriptional networks.
Author contributions:JS and GZ both contributed to conceptuation, writing and editing of the manuscript. JS prepared the figure.
Conflicts of interest:None declared.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under dentical terms.
Open peer reviewers:Özgür Demir, Gaziosmanpasa University, Turkey;omar Abdel Hamed Ahmed-Farid, Gaziosmanpasa University, Turkey.
Akbarian S, Beeri MS, Haroutunian V (2013) Epigenetic determinants of healthy and diseased brain aging and cognition. JAMA Neurol 70:711-718.
Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, Iwashita S, Kako K,Kishi T, Kasuya Y, Fukamizu A (2007) Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal 19:519-527.
Bandler RC, Mayer C, Fishell G (2017) Cortical interneuron specification: the juncture of genes, time and geometry. Curr Opin Neurobiol 42:17-24.
Barros P, Jordan P, Matos P (2009) Rac1 signaling modulates BCL-6-mediated repression of gene transcription. Mol Cell Biol 29:4156-4166.
Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K (2007) High-resolution profiling of histone methylations in the human genome. Cell 129:823-837.
Bartolini G, Ciceri G, Marín O (2013) Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron 79:849-864.
Bashtrykov P, Jankevicius G, Smarandache A, Jurkowska RZ, Ragozin S, Jeltsch A (2012) Specificity of Dnmt1 for methylation of hemimethylated CpG sites resides in its catalytic domain. Chem Biol 19:572-578.
Benes FM, Kwok EW, Vincent SL, Todtenkopf MS (1998) A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry 44:88-97.
Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL (1991) Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 48:996-1001.
Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, CuffJ, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL,Lander ES (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125:315-326.
Bhutani N, Burns DM, Blau HM (2011) DNA demethylation dynamics. Cell 146:866-872.
Bito H, Takemoto-Kimura S (2003) Ca(2+)/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium 34:425-430.
Bollati V, Schwartz J, Wright R, Litonjua A, Tarantini L, Suh H, Sparrow D,Vokonas P, Baccarelli A (2009) Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev 130:234-239.
Buss RR, Sun W, Oppenheim RW (2006) Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci 29:1-35.
Cai S, Chen R, Li X, Cai Y, Ye Z, Li S, Li J, Huang H, Peng S, Wang J, Tao Y,Huang H, Wen X, Mo J, Deng Z, Wang J, Zhang Y, Gao X, Wen X (2015)Downregulation of microRNA-23a suppresses prostate cancer metastasis by targeting the PAK6-LIMK1 signaling pathway. Oncotarget 6:3904-3917.
Carbonell A, Mazo A, Serras F, Corominas M (2013) Ash2 acts as an ecdysone receptor coactivator by stabilizing the histone methyltransferase Trr. Mol Biol Cell 24:361-372.
Casillas MA, Jr., Lopatina N, Andrews LG, Tollefsbol TO (2003) Transcriptional control of the DNA methyltransferases is altered in aging and neoplastically-transformed human fibroblasts. Mol Cell Biochem 252:33-43.
Catts VS, Weickert CS (2012) Gene expression analysis implicates a death receptor pathway in schizophrenia pathology. PLoS One 7:e35511.
Chen C, Wang Y, Zhang J, Ma L, Gu J, Ho G (2014) Contribution of neural cell death to depressive phenotypes of streptozotocin-induced diabetic mice. Dis Model Mech 7:723-730.
Chen ZX, Riggs AD (2011) DNA methylation and demethylation in mammals. J Biol Chem 286:18347-18353.
Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ (2011) Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci 31:16619-16636.
Chouliaras L, Rutten BP, Kenis G, Peerbooms O, Visser PJ, Verhey F, van Os J,Steinbusch HW, van den Hove DL (2010) Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Prog Neurobiol 90:498-510.
Civiero L, Cirnaru MD, Beilina A, Rodella U, Russo I, Belluzzi E, Lobbestael E, Reyniers L, Hondhamuni G, Lewis PA, Van den Haute C, Baekelandt V,Bandopadhyay R, Bubacco L, Piccoli G, Cookson MR, Taymans JM, Greggio E (2015) Leucine-rich repeat kinase 2 interacts with p21-activated kinase 6 to control neurite complexity in mammalian brain. J Neurochem 135:1242-1256.
Clements EG, Mohammad HP, Leadem BR, Easwaran H, Cai Y, Van Neste L,Baylin SB (2012) DNMT1 modulates gene expression without its catalytic activity partially through its interactions with histone-modifying enzymes.Nucleic Acids Res 40:4334-4346.
Costa E, Grayson DR, Guidotti A (2003) Epigenetic downregulation of GABAergic function in schizophrenia: potential for pharmacological intervention?Mol Interv 3:220-229.
Déléris P, Trost M, Topisirovic I, Tanguay P-L, Borden KLB, Thibault P,Meloche S (2011) Activation loop phosphorylation of ERK3/ERK4 by group I p21-activated kinases (PAKs) defines a novel PAK-ERK3/4-MAPK-activated protein kinase 5 signaling pathway. J Biol Chem 286:6470-6478.
De la Mota-Peynado A, ChernoffJ, Beeser A (2011) Identification of the atypical MAPK Erk3 as a novel substrate for p21-activated kinase (Pak) activity. J Biol Chem 286:13603-13611.
de la Torre-Ubieta L, Gaudilliere B, Yang Y, Ikeuchi Y, Yamada T, DiBacco S,Stegmuller J, Schuller U, Salih DA, Rowitch D, Brunet A, Bonni A (2010) A FOXO-Pak1 transcriptional pathway controls neuronal polarity. Genes Dev 24:799-813.
Dekkers MP, Barde YA (2013) Developmental biology. Programmed cell death in neuronal development. Science 340:39-41.
Dekkers MP, Nikoletopoulou V, Barde YA (2013) Cell biology in neuroscience:Death of developing neurons: new insights and implications for connectivity.J Cell Biol 203:385-393.
Du J, Johnson LM, Jacobsen SE, Patel DJ (2015) DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol 16:519-532.
Duman RS (2009) Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues Clin Neurosci 11:239-255.
Eberharter A, Becker PB (2002) Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics.EMBO Rep 3:224-229.
Fagiolini M, Jensen CL, Champagne FA (2009) Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol 19:207-212.
Fan G, Martinowich K, Chin MH, He F, Fouse SD, Hutnick L, Hattori D, Ge W, Shen Y, Wu H, ten Hoeve J, Shuai K, Sun YE (2005) DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development 132:3345-3356.
Fan G, Beard C, Chen RZ, Csankovszki G, Sun Y, Siniaia M, Biniszkiewicz D,Bates B, Lee PP, Kuhn R, Trumpp A, Poon C, Wilson CB, Jaenisch R (2001)DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J Neurosci 21:788-797.
Feng J, Fouse S, Fan G (2007) Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res 61:58R-63R.
Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G (2010)Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci 13:423-430.
Froimchuk E, Jang Y, Ge K (2017) Histone H3 lysine 4 methyltransferase KMT2D. Gene 627:337-342.
Fu Z, Tindall DJ (2008) FOXOs, cancer and regulation of apoptosis. Oncogene 27:2312-2319.
Furnari MA, Jobes ML, Nekrasova T, Minden A, Wagner GC (2013) Functional deficits in PAK5, PAK6 and PAK5/PAK6 knockout mice. PLoS One 8:e61321.
Götz M, Nakafuku M, Petrik D (2016) Neurogenesis in the Developing and Adult Brain-Similarities and Key Differences. Cold Spring Harb Perspect Biol 8:a018853.
Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, Pla R, Pierani A, Marin O (2011) A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J Neurosci 31:16570-16580.
Guidotti A, Ruzicka W, Grayson DR, Veldic M, Pinna G, Davis JM, Costa E(2007) S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis. Neuroreport 18:57-60.
Han L, Witmer PD, Casey E, Valle D, Sukumar S (2007) DNA methylation regulates MicroRNA expression. Cancer Biol Ther 6:1284-1288.
Hashimshony T, Zhang J, Keshet I, Bustin M, Cedar H (2003) The role of DNA methylation in setting up chromatin structure during development. Nat Genet 34:187-192.
Hatok J, Racay P (2016) Bcl-2 family proteins: master regulators of cell survival.Biomol Concepts 7:259-270.
Hatta M, Cirillo LA (2007) Chromatin opening and stable perturbation of core histone:DNA contacts by FoxO1. J Biol Chem 282:35583-35593.
Hermann A, Goyal R, Jeltsch A (2004) The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J Biol Chem 279:48350-48359.
Hirasawa R, Chiba H, Kaneda M, Tajima S, Li E, Jaenisch R, Sasaki H (2008)Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev 22:1607-1616.
Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14:R115.
Hua T, Kao C, Sun Q, Li X, Zhou Y (2008) Decreased proportion of GABA neurons accompanies age-related degradation of neuronal function in cat striate cortex. Brain Res Bull 75:119-125.
Hutnick LK, Golshani P, Namihira M, Xue Z, Matynia A, Yang XW, Silva AJ,Schweizer FE, Fan G (2009) DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Hum Mol Genet 18:2875-2888.
Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, Dausman J, Lee P, Wilson C, Lander E, Jaenisch R (2001) Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation.Nat Genet 27:31-39.
Jang HS, Shin WJ, Lee JE, Do JT (2017) CpG and non-CpG methylation in epigenetic gene regulation and brain function. Genes 8:E148.
Ji Q, Hu H, Yang F, Yuan J, Yang Y, Jiang L, Qian Y, Jiang B, Zou Y, Wang Y, Shao C, Gong Y (2014) CRL4B interacts with and coordinates the SIN3A-HDAC complex to repress CDKN1A and drive cell cycle progression. J Cell Sci 127:4679-4691.
Jiang CH, Tsien JZ, Schultz PG, Hu Y (2001) The effects of aging on gene expression in the hypothalamus and cortex of mice. Proc Natl Acad Sci U S A 98:1930-1934.
Jiang H, Lu X, Shimada M, Dou Y, Tang Z, Roeder RG (2013) Regulation of transcription by the MLL2 complex and MLL complex-associated AKAP95.Nat Struct Mol Biol 20:1156-1163.
Jin B, Robertson KD (2013) DNA methyltransferases, DNA damage repair, and cancer. Adv Exp Med Biol 754:3-29.
Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N,Strouboulis J, Wolffe AP (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19:187-191.
Kadriu B, Guidotti A, Chen Y, Grayson DR (2012) DNA methyltransferases1(DNMT1) and 3a (DNMT3a) colocalize with GAD67-positive neurons in the GAD67-GFP mouse brain. J Comp Neurol 520:1951-1964.
Kann O, Papageorgiou IE, Draguhn A (2014) Highly energized inhibitory inter-neurons are a central element for information processing in cortical networks.J Cereb Blood Flow Metab 34:1270-1282.
Kannan K, Jain SK (2000) Oxidative stress and apoptosis. Pathophysiology 7:153-163.
Kepecs A, Fishell G (2014) Interneuron cell types are fit to function. Nature 505:318-326.
Kole AJ, Annis RP, Deshmukh M (2013) Mature neurons: equipped for survival.Cell Death Dis 4:e689.
Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S (2011a) Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry 68:340-350.
Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S (2011b) Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res 131:165-173.
Kristiansen M, Ham J (2014) Programmed cell death during neuronal development: the sympathetic neuron model. Cell Death Differ 21:1025-1035.
Kumar R, Sanawar R, Li X, Li F (2017) Structure, biochemistry, and biology of PAK kinases. Gene 605:20-31.
Lande-Diner L, Zhang J, Ben-Porath I, Amariglio N, Keshet I, Hecht M, Azuara V, Fisher AG, Rechavi G, Cedar H (2007) Role of DNA methylation in stable gene repression. J Biol Chem 282:12194-12200.
Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D,Coleman P, Lemere CA, Hof PR, van den Hove DL, Rutten BP (2015) The epigenetics of aging and neurodegeneration. Prog Neurobiol 131:21-64.
Laughlin SB, de Ruyter van Steveninck RR, Anderson JC (1998) The metabolic cost of neural information. Nat Neurosci 1:36-41.
Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, Balk SP (2002)AR and ER interaction with a p21-activated kinase (PAK6). Mol Endocrinol 16:85-99.
Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM,Sweatt JD (2006) Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem 281:15763-15773.
Levitt P (2005) Disruption of interneuron development. Epilepsia 46 Suppl 7:22-28.
Lewis DA, Curley AA, Glausier JR, Volk DW (2012) Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35:57-67.
Li E, Bestor TH, Jaenisch R (1992) Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69:915-926.
Liao J, Karnik R, Gu H, Ziller MJ, Clement K, Tsankov AM, Akopian V, Gifford CA, Donaghey J, Galonska C, Pop R, Reyon D, Tsai SQ, Mallard W, Joung JK,Rinn JL, Gnirke A, Meissner A (2015) Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat Genet 47:469-478.
Liu W, Liu Y, Liu H, Zhang W, Fu Q, Xu J, Gu J (2015) Tumor suppressive function of p21-activated kinase 6 in hepatocellular carcinoma. J Biol Chem 290:28489-28501.
Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429:883-891.
Lujambio A, Calin GA, Villanueva A, Ropero S, Sanchez-cespedes M, Blanco D,Montuenga LM, Rossi S, Nicoloso MS, Faller WJ, Gallagher WM, Eccles SA,Croce CM, Esteller M (2008) A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci U S A 105:13556-13561.
Lv J, Xin Y, Zhou W, Qiu Z (2013) The epigenetic switches for neural development and psychiatric disorders. J Genet Genomics 40:339-346.
Marín O (2012) Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 13:107-120.
Margueron R, Reinberg D (2011) The Polycomb complex PRC2 and its mark in life. Nature 469:343-349.
Martini FJ, Valiente M, Lopez Bendito G, Szabo G, Moya F, Valdeolmillos M,Marin O (2009) Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development 136:41-50.
Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE (2003)DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302:890-893.
Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J (2010)Epigenetic changes in Alzheimer’s disease: decrements in DNA methylation.Neurobiol Aging 31:2025-2037.
Mazumdar A, Kumar R (2003) Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett 535:6-10.
Meadows JP, Guzman-Karlsson MC, Phillips S, Brown JA, Strange SK, Sweatt JD, Hablitz JJ (2016) Dynamic DNA methylation regulates neuronal intrinsic membrane excitability. Sci Signal 9:ra83.
Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P,Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O’Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, et al. (2007)Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448:553-560.
Miller MW (1995) Relationship of the time of origin and death of neurons in rat somatosensory cortex: barrel versus septal cortex and projection versus local circuit neurons. J Comp Neurol 355:6-14.
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687-702.
Mo A, Mukamel EA, Davis FP, Luo C, Henry GL, Picard S, Urich MA, Nery JR,Sejnowski TJ, Lister R, Eddy SR, Ecker JR, Nathans J (2015) Epigenomic signatures of neuronal diversity in the mammalian brain. Neuron 86:1369-1384.
Moore AZ, Hernandez DG, Tanaka T, Pilling LC, Nalls MA, Bandinelli S, Singleton AB, Ferrucci L (2016) Change in epigenome-wide DNA methylation over 9 years and subsequent mortality: results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci 71:1029-1035.
Moore LD, Le T, Fan G (2013) DNA methylation and its basic function. Neuropsychopharmacology 38:23-38.
Nakagawa T, Xiong Y (2011) Chromatin regulation by CRL4 E3 ubiquitin ligases:CUL4B targets WDR5 ubiquitylation in the nucleus. Cell Cycle 10:4197-4198.
Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A(1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393:386-389.
Nekrasova T, Jobes ML, Ting JH, Wagner GC, Minden A (2008) Targeted disruption of the Pak5 and Pak6 genes in mice leads to deficits in learning and locomotion. Dev Biol 322:95-108.
Ning X, Shi Z, Liu X, Zhang A, Han L, Jiang K, Kang C, Zhang Q (2015)DNMT1 and EZH2 mediated methylation silences the microRNA-200b/a/429 gene and promotes tumor progression. Cancer Lett 359:198-205.
Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S,Kakizuka A, Ichijo H (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16:1345-1355.
Noguchi H, Kimura A, Murao N, Matsuda T, Namihira M, Nakashima K (2015)Expression of DNMT1 in neural stem/precursor cells is critical for survival of newly generated neurons in the adult hippocampus. Neurosci Res 95:1-11.
Numata S, Ye T, Hyde TM, Guitart-Navarro X, Tao R, Wininger M, Colantuoni C, Weinberger DR, Kleinman JE, Lipska BK (2012) DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet 90:260-272.
Oliver KL, Lukic V, Freytag S, Scheffer IE, Berkovic SF, Bahlo M (2016) In silico prioritization based on coexpression can aid epileptic encephalopathy gene discovery. Neurology Genetics 2:e51.
Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, Jonsdottir GA, Stewart R, Thomson JA (2007) Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell 1:299-312.
Pensold D, Symmank J, Hahn A, Lingner T, Salinas-Riester G, Downie BR,Ludewig F, Rotzsch A, Haag N, Schubert NAK, Hübner CA, Pieler T, Zimmer G (2016) The DNA methyltransferase 1 (DNMT1) controls the shape and dynamics of migrating POA-derived interneurons fated for the murine cerebral cortex. Cereb Cortex doi: 10.1093/cercor/bhw341.
Petak I, Danam RP, Tillman DM, Vernes R, Howell SR, Berczi L, Kopper L,Brent TP, Houghton JA (2003) Hypermethylation of the gene promoter and enhancer region can regulate Fas expression and sensitivity in colon carcinoma. Cell Death Differ 10:211-217.
Peyre E, Silva CG, Nguyen L (2015) Crosstalk between intracellular and extracellular signals regulating interneuron production, migration and integration into the cortex. Front Cell Neurosci 9:129.
Pfisterer U, Khodosevich K (2017) Neuronal survival in the brain: neuron type-specific mechanisms. Cell Death Dis 8:e2643.
Pugliese M, Carrasco JL, Geloso MC, Mascort J, Michetti F, Mahy N (2004)Gamma-aminobutyric acidergic interneuron vulnerability to aging in canine prefrontal cortex. J Neurosci Res 77:913-920.
Purkait S, Sharma V, Kumar A, Pathak P, Mallick S, Jha P, Sharma MC, Suri V, Julka PK, Suri A, Sharma BS, Sarkar C (2016) Expression of DNA methyltransferases 1 and 3B correlates with EZH2 and this 3-marker epigenetic signature predicts outcome in glioblastomas. Exp Mol Pathol 100:312-320.
Qing H, Gong W, Che Y, Wang X, Peng L, Liang Y, Wang W, Deng Q, Zhang H, Jiang B (2012) PAK1-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Tumour Biol 33:985-994.
Rakyan VK, Down TA, Maslau S, Andrew T, Yang TP, Beyan H, Whittaker P,McCann OT, Finer S, Valdes AM, Leslie RD, Deloukas P, Spector TD (2010)Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res 20:434-439.
Rhee KD, Yu J, Zhao CY, Fan G, Yang XJ (2012) Dnmt1-dependent DNA methylation is essential for photoreceptor terminal differentiation and retinal neuron survival. Cell Death Dis 3:e427.
Rozycka A, Liguz-Lecznar M (2017) The space where aging acts: focus on the GABAergic synapse. Aging cell 16:634-643.
Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A (2007) Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry 12:385-397.
Schürmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM (2000) p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol 20:453-461.
Segal M (2010) Dendritic spines, synaptic plasticity and neuronal survival: activity shapes dendritic spines to enhance neuronal viability. Eur J Neurosci 31:2178-2184.
Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA (2010) DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 463:563-567.
Shimoda N, Izawa T, Yoshizawa A, Yokoi H, Kikuchi Y, Hashimoto N (2014)Decrease in cytosine methylation at CpG island shores and increase in DNA fragmentation during zebrafish aging. Age (Dordr) 36:103-115.
Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, Jaenisch R, Laird PW, Akbarian S (2007) DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS One 2:e895.
Smallwood A, Estève P-O, Pradhan S, Carey M (2007) Functional cooperation between HP1 and DNMT1 mediates gene silencing. Genes Dev 21:1169-1178.
So AY, Jung JW, Lee S, Kim HS, Kang KS (2011) DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs.PLoS One 6:e19503.
Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, Alfaro-Cervello C, Tang Y, Garcia-Verdugo JM, Rubenstein JL, Baraban SC, Alvarez-Buylla A (2012) Intrinsically determined cell death of developing cortical interneurons. Nature 491:109-113.
Sullivan EM, O’Donnell P (2012) Inhibitory interneurons, oxidative stress, and schizophrenia. Schizophr Bull 38:373-376.
Tang Y, Zhou H, Chen A, Pittman RN, Field J (2000) The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem 275:9106-9109.
Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2:222-228.
Trowbridge JJ, Snow JW, Kim J, Orkin SH (2009) DNA methyltransferase 1 is essential for and uniquely regulates hematopoietic stem and progenitor cells.Cell Stem Cell 5:442-449.
Tsumura A, Hayakawa T, Kumaki Y, Takebayashi S, Sakaue M, Matsuoka C,Shimotohno K, Ishikawa F, Li E, Ueda HR, Nakayama J, Okano M (2006)Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells 11:805-814.
Uribe E, Wix R (2012) Neuronal migration, apoptosis and bipolar disorder. Rev Psiquiatr Salud Ment 5:127-133.
van Noesel MM, van Bezouw S, Salomons GS, Voute PA, Pieters R, Baylin SB,Herman JG, Versteeg R (2002) Tumor-specific down-regulation of the tumor necrosis factor-related apoptosis-inducing ligand decoy receptors DcR1 and DcR2 is associated with dense promoter hypermethylation. Cancer Res 62:2157-2161.
Veldic M, Guidotti A, Maloku E, Davis JM, Costa E (2005) In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A 102:2152-2157.
Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A,Costa E (2004) DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A 101:348-353.
Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F (2006) The Polycomb group protein EZH2 directly controls DNA methylation. Nature 439:871-874.
Voigt P, Tee WW, Reinberg D (2013) A double take on bivalent promoters.Genes Dev 27:1318-1338.
Wamsley B, Fishell G (2017) Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat Rev Neurosci 18:299-309.
Xin Y, O’Donell A, Ge Y, Chanrion B, Dwork A, Arango V, Mann JJ, Haghighi F (2013) DNA demethylation of neuronal cell death genes in depression. Epigenetics Chromatin 6:P113.
Xu X, Zhan M, Duan W, Prabhu V, Brenneman R, Wood W, Firman J, Li H,Zhang P, Ibe C, Zonderman AB, Longo DL, Poosala S, Becker KG, Mattson MP (2007) Gene expression atlas of the mouse central nervous system: impact and interactions of age, energy intake and gender. Genome Biol 8:R234.
Ye DZ, Jin S, Zhuo Y, Field J (2011) p21-Activated kinase 1 (Pak1) phosphorylates BAD directly at serine 111 in vitro and indirectly through Raf-1 at serine 112. PLoS One 6:e27637.
Yuan J, Lipinski M, Degterev A (2003) Diversity in the mechanisms of neuronal cell death. Neuron 40:401-413.
Yuan Z, Lehtinen MK, Merlo P, Villén J, Gygi S, Bonni A (2009) Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem 284:11285-11292.
Zaret KS, Mango SE (2016) Pioneer transcription factors, chromatin dynamics,and cell fate control. Curr Opin Genet Dev 37:76-81.
Zhang M, Siedow M, Saia G, Chakravarti A (2010) Inhibition of p21-activated kinase 6 (PAK6) increases radiosensitivity of prostate cancer cells. Prostate 70:807-816.
Zhang P, Lee H, Brunzelle JS, Couture JF (2012) The plasticity of WDR5 peptide-binding cleftenables the binding of the SET1 family of histone methyl-transferases. Nucleic Acids Res 40:4237-4246.
杂志排行
中国神经再生研究(英文版)的其它文章
- Design and criteria of electrospun fibrous scaffolds for the treatment of spinal cord injury
- Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogenactivated protein kinase and nuclear factor kappa B signaling pathways
- The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction
- MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury
- Interferon regulatory factor 2 binding protein 2: a new player of the innate immune response for stroke recovery
- Endogenous retinal neural stem cell reprogramming for neuronal regeneration
