The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction
2017-12-15MarcoCascellaSabrinaBimonte
Marco Cascella, Sabrina Bimonte*
Division of Anesthesia and Pain Medicine Istituto Nazionale Tumori – IRCCS - Fondazione G. Pascale, Via Mariano Semmola, Naples, Italy
How to cite this article:Cascella M, Bimonte S (2017) The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction. Neural Regen Res 12(11):1780-1785.
Introduction
The term postoperative cognitive dysfunction (POCD) encompasses a wide spectrum of clinical conditions featuring a decline in a variety of neuropsychological tasks after anesthesia and surgery in elderly patients. Memory and information processing are the most involved cognitive domains in such decline and their impairment may vary from mild and short-lived clinical manifestations to severe and permanent damages (Cascella et al., 2017; Evered et al., 2017).
Because POCD is a multifactorial process, a large number of predisposing, causal, and precipitating factors are involved in its pathophysiology. However, the neuroinflam-mation and the microglial activation seem to play a key role in the POCD’s genesis as they trigger and amplify a complex cascade involving immuno-hormonal activation, microcircle alterations, hippocampal oxidative stress activation and, finally, an increased permeability of the blood brain barrier (BBB) (e.g., alterations of tight junctions) (Wang et al., 2017b) which promotes the migration of macrophages into the brain parenchyma (Vacas et al., 2013). In turn, the neuronal damages and apoptosis, and the impaired neurotransmission in hippocampus and other cerebral regions(e.g., medial prefrontal cortex, mPFC, amigdala and gyrus cinguli) (Ling et al., 2015) are the acme of such increased and deregulated inflammatory response (Fodale et al., 2017)(Figure 1).
Although it is still unclear whether or not POCD represents an unmasking of early dementia or a predictor of later dementia, several preclinical (Hovens et al., 2013) and clinical studies (Patel et al., 2016) demonstrated that perioperative neuroinflammation may accelerate the trajectory of cognitive decline. Nevertheless, in this scenario the specific role of anesthesia remains to be understood. Thus, we must establish if anesthesia represents a major actor or just a co-participant in a complex framework of such perioperative homeostasis perturbation. The clinical implication has a high impact.
The Impact of General Anesthetics
The role of anesthetics in the genesis of POCD in humans is debated (Yang and Fuh, 2015) and most of evidences proving a linkage between anesthesia and POCD are preclinical(Seitz et al., 2001). Moreover, previous reverse analysis of case-control Alzheimer’s disease (AD) studies failed to report an association between general anaesthesia and AD(Breteler et al., 1991). On the contrary, a recent meta-analysis of epidemiological studies found, in three studies, a significant nonlinear relationship between times of exposure to anesthetics and augmented risk of AD (P< 0.0001) (Jiang et al., 2017).
A huge number of preclinical studies have been conducted on the topic and many mechanisms have been proposed to explain the potential neurodegenerative effects of anesthetics in adults whereas another interesting field of research concerns the neurodegeneration and the interference with the synaptogenesis, in the developing rat brain (Satomoto and Makita, 2016). Because the linkage between anesthesia and cognitive dysfunction in new-borns is not the aim of this article, the discussion is focused only on anesthetics and POCD.
In 2004, the Eckenhof f’s investigation proved that at the concentration used clinically several inhaled anesthetics including halothane and isoflurane may induce the oligomerization of amyloid β (Aβ) and increase Aβ-induced cytotoxicity in pheochromocytoma cells (Eckenhoff et al.,2004). Although the inhaled agent desflurane did not impair learning and memory in mice (Zhang et al., 2012), this inhaled anesthetic induced caspase activation and increased amyloid precursor protein (APP) proteolysis in H4 cells expressing APP (H4-APP cells) (Zhang et al., 2008). Again,anin vitroexperiment on H4-APP cells proved that nitrous oxide and isoflurane induced the activation of caspase-3 and apoptosis, and increased the levels of aspartyl protease β-site APP-cleaving enzyme (BACE) which is an α-secretase implicated in the proteolysis of APP in Aβ (Zhen et al., 2009).
In addition, inhaled anesthetics may also increase tau hyper-phosphorylation and neurofibrillary tangles (NFTs)(Planel et al., 2007). However, the mechanisms underlying the anesthetic-induced cognitive impairment are very complex. For instance, it has been demonstrated that sevoflurane induced a wide range of hippocampal alterations in adult rats, including an increased endoplasmic reticulum stress-apoptosis and intracellular alterations such as the down-regulation of the cyclic adenosine monophosphate(cAMP)/cAMP response element-binding protein (CREB)signalling pathway (Xiong et al., 2013). Specific changes have been also demonstrated following the use of intravenous agents. A prolonged infusion (over 4 hours) of propofol elicited cognitive deficit through the inhibition of hippocampal autophagy, especially in the CA1 region (Yang et al., 2017a). The autophagy is a catabolic process in which aggregated proteins, lipids, and organelles are engulfed within double-membrane vesicles (phagophore) in order to balance their synthesis in cells. This trafficking and degradative pathway is implicated in the regulation of metabolic processes in health, disease and aging (Papáčková and Cahová, 2014).The inhaled agents are also able to inhibit the autophagy as isoflurane or sevoflurane exposure could impair spatial cognitive function and hippocampal phagophore formation in aged rats (Zhang et al., 2016). This evidence is significant because hippocampal autophagic dysregulation is also implicated in the pathogenesis of neurodegenerative diseases including AD in which it occurs prior to the appearance of Aβ and tau pathology whereas this proapoptotic pathway seems to be independent by the Aβ accumulation during AD progression (Mufson et al., 2012). Thus, preclinical studies may suggest that anesthetics could induce cognitive impairment firstly through alterations in the intracellular signaling pathways and hippocampal autophagic dysregulation, and finally through Aβ and NFTs formation. Consequently, the caspase activation with increased BACE activity and subsequent aberrant APP processing lead, in turn, to further apoptosis(Jiang and Jiang, 2015).
Interestingly, clinical and preclinical studies indicated that anesthesia may be protective against inflammation-in-duced POCD. For instance, small doses of ketamine reduced the incidence of POCD in patients who underwent cardiac surgery, possibly through the anti-inflammatory effect of the agent (Hudetz et al., 2009). In addition, propofol attenuated the experimentally induced impairment of learning and memory by suppressing interleukin (IL)-1β and tumor necrosis factor (TNF)-α production, and by decreasing the concentration of glutamate in the hippocampus (Zhu et al.,2015). Again, dexmedetomidine (combined with flurbiprofen axetil) reduced POCD in a recent clinical setting (Ma et al., 2017). Investigations on the potential neuroprotective role of endovenous anesthetics against POCD should be encouraged. For example, it has been demonstrated that propofol and thiopental did not interfere with cortical APP protein (Palotás et al., 2005) and a prolonged treatment with propofol had limited impact on survival of rat neural progenitor cells,in vitro(Palanisamy et al., 2016). Moreover,recent data showed that in an AD transgenic mouse model (3xTgAD) the use of propofol is associated with better cognitive outcomes compared with inhalation an aesthesia (Mardini et al., 2017).
Mechanisms of Hippocampal and Extra-hippocampal Dysfunction
During the last decade most evidences demonstrated that the aging process is associated with an increased neuroin-flammatory response involving microglial activation and proinflammatory cytokines production, such as IL-1β, IL-6 and TNF-α (Wang et al., 2015). On the contrary, the anti-inflammatory cytokine IL-4, which prevents the microglial priming and the subsequent activation of the deleterious microglial phenotype M1, decrease during aging (Czeh et al., 2011). Furthermore, other factors (e.g., alterations in immune response) are responsible for the microglial senescence expressed as an upregulation of pro-inflammatory brain factors (Koellhoffer et al., 2017). Interestingly, the surgery-induced cytokine pattern follows the same scheme consisting of an increased release of proinflammatory cytokine signaling combined with a decrease of the anti-inflammatory response (Bastian et al., 2008). According to this point of view, hippocampal neuroinflammation due to surgery could represent a triggering factor that exacerbates a pre-existing condition. For instance, studies assessing functional Magnetic Resonance Imaging (fMRI), demonstrated that preoperative hippocampal leukoaraiosis/lacunae were significantly associated with the occurrence of POCD(Price et al., 2014). Functionally, failures to adjust the proinflammatory processes and subsequent M1 phenotype over activation lead to several hippocampal alterations. Specific neuronal impairments in the CA1 area include, for example,the inhibition of the long-term potentiation (LTP) and dendritic branching, which are involved in memory formation and maintenance (Lemaire et al., 2012). In addition to the hippocampal spatial memory, other cognitive domains such as the visual memory and the reversal learning (depending on the striatal function) can be affected in POCD. However, these impairments are often temporary and short-lived(Hovens et al., 2015). This piece of evidence suggests that neuroinflammation (and restore mechanisms) may follow different pathways (e.g., different ways of microglial activation) in different brain regions.
Regarding to the neurotransmission mechanisms affected in the POCD’s pathogenesis, preclinical investigations demonstrated that postoperative memory deficits may be due to up-regulation of hippocampal alpha-5 subunit of γ-aminobutyric acid type A receptors (α5GABAARs) which inhibit the LTP phenomenon (Zurek et al., 2014). This is a very interesting finding as most general anesthetics operate as positive allosteric modulators of inhibitory GABAARs and subtypes of these receptors are responsible for several behavioral endpoints, including amnesia (Bonin and Orser, 2008). Furthermore, the glutamatergic transmission is also involved; indeed, an increased hippocampal glutamate concentration and an up-regulation of glutamate aspartate transporter (GLAST) were associated with isoflurane-induced spatial learning/memory impairment in adult rats(Qu et al., 2013). The pathogenesis of the impairments in the neurotransmission systems is very complex due to the several functional and metabolic pathways involved. For instance, sevoflurane decreased the expression in mPFC of the postsynaptic density protein (PSD)-95 which interacts with neuroligin, glutamatergic receptors, and potassium channels and plays a paramount role in synaptic plasticity(Ling et al., 2015). Moreover, altered levels of N, N-diethylacetamide, n-ethylacetamide, aspartic acid, malic acid and arabinonic acid have been found in the hippocampus of isoflurane-treated rats (Hu et al., 2014). The study of these alterations could be an interesting future perspective, especially for the discovery of biomarkers useful to predict the occurrence and the progress of cognitive dysfunction.
Perspectives and Future Directions
To date, no conclusive proof has been found to validate the hypothesis that general anesthetics may induce cognitive dysfunction. At the same time, due to the results of a huge number of the preclinical studies on this topic, it is difficult to assert with certainty the absence of any neurodegenerative effect of anesthetics. Probably, the problem concerns on what we are searching for and how we are searching. It is necessary to improve a cross-talk between preclinical and clinical research. Thus, furtherin vitroandin vivoinvestigations are extremely needed to demonstrate the potential mechanism(s) responsible for the anesthetic neuronal damage and the different neurodegenerative effect depending on different clinical uses (e.g., time of exposure and concentration). The neurotoxic effects of inhaled anesthetics, for instance, may occur in specific experimental conditions. Indeed, the exposure to 1.5% sevoflurane in rats did not cause significantly different cognitive performance compared with a higher concentration (up to 3%) which induced cognitive impairment (Pend et al., 2011). Furthermore, in human neuroglioma cells and mouse primary neurons, although a treatment with 2% isoflurane (for 6 hours or 30 minutes)increased the Aβ-induced caspase-3 activation and apoptosis, an exposure for the same time but at a lower concentration (0.5%) showed protection against neurotoxicity (Xu et al., 2011). Probably, further studies on the mechanisms by which inhaled anesthetics accelerate the protein oligomerization (e.g., protein-protein interaction) could be useful to elucidate many doubts (Carnini et al., 2006).
One question regarding these studies is to determine whether the observed POCD is caused by general anesthetic or surgery, or by both. While the role of anesthetics on POCD is still not conclusive, research on the molecular mechanisms of anesthetics could represent an interesting chance to investigate on the neurodegenerative/neuroprotective effect of these drugs. Because ion channels are important targets for anesthetic drugs, this can be considered one of the potential mechanisms of the impact of general anesthetics on POCD, assuming that the association is real. Expanding elaboration may lead more research in this direction in the future. The operating mechanisms of anesthetics represent a fascinating field of study which could potentiate the research on POCD. In this way, the effects of anesthetics on specific GABAergic transmission pathways could give us surprising findings. Accumulating evidence suggests that the GABAergic transmission gives a significant contribution to the pathogenesis of AD (Govindpani et al.,2017). For instance, it has been proved that the dysfunction(i.e., reduced gamma oscillatory activity) of the parvalbumin(PV) inhibitory GABAergic interneurons (implicated in the information processing of attention, perception and working memory) induced cognitive dysfunction in a mouse AD model (Verret et al., 2012). Moreover, the exposure of mice to ketamine induced dysfunction of fast-spiking inhibitory interneurons through the activation of the oxidative stress(Behrens et al., 2007). It represents a good example of crosstalk between scientists of different areas of study.
The clinical research, possibly supported by epidemiology,should be focused on the evaluation of factors which may lead bias in the analysis of the correlation between anesthesia and POCD. To this regard, preclinical findings showed that male rats suffered for a more severe POCD than female rats (Xie et al., 2015). Again, the specific weight of risk factors such as age, presymptomatic neurodegenerative disease or dementia should be better investigated. A meta-analysis of clinical studies concluded that the apolipoprotein E(APOE) ε4 carrier status which is a genetic risk factor for sporadic or late onset AD, was associated with a significantly increased POCD risk about 1-week postsurgery (Cao et al.,2014). Other investigations should be focused on the identification of prognostic and predictive biomarkers. Specific miRNA could represent valid markers for early diagnosis of POCD. For example, miR-572 has been found to be involved in the development and restoration of POCD (Yu et al., 2015). In addition, desflurane induced downregulation of the microRNA precursor, miR-214, which normally inhibits the expression of the pro-apoptotic gene, Bax (Yu and Zhang, 2013). In addition to a potential diagnostic role, the investigation on miRNA could lead to interesting findings because it is reasonable to assume that several non-coding RNAs might be involved in the pathogenesis of POCD.
Moreover, further studies are needed to better explain the mechanisms responsible for the alterations in the hippocampal and extra-hippocampal neuronal functions in both aging and POCD (Hovens et al., 2014). For instance, the correlation between immune senescence and neuroinflammation could represent an interesting field of study (Ron-Harel and Schwartz, 2009).
The assumed veracity of the hypothesis: anesthesia (and/or surgery) = neurocognitive dysfunction calls for search of prophylactic strategies. In this direction, one possible strategy could be the prevention or the limitation of the neuroinflammation cascade. Taking into the account the pivotal role of microglia in hippocampal neuroinflammation, targeting microglia, directed towards microglial priming and the aberrant microglial response, could represent an effective strategy to mitigate the development of POCD.For this purpose, recent data demonstrated that the nuclear factor (NF)-κB signalling pathway is a potential therapeutic target in POCD (Zheng et al., 2017). Because different oxygen species in the hippocampus contribute to microglial activation in POCD, specific NADPH oxidase inhibitors already investigatedin vivo(e.g., apocynin) (Qiu et al., 2017)could be studied in clinical trials. Again, the peroxisome proliferator-activated receptor-γ (PPARγ), a nuclear hormone receptor which modulates the oxidative metabolism and inflammation, is downregulated in POCD whereas PPARγ activators may prevent or attenuate neurodegeneration (Iglesias et al., 2017). Thus, the activation of PPARγ could represent an effective strategy to prevent or treat POCD (Zhang et al., 2017). The microglial function is also mediated by the activity of the cannabinoid receptor type 2 (CB2R) which mitigates the production of inflammatory mediators and induces the production of anti-inflammatory and immunomodulatory cytokines in different neurodegenerative conditions, including AD (Wang et al., 2017a).Different endocannabinoids such as N-arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG) have been reported to play a significant role in the modulation of memory consolidation (Basavarajappa et al., 2014). On this basis, the endocannabinoid system is another potential target for POCD’s treatment. Further, it has been showed that histone deacetylases (HDACs) expression can interfere with neuroinflammation by modulation cytokine synthesis and release (Suh et al., 2010). Consequently, becausein vitroandin vivostudies found that HDAC inhibitors (e.g., trichostatin A) prevented the microglial activation and ameliorated the cytokine-associated changes in cognitive function (Hsing et al., 2015), the epigenetic modulation could represent another possible approach for inducing the polarization of the microglial neuroprotective phenotype M2, and a subsequent POCD prevention. Thus, the imbalance in the regulation of the neuroin-flammatory processes could be addressed by enhancing the protective pathways. Accumulating evidence showed that the increased transport of peripheral mediators across the BBB is also under control of afferent pathways such as the vagal nerve (Banks, 2015). Indeed, the activation of the so-called cholinergic reflex by stimulating the α7 subtype of nicotinic acetylcholine receptors (α7 nAChR)in macrophages inhibited the NF-κB signaling pathway,preventing the further hippocampal M2 polarization and POCD in rats (Terrando et al., 2011). On the contrary, the release of the anti-inflammatory cytokine IL-10 is not sup-pressed by the cholinergic stimulation (Tracey, 2009). Recently, ad hoc animal models for studying the BBB permeability have been developed (Yang et al., 2017b). It is possible to assume that the correlation between systemic inflammation, neuroinflammation and their neural modulation, as well as the possibility of modulating the hippocampal cholinergic transmission should be better investigated by using these new approaches (Figure 1).
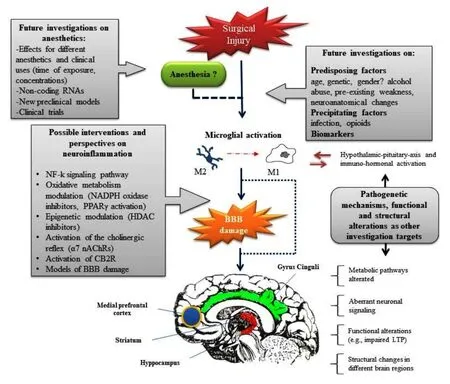
Figure 1 Mechanisms of POCD and research perspectives.The neuroinflammation due to surgery and expressed as microglial activation (towards the deleterious phenotype M1), increased oxidative stress activation, microcircle alterations and BBB damage. This leads to an induction of aberrant neuronal signaling and structural alterations in several brain regions (e.g.,hippocampus, striatum, medial prefrontal cortex, and gyrus cinguli), thus representing the proposed mechanism of POCD. There is a link between inflammation and the stress response because cytokines release can activate the hypothalamic-pituitary-axis whereas stress-induced neuronal responses are associated with microglia activation and increased neuroin-flammatory signaling. The role of general anesthetics remains questioned. Future investigations on anesthetics, mechanisms of neuroinflammation, functional and structural alterations in different brain regions, as well as predisposing and precipitating factors are needed. POCD: Postoperative cognitive dysfunction; BBB: bloods brain barrier; Ach: Acetylcholine; LTP: long-term potentiation; NF-k:nuclear factor-κ; CB2R: cannabinoid receptor type 2; PPARγ peroxisome proliferator-activated receptor-γ; HDAC: histone deacetylase.
Conclusion
Although implicated mechanistically in preclinical investigations, it seems that anesthetics play a smaller role than surgery in the POCD’s genesis. However, the lack of correspondence between the results of preclinical and clinical studies could suggest that the effect of an acute insult (i.e.,anesthetic) is subsequently compensated by not still understood compensatory mechanisms. In other words, assuming that the multifactorial pathogenesis of POCD involves an imbalance between pathogenetic factors and protective mechanisms, we should investigate deeper on the mechanisms underlying the inflammatory homeostasis. Moreover, several ways have been proposed for interfering with the pathogenetic cascade of POCD, especially in order to counteract neuroinflammation processes. Other interesting perspectives concern the study of predisposing and precipitating factors as well as the identification of prognostic and predictive biochemical/neuroanatomical biomarkers.
Author contributions:The present article was written by MC and SB.
Conflicts of interest:None declared.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer review report:
Reviewer:Evgeniya Pushchina, A. V. Zhirmunsky Institute of Marine Biology FEB RAS, Russia.
Comments to authors:The review presents current and interesting information on postoperative cognitive dysfunction caused by the use of general anesthetics. The influence of various factors on the occurrence of POCD is considered. The review presents data about the complex and multifactor effect to POCD, in particular, neuroin-flammatory and microglial activation, which have complex and unrelated effects on the cascade, including immuno-hormonal activation, changes in microcirculation, hippocampus - oxidative activation of stress, and other factors.This work is of unconditional interest to a wide range of readers and contains relevant and useful information.
Banks WA (2015) The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Behav Immun 44:1-8.
Basavarajappa BS, Nagre NN, Xie S, Subbanna S (2014) Elevation of endogenous anandamide impairs LTP, learning, and memory through CB1 receptor signaling in mice. Hippocampus 24:808-818.
Bastian D, Tamburstuen MV, Lyngstadaas SP, Reikerås O (2008) Systemic and local cytokine kinetics after total hip replacement surgery.Eur Surg Res 41:334-340.
Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL (2007) Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318:1645-1647.
Bonin RP, Orser BA (2008) GABA(A) receptor subtypes underlying general anesthesia. Pharmacol Biochem Behav 90:105-112.
Breteler MM, van Duijn CM, Chandra V, Fratiglioni L, Graves AB,Heyman A, Jorm AF, Kokmen E, Kondo K, Mortimer JA, et al. (1991)Medical history and the risk of Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 20 Suppl 2:S36-42.
Cao L, Wang K, Gu T, Du B, Song J (2014) Association between APOE epsilon 4 allele and postoperative cognitive dysfunction: a meta-analysis. Int J Neurosci 124:478-485.
Carnini A, EckenhoffMF, EckenhoffRG (2006) Interactions of volatile anesthetics with neurodegenerative-disease-associated proteins. Anesthesiol Clin 24:381-405.
Cascella M, Muzio MR, Bimonte S, Cuomo A, Jakobsson JG (2017)Postoperative delirium and postoperative cognitive dysfunction: updates in pathophysiology, potential translational approaches to clinical practice and further research perspectives. Minerva Anestesiol doi: 10.23736/S0375-9393.17.12146-2.
Czeh M, Gressens P, Kaindl AM (2011) The yin and yang of microglia.Dev Neurosci 33:199-209.
EckenhoffRG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, EckenhoffMF (2004) Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology 101:703-709.
Evered L, Scott DA, Silbert B (2017) Cognitive decline associated with anesthesia and surgery in the elderly: does this contribute to dementia prevalence? Curr Opin Psychiatry 30:220-226.
Fodale V, Tripodi VF, Penna O, Fama F, Squadrito F, Mondello E,David A (2017) An update on anesthetics and impact on the brain.Expert Opin Drug Saf 16:997-1008.
Govindpani K, Calvo-Flores Guzmán B, Vinnakota C, Waldvogel HJ,Faull RL, Kwakowsky A (2017) Towards a better understanding of GABAergic remodeling in Alzheimer’s disease. Int J Mol Sci 18:E1813.
Hovens IB, Schoemaker RG, van der Zee EA, Heineman E, Nyakas C,van Leeuwen BL (2013) Surgery-induced behavioral changes in aged rats. Exp Gerontol 48:1204-1211.
Hovens IB, Schoemaker RG, van der Zee EA, Absalom AR, Heineman E, van Leeuwen BL (2014) Postoperative cognitive dysfunction: Involvement of neuroinflammation and neuronal functioning. Brain Behav Immun 38:202-210.
Hovens IB, van Leeuwen BL, Nyakas C, Heineman E, van der Zee EA,Schoemaker RG (2015) Postoperative cognitive dysfunction and microglial activation in associated brain regions in old rats. Neurobiol Learn Mem 118:74-79.
Hsing CH, Hung SK, Chen YC, Wei TS, Sun DP, Wang JJ, Yeh CH(2015) Histone deacetylase inhibitor trichostatin a ameliorated endotoxin-induced neuroinflammation and cognitive dysfunction.Mediators Inflamm 2015:163140.
Hu R, Huang D, Tong J, Liao Q, Hu Z, Ouyang W (2014) Aspartic acid in the hippocampus: a biomarker for postoperative cognitive dysfunction. Neural Regen Res 9:143-152.
Hudetz JA, Iqbal Z, Gandhi SD, Patterson KM, Byrne AJ, Hudetz AG,Pagel PS, Warltier DC (2009) Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol Scand 53:864-872.
Iglesias J, Morales L, Barreto GE (2017) Metabolic and inflammatory adaptation of reactive astrocytes: role of PPARs. Mol Neurobiol 54:2518-2538.
Jiang J, Jiang H (2015) Effect of the inhaled anesthetics isoflurane,sevoflurane and desflurane on the neuropathogenesis of Alzheimer’s disease (review). Mol Med Rep 12:3-12.
Jiang J, Dong Y, Huang W, Bao M (2017) General anesthesia exposure and risk of dementia: a meta-analysis of epidemiological studies.Oncotarget 8:59628-59637.
Koellhoffer EC, McCullough LD, Ritzel RM (2017) Old maids: aging and its impact on microglia function. Int J Mol Sci 18:E769.
Lemaire V, Tronel S, Montaron MF, Fabre A, Dugast E, Abrous DN(2012) Long-lasting plasticity of hippocampal adult-born neurons. J Neurosci 32:3101-3108.
Ling YZ, Ma W, Yu L, Zhang Y, Liang QS (2015) Decreased PSD95 expression in medial prefrontal cortex (mPFC) was associated with cognitive impairment induced by sevoflurane anesthesia. J Zhejiang Univ Sci B 16:763-771.
Ma XD, Li BP, Wang DL, Yang WS (2017) Postoperative benefits of dexmedetomidine combined with flurbiprofen axetil after thyroid surgery. Exp Ther Med 14:2148-2152.
Mardini F, Tang JX, Li JC, Arroliga MJ, Eckenhoff RG, Eckenhoff MF (2017) Effects of propofol and surgery on neuropathology and cognition in the 3xTgAD Alzheimer transgenic mouse model. Br J Anaesth 119:472-480.
Mufson EJ, He B, Nadeem M, Perez SE, Counts SE, Leurgans S, Fritz J,Lah J, Ginsberg SD, Wuu J, ScheffSW (2012) Hippocampal proNGF signaling pathways and beta-amyloid levels in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol 71:1018-1029.
Palanisamy A, Friese MB, Cotran E, Moller L, Boyd JD, Crosby G, Culley DJ (2016) Prolonged treatment with propofol transiently impairs proliferation but not survival of rat neural progenitor cells in vitro.PLoS One 11:e0158058.
Palotás M, Palotás A, Bjelik A, Pákáski M, Hugyecz M, Janka Z,Kálmán J (2005) Effect of general anesthetics on amyloid precursor protein and mRNA levels in the rat brain. Neurochem Res 30:1021-1026.
Papáčková Z, Cahová M (2014) Important role of autophagy in regulation of metabolic processes in health, disease and aging. Physiol Res 63:409-420.
Patel D, Lunn AD, Smith AD, Lehmann DJ, Dorrington KL (2016)Cognitive decline in the elderly after surgery and anaesthesia: results from the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Anaesthesia 71:1144-1152.
Peng S, Zhang Y, Sun DP, Zhang DX, Fang Q, Li GJ (2011) The effect of sevoflurane anesthesia on cognitive function and the expression of insulin-like growth factor-1 in CA1 region of hippocampus in old rats. Mol Biol Rep 38:1195-1199.
Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, Krishnamurthy P, Herman M, Wang L, Schachter JB, Nelson RB, Lau LF, DuffKE (2007) Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci 27:3090-3097.
Price CC, Tanner JJ, Schmalfuss I, Garvan CW, Gearen P, Dickey D,Heilman K, McDonagh DL, Libon DJ, Leonard C, Bowers D, Monk TG (2014) A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology 120:601-613.
Qiu LL, Luo D, Zhang H, Shi YS, Li YJ, Wu D, Chen J, Ji MH, Yang JJ(2016) Nox-2-mediated phenotype loss of hippocampal parvalbumin interneurons might contribute to postoperative cognitive decline in aging mice. Front Aging Neurosci 8:234.
Qu X, Xu C, Wang H, Xu J, Liu W, Wang Y, Jia X, Xie Z, Xu Z, Ji C,Wu A, Yue Y (2013) Hippocampal glutamate level and glutamate aspartate transporter (GLAST) are up-regulated in senior rat associated with isoflurane-induced spatial learning/memory impairment.Neurochem Res 38:59-73.
Ron-Harel N, Schwartz M (2009) Immune senescence and brain aging:can rejuvenation of immunity reverse memory loss? Trends Neurosci 32:367-375.
Satomoto M, Makita K (2016) Anesthesia-induced neurotoxicity in an animal model of the developing brain: mechanism and therapies.Neural Regen Res 11:1407-1408.
Suh HS, Choi S, Khattar P, Choi N, Lee SC (2010) Histone deacetylase inhibitors suppress the expression of inflammatory and innate immune response genes in human microglia and astrocytes. J Neuroimmune Pharmacol 5:521-532.
Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M,Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M (2011)Resolving postoperative neuroinflammation and cognitive decline.Ann Neurol 70:986-995.
Tracey KJ (2009) Reflex control of immunity. Nat Rev Immunol 9:418-428.
Vacas S, Degos V, Feng X, Maze M (2013) The neuroin-flammatory response of postoperative cognitive decline. Br Med Bull 106:161-178.
Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N,Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ (2012) Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149:708-721.
Wang B, Li S, Cao X, Dou X, Li J, Wang L, Wang M, Bi Y (2017a)Blood-brain barrier disruption leads to postoperative cognitive dysfunction. Curr Neurovasc Res doi: 10.2174/156720261466617100910 5825.
Wang L, Liu BJ, Cao Y, Xu WQ, Sun DS, Li MZ, Shi FX, Li M, Tian Q, Wang JZ, Zhou XW (2017b) Deletion of type-2 cannabinoid receptor induces Alzheimer’s disease-like tau pathology and memory impairment through AMPK/GSK3beta pathway. Mol Neurobiol doi:10.1007/s12035-017-0676-2.
Wang WY, Tan MS, Yu JT, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3:136.
Xie H, She GM, Wang C, Zhang LY, Liu CF (2015) The gender difference in effect of sevoflurane exposure on cognitive function and hippocampus neuronal apoptosis in rats. Eur Rev Med Pharmacol Sci 19:647-657.
Xiong WX, Zhou GX, Wang B, Xue ZG, Wang L, Sun HC, Ge SJ (2013)Impaired spatial learning and memory after sevoflurane-nitrous oxide anesthesia in aged rats is associated with down-regulated cAMP/CREB signaling. PLoS One 8:e79408.
Xu Z, Dong Y, Wu X, Zhang J, McAuliffe S, Pan C, Zhang Y, Ichinose F, Yue Y, Xie Z (2011) The potential dual effects of anesthetic isoflu-rane on Abeta-induced apoptosis. Curr Alzheimer Res 8:741-752.
Yang CW, Fuh JL (2015) Exposure to general anesthesia and the risk of dementia. J Pain Res 8:711-718.
Yang N, Li L, Li Z, Ni C, Cao Y, Liu T, Tian M, Chui D, Guo X (2017a)Protective effect of dapsone on cognitive impairment induced by propofol involves hippocampal autophagy. Neurosci Lett 649:85-92.Yang S, Gu C, Mandeville ET, Dong Y, Esposito E, Zhang Y, Yang G,Shen Y, Fu X, Lo EH, Xie Z (2017b) Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front Immunol 8:902.
Yu X, Liu S, Li J, Fan X, Chen Y, Bi X, Liu S, Deng X (2015) MicroRNA-572 improves early post-operative cognitive dysfunction by down-regulating neural cell adhesion molecule 1. PLoS One 10:e0118511.
Yu Y, Zhang Y (2013) Desflurane accelerates neuronal cytotoxicity of Abeta by downregulating miR-214. Neurosci Lett 554:28-33.
Zhang B, Dong Y, Zhang G, Moir RD, Xia W, Yue Y, Tian M, Culley DJ, Crosby G, Tanzi RE, Xie Z (2008) The inhalation anesthetic desflurane induces caspase activation and increases amyloid beta-protein levels under hypoxic conditions. J Biol Chem 283:11866-11875.
Zhang X, Zhou Y, Xu M, Chen G (2016) Autophagy is involved in the sevoflurane anesthesia-induced cognitive dysfunction of aged rats.PLoS One 11:e0153505.
Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z (2012) Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 71:687-698.
Zhang Z, Yuan H, Zhao H, Qi B, Li F, An L (2017) PPARgamma activation ameliorates postoperative cognitive decline probably through suppressing hippocampal neuroinflammation in aged mice. Int Immunopharmacol 43:53-61.
Zhen Y, Dong Y, Wu X, Xu Z, Lu Y, Zhang Y, Norton D, Tian M, Li S, Xie Z (2009) Nitrous oxide plus isoflurane induces apoptosis and increases beta-amyloid protein levels. Anesthesiology 111:741-752.
Zheng JW, Meng B, Li XY, Lu B, Wu GR, Chen JP (2017) NF-kappaB/P65 signaling pathway: a potential therapeutic target in postoperative cognitive dysfunction after sevoflurane anesthesia. Eur Rev Med Pharmacol Sci 21:394-407.
Zhu X, Hao X, Luo J, Min S, Xie F, Zhang F (2015) Propofol inhibits inflammatory cytokine-mediated glutamate uptake dysfunction to alleviate learning/memory impairment in depressed rats undergoing electroconvulsive shock. Brain Res 1595:101-109.
Zurek AA, Yu J, Wang DS, Haffey SC, Bridgwater EM, Penna A, Lecker I, Lei G, Chang T, Salter EW, Orser BA (2014) Sustained increase in alpha5GABAA receptor function impairs memory after anesthesia. J Clin Invest 124:5437-5441.
杂志排行
中国神经再生研究(英文版)的其它文章
- Design and criteria of electrospun fibrous scaffolds for the treatment of spinal cord injury
- Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogenactivated protein kinase and nuclear factor kappa B signaling pathways
- Taking out the garbage: cathepsin D and calcineurin in neurodegeneration
- MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury
- Interferon regulatory factor 2 binding protein 2: a new player of the innate immune response for stroke recovery
- Endogenous retinal neural stem cell reprogramming for neuronal regeneration
