乙醇生物标志物的研究及应用进展
2017-12-06冯雪伊
冯雪伊,沈 敏
(1.司法鉴定科学研究院 上海市法医学重点实验室 上海市司法鉴定专业技术服务平台,上海200063;2.上海市公安局 静安分局 刑侦支队刑事科学技术研究所,上海200070)
鉴定综述Review
乙醇生物标志物的研究及应用进展
冯雪伊1,2,沈 敏1
(1.司法鉴定科学研究院 上海市法医学重点实验室 上海市司法鉴定专业技术服务平台,上海200063;2.上海市公安局 静安分局 刑侦支队刑事科学技术研究所,上海200070)
酒精滥用已成为重大的公共卫生问题,是世界卫生组织公布的第三大致死致残元凶,因而在所涉交通事故、酗酒肇事、职业卫生、毒品滥用、毒物中毒等案(事)件中,体内乙醇检测及评价已成为法医毒物鉴定的重要任务。与乙醇相比,其体内生物标志物的检测表现出更多的优势,具有灵敏度高、特异性强、检测时限宽、鉴别能力强、应用领域广等特征。综述了乙醇的直接生物标志物乙基葡萄糖醛酸苷、硫酸乙酯和脂肪酸乙酯的研究及应用情况,以期推动法医毒物鉴定的进步,为诉讼及社会证明活动提供更加科学、可靠、有效的证据信息。
乙醇生物标志物;酒精滥用;乙基葡萄糖醛酸苷;硫酸乙酯;脂肪酸乙酯
酒精(乙醇)饮料的滥用是全世界广泛关注的公共卫生问题,过量饮酒不仅会造成个体生理机能损害,还会影响正常的行为判断能力从而带来社会、经济、法律等一系列相关问题[1]。中国人口基数大、酒文化源远流长,现代化的生活方式促进了酒精的需求和消耗,加之国家对酒管控政策相对薄弱,我国饮酒人数日益增长,为酒精相关问题的产生提供了环境条件。《柳叶刀》公布了中国人均饮酒量自1987年以来翻升三倍以上[2];2013年全球疾病负担报告显示,中国15岁至49岁人群中因饮酒导致的伤残调整寿命年损失数最大,造成年均12万人死亡[3]。常规的酒精检测限于乙醇清除速率快(10 mg/dL·h[4]),一次过量饮酒24 h后便难以检出,因而单凭血液乙醇浓度在某些情况下无法认定个体摄酒与否。与此同时,利用受试者的生物样本对乙醇生物标志物进行检测,可区分日常饮酒、酗酒、慢性酒精依赖以及外源性乙醇污染等情况;在死后毒物学领域,则可为揭示血液乙醇来源,判断生前饮酒和死后生成等提供辅助证据。
国内外专家学者将乙醇生物标志物分为两类:间接生物标志物和直接生物标志物。其中间接生物标志物是指一类能指示因酗酒引起的正常生理过程或病理过程的改变、抑或是影响药物反应的物质,常见的有丙氨酸转氨酶(Alanine aminotransferase,ALT)、天门冬氨酸转氨酶(Aspartate aminotransferase,AST)、平均红细胞体积(Mean Corpuscular Volume,MCV)、γ-谷氨酰转移酶(γ-glutamyl transferase,γ-GT)、糖缺乏性转铁蛋白(Carbohydrate-deficient transferring, CDT)、总血清唾液酸(Total serum sialic acid,TSA)等。由于此类标志物不是由乙醇代谢形成,而是由酒精性器官损伤而间接产生,仅能标志长期酗酒行为。在诊断酒精相关障碍方面的敏感性及特异性有限,还受性别、年龄以及非饮酒导致的共病障碍(酶水平升高)等影响,在实际使用中往往出现假阴性或假阳性等问题[5-7]。直接生物标志物为酒精的代谢产物,仅在有乙醇摄入的情况下存在,在体内停留时间长于乙醇,主要包括乙基葡萄糖醛酸苷(Ethyl glucuronide,EtG)、硫酸 乙 酯 (Ethyl sulfate,EtS)、脂肪酸乙酯(Fatty acid ethyl esters, FAEEs)和磷脂酰乙醇(Phosophatidylethanol,PEth)。 直接标志物不受疾病、生理因素影响,敏感性和特异性较间接标志物大大提高,能区分各类饮酒情况,包括少量饮酒行为,在戒瘾监测、法医学鉴定、驾驶能力评估、复职检测、子女监护权判定、肝脏移植评估、胎儿酒精综合症研究等领域均有所涉及,为预防、诊断、治疗相关酒精问题提供了全新的思路和方法[8-9]。本文将围绕常用于鉴别酒精滥用的直接生物标志物EtG、EtS和FAEEs的研究及应用进展,作一综述。
1 EtG和EtS
1.1 EtG和EtS的来源
酒精摄入人体后,大部分经氧化途径由肝脏代谢(90%~95%),或经肾脏、皮肤及呼吸道排出体外(5%)。仅少部分乙醇经非氧化途径转变成乙基葡萄糖醛酸苷(Ethyl glucuronide,EtG)(约 0.5%~1.6%)和硫酸乙酯(Ethyl sulfate,EtS)(约 0.1%)。 乙基葡萄糖醛酸在UDP-葡萄糖醛酸转移酶(UGT)的催化下与乙醇共轭生成EtG,胞质中的硫酸磺基转移酶催化硫酸与磷酸腺苷共轭生成EtS,乙醇的清除速率远大于EtG,EtG能够在乙醇无法检出的情况下成为酒精摄入的有力证据[10-11]。EtG和EtS均为高灵敏标志物,即使在低水平的乙醇暴露下同样能够检出[12]。
1.2 生物样品中EtG和EtS的研究现状
1.2.1 尿液分析
EtG主要经尿排除,占摄入酒精量的0.02%~0.06%,早在1995年Schmidt等首先在尿液中检测到 EtG;2004年,Helander等在人体尿液中测得EtS。人体摄入0.25 g/kg(乙醇摄入量/体重)酒精后,尿中EtG和EtS的检测时限可达24 h,当摄入量为0.5 g/kg时,检测时限可达48 h[13-15]。文献报道乙醇在人体内消除后3~5 d内仍能检出EtG,有利于延长酒精摄入的检测时限[16-17]。EtG和EtS的代谢形成途径不同,因此在认定近期酒精摄入的案件当中,同时检测尿中的EtG和EtS有助于提高分析方法的可靠性。Helander等[16]对一名健康男性饮酒者进行尿液分析,空腹单次饮酒0.5 g/kg后,乙醇在2 h出现峰值浓度,于8 h后难以检出。EtG与EtS的浓度-时间曲线的走势相似,EtS的浓度略高于EtG,两者均于摄酒后1 h检出,峰值浓度均出现在4 h。检测时限相近,饮酒后29 h仍能检出,32 h后无法检出。这与Wurst等[18]报道的结果有所差异,平均饮酒0.2 g/kg后,EtG和EtS的尿液检测时限为36 h,导致的原因可能包括个体饮酒量的差异、机体代谢能力的差异等因素。另外,Helander等还分析了54例临床酒精检测的尿样,EtG与EtS的浓度之间存在良好的相关性(r2=0.839;p<0.0001),平均浓度比(CEtG∶CEtS)约为 1.5。 Kummer等[19]对 27 例摄酒志愿者进行尿液分析,EtG和EtS浓度相关性高(r=0.996,p<0.001)。EtG和EtS均为乙醇的直接代谢产物,是尿液分析的主要检测对象,具有较高的检测灵敏度,尤其在样品收集延迟的情况下,乙醇因快速代谢而难以检出,EtG及EtS的检测分析就具有重要的证据价值。但是,较高的灵敏度容易导致假阳性结果,此时设定合理的检测界限值(阈值,cut-off)至关重要。目前国际上没有统一的尿液阈值标准,一般认为 0.1 μg/mL EtG、0.05 μg/mL EtS 能够有效排除反复饮酒情况[20];同时对禁酒者的尿液分析发现,0.1 μg/mL EtG[21]和 0.1 μg/mL EtS[22]可区分非饮酒和饮酒情况。
1.2.2 血液分析
EtG和EtS的血液分析窗口较短,低剂量酒精摄入下达10 h,高剂量重复摄入下可达24 h[23-25],血中EtS的检测时限为乙醇的两倍,而EtG的检测时限更长。Høiseth等[15]对10名空腹饮酒0.5 g/kg的志愿者进行血尿分析,血中EtG的峰值浓度时间在4 h,乙醇峰值浓度时间为0.5~2.0h;EtG的消除半衰期为2.2 h,使得EtG比乙醇的可检测时限更长,血中乙醇检出时限为5.0~7.0 h,EtG的检出时限可达10~14 h。尿液的EtG浓度水平明显高于血液,约为血中EtG浓度的145倍。Halter等[23]对13名适量饮酒的志愿者进行血尿分析,血中EtG和EtS的峰值浓度时间分别为4 h和3 h,与乙醇峰值浓度时间分别相差2.3 h和1.2 h;尿中EtG和EtS的峰值浓度时间分别为6.2 h和5.3 h。虽然尿液的检测窗口更长、浓度更高,然而尿液分析存在一定的不可控因素,如个体频繁饮水、频繁排尿等均会导致尿液中待分析物的浓度降低,造成假阴性结果。同时,血液中两种物质的检测时长通常为24 h,血液的短时效性更有利于认定个体近期饮酒甚至当下饮酒的情况。在Lostia等[25]的报道中,19位饮酒者分成两组,分别给与了不同的饮酒量0.76 g/kg和1.53 g/kg,EtG和EtS的血、尿峰值浓度时间随着剂量的提高均出现延后现象,而Høiseth等[26]对两种剂量0.5 g/kg和1.0 g/kg的酒精代谢物研究显示,高剂量组血液峰值浓度时间有所延后,尿液峰值浓度没有变化。此种剂量影响药代动力学因素应受到重视,有助于更客观、真实地反应浓度随时间变化趋势,从而进行相关计算和模型建立。相关文献报道的血、尿中EtG和EtS的药代数据见表1。
1.2.3 毛发分析
毛发作为一种反映长程信息的检材,检测窗口长(数月至数年),根据人体毛发的生长速度,能提供酒精摄入史、区分日常饮酒与酗酒等重要信息,弥补了血、尿分析的不足。EtG主要通过汗液侵蚀发干或通过血液扩散到生长细胞的途径进入毛发。EtG进入毛发以后能稳定存在,但由于其分子具有弱酸性(pKa=2.84),EtG以极小的速率进入至毛发,毛发中的EtG含量少,需要高灵敏的分析手段。近年来,毛发中EtG已成为判断酒精摄入与否的重要标志,高灵敏的分析技术不断涌现,检出限已从原来的20~50 pg/mg降低至0.7 pg/mg[27-28],然而要鉴别长期酗酒、日常饮酒以及非饮酒情况,还需标准化的界值来限定,从而防止假阴性或者假阳性结果的产生。国际毛发分析协会 (Society of Hair Testing,SoHT)将7pg/mg作为区分饮酒(乙醇摄入量>10g/d)和非饮酒的阈值,也是鉴别戒酒者是否复饮的一个重要界定指标;同时将30 pg/mg作为区分长期酗酒(乙醇摄入量>60 g/d)与日常饮酒的阈值[29]。此外,不同学者在结合文献报道及实验数据的基础上总结出了不同的临界值。如Kharbouche等[30]检测了125个受试者头发样本,设定9pg/mg为区分风险性饮酒(乙醇摄入量>20/30 g/d)与非饮酒的阈值,25 pg/mg为长期酗酒(乙醇摄入量>60 g/d)的阈值。Politi等[30]使用饮酒问卷调查表(Drinking Questionaire)进行数据分析后得出4 pg/mg和5 pg/mg分别作为鉴别饮酒量(乙醇摄入量>30 g/d及40 g/d)的阈值[31]。Stewart等[32]对200名肝病患者进行毛发分析,设定15 pg/mg作为饮酒量(乙醇摄入量>28 g/d)的阈值,分析结果准确度高,同时指出性别及疾病因素(如肝硬化)对毛发中的EtG含量检测有所影响[32]。最近有学者指出7 pg/mg作为区分禁酒与饮酒的阈值还有待进一步考察[33-34]。
1.2.4 指甲分析
指甲与毛发同为硬质角蛋白结构,能够稳定固化外源性物质,不受代谢及排泄影响,检测窗口长。与尿液检材相比,指甲与毛发更能提供丰富的饮酒史信息,有利于鉴别长期饮酒和短期酗酒等情况,同时在戒酒治疗监测、临床酒精检测等方面有所帮助。据Morini等[35]报道,毛发可提供的饮酒信息仅限于长期过量饮酒(15~20 g/d),无法甄别短期酗酒情况,而指甲中EtG的浓度远高于毛发,有利于区分不同的饮酒习惯,提供更多的酒精滥用信息。Berger等[36]对447名受试者进行EtG检测,毛发中的EtG含量为0~180.5 pg/mg,指甲中的含量为0~397.08 pg/mg;Berger指出指甲中的EtG的定量分析能够区分不同程度的风险饮酒情况,对鉴别高风险饮酒及持续性风险饮酒人群的准确度分别为100%和82%~86%,高于毛发。
1.2.5 其他生物检材
非传统生物样品如胚胎组织、胎粪、玻璃体液等也是EtG和EtS的重要检材。孕期酒精暴露会导致胎儿酒精谱系障碍(FAS),引起新生儿严重的健康问题如颅面畸形、生长阻滞、认知以及社交障碍等,因此在认定孕期饮酒的证据信息显得尤为重要。当新生儿出现FAS症状,孕妇较多隐瞒饮酒史而耽误治疗,往往需要通过胚胎组织进行检测。Goecke等[37]对577名妊娠后女性的胎粪进行乙醇标志物检测,发现仅有对EtG的分析符合饮酒史的记录,同时设定120 ng/g(EtG/胎粪)为孕期饮酒的标志,具有较高的准确度。Morini等[38]分析了70份早期胎盘和胚胎组织(各35份)中的EtG和EtS,两者的检测浓度较高,且两者在胎盘中的浓度均高于胚胎组织,证实EtG与EtS均能够穿透胎盘屏障进入胚胎组织,强调EtG和EtS的来源不仅限于胎儿代谢产物更包括母体代谢产物,为孕期饮酒提供有力证据。
玻璃体液中的EtG和EtS也是酒精分析的重要内容,是死后乙醇检测的主要手段。死后生物检材中的乙醇浓度受多种因素影响而发生改变,如乙醇的生物转换导致的浓度下降、血液腐败以及上消化道扩散导致的乙醇浓度升高等,成为衡量死前酒精摄入的主要难题。眼睛的解剖学结构决定其具有减速扩散的机能,因此相对于其他生物检材,外源性物质进入玻璃体液能够稳定存在,较少受尸体腐败影响[39]。Thierauf等[40]分析了人死后血液、尿液以及玻璃体液中的乙醇、EtG和EtS,发现血、尿中均存在乙醇、EtG和EtS,玻璃体液中只存在EtG和EtS,且两者在玻璃体液中的浓度水平较高,表明EtG和EtS系死者生前饮酒后代谢生成并进入玻璃体液,证实了死者在生前有饮酒情况。
1.3 EtG和EtS的分析方法
EtG和EtS均为极性小分子,pKa分别为2.84和-3.14,因而血、尿分析无法有效适用于液液提取(LLE)或固相萃取(SPE);尿液直接稀释进样法容易掺杂大量杂质、引起基底效应偏高,同时会对仪器产生伤害;沉淀蛋白法是EtG和EtS的主要前处理方法[23,41],沉淀蛋白后上清液用液相色谱-串联质谱(LC-MS/MS)分析后没有明显的基质干扰[42]。Freire等[43]采用微波辅助提取技术(MAE)处理尿液样品中的EtG,操作简便快速,大大缩短提取时间、节省溶剂消耗的同时保证较高的回收效率,但是该方法采用了气相色谱-质谱(GC-MS)进行分析,样品需要衍生化处理且方法灵敏度较低(5 ng/mL)。MAE同样适用于毛发样品处理[44],其他常见处理方法主要为 SPE[27,45-46]、超声提取法[47-49]以及两者的结合使用[27,50-52],固相萃取柱主要有氨基柱、OASIS MAX、Clean Screen(EtG Carbon);顶空-固相微萃取法(HSSPME)也是毛发处理的重要方法,常与气相色谱-串联质谱法(GC-MS/MS)联用[53-55]。 指甲、胚胎组织、胎粪以及玻璃体液的检材处理方法则主要集中于沉淀蛋白[38,40]、超声提取[35,56]、SPE[57-58]等。
生物检材中EtG和EtS的分析手段主要为GC-MS[43-44,59-60]、LC-MS[13,61],后期更多采用 LC-MS/MS[19,22,35,40,58,62-64],避免气相色谱的衍生化处理,同时提高了分析方法的灵敏度。Cappelle等[65]比较了GC-MS和GC-MS/MS两种方法分析毛发中的EtG,检出限(LOD)和定量限(LOQ):GC-MS/MS 为0.005 pg/mg和 0.017pg/mg;GC-MS为 0.022pg/mg和0.075pg/mg。Delphine等[66]建立了GC-MS/MS法分析毛发中的EtG,并与GC-MS法比较,两者定量结果偏差较大,在区分非饮酒和适量饮酒人群(阈值7 pg/mg)时,GC-MS/MS方法灵敏度更高。LC-MS/MS在分析血液中的EtG时,其灵敏度与GC-MS/MS相比没有较大差别,然而其无需衍生化处理,且定量精密度更高[64,67]。
LC-MS/MS分析多数采用反相色谱/负离子电喷雾模式(ESI)监测,由于EtG和EtS极性较大,只有在高水相比例下才能获得较好的保留,然而在电离过程中,小分子分析物向气相转移需要在高有机相比例的协助下带电,水相比例较多不利于电离的促进,同时增加了基质干扰的可能[18,63]。许多文献报道柱后添加有机溶剂有利于促进电离进程,提高分析灵敏度[27,35,40,56,68-69],2-丙醇、乙腈为主要的柱后添加溶剂。为避免电喷雾离子源对分析灵敏度的影响、同时简化分析步骤,有文献报道采用大气压化学电离源(APCI)作为ESI的替代源,在分析水性组分时表现出较好的响应效果,获得更高的检测灵敏度,不受离子抑制效应的影响[22,70]。此外,负化学电离源(NCI)常用于GC-MS/MS,替代了之前的电子轰击源(EI),能大大提高毛发中EtG的检测灵敏度[52,55]。
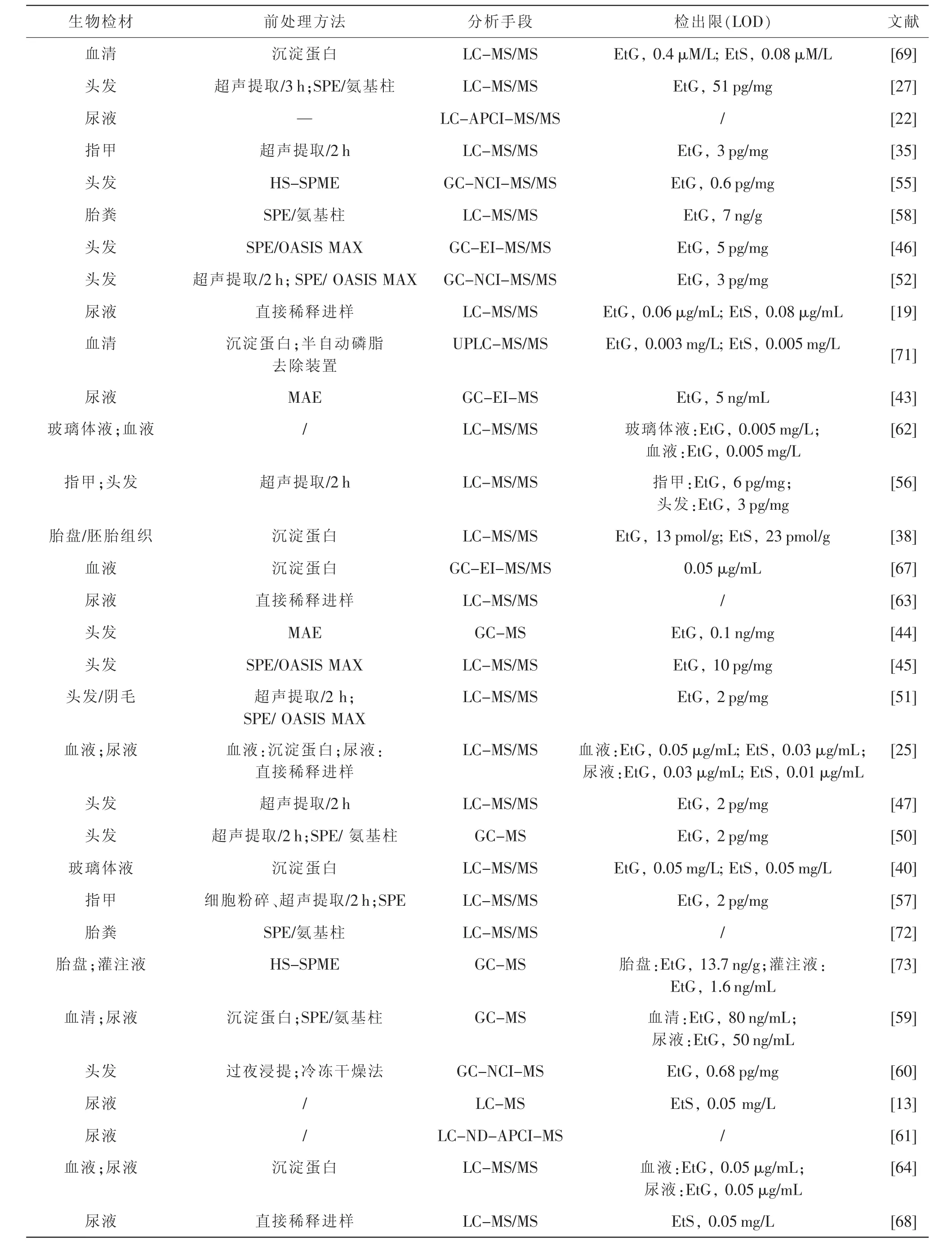
表3 文献报道不同生物检材中EtG和EtS的分析方法
2 FAEEs
2.1 FAEEs的形成过程
FAEEs是乙醇经酶促作用与体内的内源性脂肪酸、甘油三脂、脂蛋白、磷脂发生酯化反应而形成的一系列脂肪酸乙酯(图1)。FAEEs由20多种化合物组成,包括月桂酸乙酯(E12)、肉豆蔻酸乙酯(E14)、棕榈酸乙酯(E16)、棕榈油酸乙酯(E16:1)、硬脂酸乙酯(E18)、油酸乙酯(E18:1)、亚油酸乙酯(E18:2)、亚麻酸乙酯(E18:3)、花生四烯酸乙酯(E20:4)、二十二碳六烯酸(E22:6)等等。这些均为亲脂性化合物,能够穿透皮脂腺进而分布于全身。
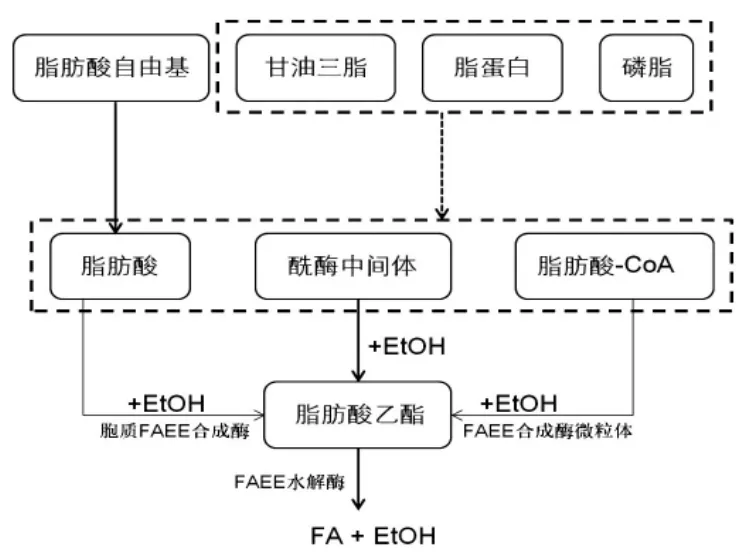
图1 FAEE的形成及水解过程
2.2 生物样品中FAEEs的研究现状
2.2.1 毛发分析
2001年Pragst等[74]首次在毛发中检出四种FAEE(E14、E16、E18、E18:1),说明了 FAEE 在证明酒精摄入方面的检测可行性。毛发中的黑色素呈弱酸性,易与呈碱性的物质或阳离子结合,FAEE为中性、弱极性物质,进入毛发后能够稳定存在,不受发色影响,因而毛发中的FAEE定性定量分析结果较血、尿中FAEE的检测更为普遍化。但是,由于个体在日常生理代谢过程中会产生副产物乙醇,因而在严格禁酒的情况下,个体毛发中也会检出FAEE等假阳性情况[75-76]。因此,设定合理的判断阈值对于正确区分禁酒与饮酒的情况至关重要。2009年,SoHT将四种 FAEEs(E14、E16、E18、E18:1)的总浓度 CFAEEs 定为区分不同饮酒情况的标准:当CFAEEs≥1ng/mg,表明酗酒;CFAEEs≤0.8ng/mg,表明适量饮酒;CFAEEs≤0.4 ng/mg,表明禁酒。 2012年,SoHT发布了最新的戒酒阈值标准,贴根0~3cm头发中FAEE>0.2ng/mg或贴根0~6 cm头发中FAEE>0.4 ng/mg则表明有酒精复饮情况。文献报道许多学者对于毛发中FAEE的界定标准考虑了毛发长度的因素。Elisabetta等[77]、Pragst等[78]均采用了 0.5 ng/mg FAEE(贴根 0~3 cm)作为区分日常饮酒和酗酒的标准,Elisabetta等的分析特异性高达87%;Süsse等[79]则采用了界定值 1.0 ng/mg FAEE(贴根 3~6 cm)。根据毛发的不同长度,分段设定检测阈值,综合分析,提供更加全面可靠的酒精滥用信息。
2.2.2 胎粪分析
目前大多数文献中,E16、E18:1这两种物质被认为是毛发中FAEEs检测的主要目标物,能够较好地认定酒精摄入行为[79-80]。在胎粪检测中,E18:2的检测浓度较高,是孕期酒精暴露的重要标志物,而E12、E14由于在非饮酒产妇中检出比率较大,因此不将其纳入检测目标物之列[81-83]。其他学者提出E18:2无法作为FAEEs检测的主要目标物,如Chan等[84]发现 80%的胎粪样品中无法检出 E18、E18:1、E18:2;Algar等[85]发现 E18:1、E16 浓度水平较高。 文献报道关于何种FAEE作为胎儿酒精暴露的主要标志物不尽相同[86-89],出现差异的原因可能与孕妇饮食习惯有关。因此后期有学者提出,胎粪中FAEEs的 界 定 值 以 七 种 FAEE (E16、E16:1、E18、E18:1、E18:2、E18:3、E20:4) 总 量 为 计 ,Hutson 等[90]设 定50 ng/g、Chan 等[91]设定 2 nmol/g(约 600 ng/g)来区分孕期酒精暴露与否的情况。其他学者则根据具体样品设定不同FAEE总量的限定值。Bakdash等[81]以四种 FAEE(E16、 E18、 E18:1、 E18:2)的总量 500 ng/g为摄酒判断阈值;Moore等[92]采用六种FAEE(E16、E16:1、E18、E18:1、 E18:2、 E20:4)的总量 10,000 ng/g为孕期饮酒的判断阈值。
2.3 FAEEs的分析方法
FAEE为弱极性的酯类化合物,在碱性条件下易水解而难以稳定存在。经典的FAEE提取方法主要为LLE和SPE,常见的提取溶剂为非极性或弱极性溶剂如丙酮、正己烷、二氯甲烷等[92-96],萃取柱主要为 氨 基 柱 、CUNAX 153;HS-SPME[90,97-100]以及MAE[101-103]也是毛发、胎粪以及表皮组织中FAEE的常见处理方法。FAEEs具有相对较高的挥发性,采用HS-SPME提取FAEEs时,为了防止其在高温下发生水解,调节缓冲溶剂至中性,使FAEE保持分子的稳定状态,同时加入少量NaCl,通过盐化处理促进气化进程;另外,毛发脂质层中含有游离的羧酸基团,调节缓冲液至中性有利于羧酸离解,使其呈离子状态而不利于气化。
FAEE适用于GC分析,文献报道采用氢火焰离子化检测器(FID)具有较高的灵敏性和特异性[104-105]。采用 GC-MS 可提高分析灵敏度[95,97,101,106],Zimmermann等[107]使用GC-MS/MS将毛发中FAEEs的检测限降低至 0.002~0.030 ng/mg,成功应用于区分酗酒、适量饮酒以及非饮酒情况。LC-MS/MS 分析[58,85,94,108]采用正离子电喷雾模式监测,提高分析灵敏度的同时,与其他酒精标志物EtG、EtS等同时检测能提高鉴别酒精摄入的可靠性。
3 实际案例及应用
毛发检测是鉴别酗酒和戒酒的重要手段,其检测结果关系到认定酒精滥用与否,从而影响到离婚诉讼、儿童监护权等法律问题。2009年伦敦法院开庭审理了一起离婚案件,当事人是一位母亲,2008年11月前有长期酗酒史,而后进行戒酒治疗。在争夺儿子抚养权方面,需要证明成功禁酒至少一年,没有复饮情况[109]。2009年9月收集该当事人的贴根头发进行GC-MS/MS分析,结果显示,贴根0~1 cm毛发中检出22pg/mgEtG(10 pg/mg为区分饮酒与禁酒阈值)。该当事人否认复饮情况,随即于2009年10月、2010年2月、5月和7月进行后续分析,结果显示在 0~3cm 毛发中 EtG 含量小于 2.4~3.3 pg/mg。前后不一致的分析报告显示毛发分析结果应经反复确证,并与其他生物检材或是生物标志物联合分析、相互印证,同时尤其要注意受检者是否曾使用过含酒精成分的头发美容或清洗用品,以排除假阳性结果的产生。案例中当事人曾经在此戒酒期间服用过治疗哮喘的药物(Clenil Modulite 100),该药含有9%乙醇,相当于日常摄入36 mg/天的酒精;同时使用过含气泡的洗浴用水,可能含有酒精成分。这类影响因素均未被列入考量范围。
毛发分析的溯源性同样适用于肝脏移植前的戒酒检测。原位肝移植的候选人要求在移植手术前有6个月的连续禁酒期,接受肝移植手术的病人通常患有肝硬化,因而此类病人在术前禁止有任何饮酒行为。Sterneck等[110]对63名患酒精性肝硬化的肝移植病人以及25名非酒精性肝硬化病人进行毛发、尿液和血液分析。分析前,仅有19名病人承认在半年内喝过酒,而检测结果表明有39名病人曾有饮酒行为发生。其中,18名病人的头发中检出EtG(30 pg/mg为区分饮酒与禁酒阈值);对于44名先前否认饮酒的病人,23名的头发、尿液、或血液中至少检出一项酒精相关标志物;9名先前否认饮酒的病人,头发中均检出EtG;25名非酒精性肝硬化病人的头发中未检出EtG;毛发分析特异性达98%,阳性预测率为92%。Sterneck等研究结果表明毛发中EtG的分析对术前饮酒行为的鉴别具有较高的特异性和可靠性,同时毛发的分段分析法可作为认定长期饮酒的一项重要分析手段。
在国外,酒精滥用的认定影响驾驶执照的重新授予。比利时驾驶执照重新授予法规定,驾驶员因酒驾被吊销驾驶执照的,应当在重新授予前持续有6个月的禁酒期。Kummer等[111]分析了50例待重新授予驾照人员的生物样本,联合多样化的分析手段,分别对其毛发中的EtG、尿液中的EtG、EtS、干血点中的PEth进行检测,同时对血液中的间接生物学标志物 CDT、γ-GT、ALT/AST、MCV 进行检测。 根据不同的检材特性,尿液分析提供了近期饮酒信息,26例尿样检出EtG或EtS,表明受试者在取样前几天有摄酒情况。29例干血点样品检出PEth16∶0/18:1,表明受试者近一个月内违反了禁酒规定。24例毛发样品中,在贴根0~6 cm段内检出 EtG(>9 pg/mg为复饮阈值;>38 pg/mg为酗酒阈值),表明受试者在近6个月内有过复饮情况;此外毛发检测窗口(6个月)完整覆盖法定规定的禁酒期限,可提供充足的戒酒依从性信息。结合间接生物学标志物的检测结果,共10名受试者超过所有检测项目的正常值,认定其在6个月内有饮酒情况。
许多性侵案件常与醉酒事件有关,酒精与其他安眠类药物被用作性侵辅助性工具,被害者往往在案发时失去思维意识、没有反抗能力,报案后通常需要鉴定其摄酒或摄药程度以排除被害者自愿的可能性。在这种特定的情形下,选择适合的检材至关重要。尿液分析的检测窗口长达几天时间,往往尿中检出EtG、EtS无法排除案发前几天有摄酒的情况。血液分析的检测时限在24 h内,且血药浓度能客观反映个体饮酒水平,因而血液中检出EtG、EtS通常能够认定受检者在24 h内有摄酒情况。Hegstad等[71]对49名自称遭受酒后性侵的女性进行血液分析,血液中的EtG和EtS含量均高于乙醇本身,乙醇无法检出时,EtG和EtS能检出,检测时限大于24 h。乙醇浓度与EtG和EtS的浓度具有相关性(P<0.001),EtG 和 EtS 的浓度和清除时间也具有一定的相关性(P<0.001)。
在尸检调查中,死后生物检材的分析往往涉及检材腐败,腐败产生的干扰物容易导致假阳性结果,如血液腐败会产生内源性乙醇,对于死前饮酒的鉴别带来一定的干扰。乙醇的直接标记物EtG和EtS在人体死亡后,无法由机体代谢形成,可以用来鉴别死前饮酒与否的情况。Høiseth等[112]对36例怀疑摄酒致死的尸体进行血、尿分析,其中19例血、尿样中的EtG和EtS为阳性,16例为阴性。说明19名死者生前有饮酒倾向,而另外16名生前的饮酒概率较小。仅有一例的尿样中检出EtG和EtS,血样中只检出EtS,EtG无法检出,其原因可能是个体死亡后,部分EtG因血液腐败而降解所致。Vezzoli等[62]分析了63例尸体的静脉血和玻璃体液,对其中的EtG进行LC-MS/MS分析,并对乙醇、以及尸体腐败标记物(乙醛、丙醇)进行HS-GC分析。结果表明,17例血样和玻璃体液中,EtG和EtS均无检出,表明死者生前未曾饮酒;19例血样中检出乙醇和EtG,浓度范围分别为 0.05~0.30 g/L 和 0.02~3.27 mg/L;玻璃体液中检出 EtG,浓度范围 0.01~2.88 mg/L,乙醛和丙醇均未检出,表明死者生前有饮酒经历;13例血样中检出乙醇,浓度大于0.05 g/L,而血液与玻璃体液中的EtG浓度<0.01 mg/L,其中8例检材中同时检出乙醛及丙醇,表明死者生前饮酒概率较小,体内乙醇可能由组织腐败产生。
在实际检测中时常会出现非饮酒性酒精摄入的情况,含酒精的洗手液、漱口水会导致尿液EtG和EtS假阳性[113-114];含酒精的美发用品(洗发水、生发液等)会造成毛发EtG和EtS假阳性[115],另值得注意的是,烫发、美发和频繁洗头等美发过程则会造成EtG、EtS的浸出和分解,导致其浓度损耗而出现假阴性结果[116]。因此,在检测值处于阈值附近时,应当充分考虑非饮酒性酒精摄入的干扰因素,尤其当受试者否认饮酒时,需结合病史,进行多标志物联合分析,防止产生假阳性情况。
4 小结
生物检材中EtG、EtS和FAEEs可作为人体摄入乙醇的直接生物标志物,其分析手段不断完善,检测灵敏度和特异性较高,能够鉴别少量饮酒情况。毛发分析在区分饮酒与禁酒、酗酒与日常饮酒以及鉴别戒酒后复饮等方面发挥了重要的界定作用;尿液及血液分析在近期饮酒行为认定方面能提供充足证据;其他非传统生物检材在鉴别特殊饮酒情况如死前饮酒、孕期饮酒等方面具有无可替代的证据作用。EtG、EtS和FAEEs的分析与检测已广泛应用于职业卫生、法医鉴定、考证测试、临床监测、戒瘾治疗等领域。随着酒精滥用的危害不断提升,酗酒认定具有重要的现实意义,也对酒精滥用的分析提出更大的挑战。在实际应用过程中应当结合具体案情界定阈值;必要时联合多种乙醇生物标志物(如直接标志物与间接标志物 γ-GT、CDT、MCV、ALT、AST等)以及病史进行多角度分析,同时应注意排除非饮酒性酒精摄入的干扰因素(如含酒精的美发产品、卫生用品等),为酗酒行为认定和涉酒案(事)件的处置提供更加科学、可靠、有效的证据信息。
[1]World Health Organization (WHO).Global Status Report on Alcohol and Health 2014[EB/OL].(2014-05-12)[2017-04-12].http://www.who.int/substance_abuse/publications/global_alcohol_report/en/
[2]Collin J, Casswell S.Alcohol and the Sustainable Development Goals[J].Lancet, 2016,24(1):2582-2583.
[3]Institute for Health Metrics and Evaluation.Global Burden of Disease Study 2013:GBD Comparison Results 1990–2013[EB/OL].(2016-07-07)[2017-04-17]http://vizhub.healthdata.org/gbd-compare/
[4]郭景元.实用法医学[M].上海科学技术出版社,1980.
[5]李民,王延伟,吕永强,等.酗酒标志物研究进展[J].临床军医杂志,2014,42(12):1301-1303.
[6]Conigrave KM, Davies P, Haber P, et al.Traditional Markers of Excessive Alcohol Use[J].Addiction, 2003,98(2):31-43.
[7]Rinck D,Frieling H,Freitag A,et al.Combinations of Carbohydrate-deficient Transferrin,Mean Corpuscular Erythrocyte Volume, Gamma-glutamyltransferase, Homocysteine and Folate Increase the Significance of Biological Markers in Alcohol Dependent Patients[J].Drug&Alcohol Dependence, 2007,89(1):60-65.
[8]Pragst F, Balikova MA.State of the Art in Hair Analysis for Detection of Drug and Alcohol Abuse[J].Clinica Chimica Acta, 2006,370:17-49.
[9]Thon N,Weinmann W,Yegles M,et al.Direct Metabolites of Ethanol as Biological Markers of?Alcohol Use:Basic Aspects and Applications[J].Fortschr Neurol Psychiatr,2013, 81(9):493-502.
[10]Schneider H,Glatt H.Sulpho-conjugation of Ethanol in Humans in Vivo and by Individual Sulphotransferase Forms in Vitro[J].Biochemical Journal, 2004,383(3):543-549.
[11]Foti RS, Fisher MB.Assessment of UDP-glucuronosyltransferase Catalyzed Formation of Ethyl Glucuronide in Human Liver Microsomes and Recombinant UGTs[J].Forensic Science International, 2005,153(2-3):109-116.
[12]Thierauf A,Halter CC,Rana S,et al.Urine Tested Positive for Ethyl Glucuronide after Trace Amounts of Ethanol[J].Addiction, 2009, 104 (12):2007-2012.
[13]Helander A, Beck O.Ethyl Sulfate:A Metabolite of Ethanol in Humans and a Potential Biomarker of Acute Alcohol Intake[J].Journal of Analytical Toxicology, 2005,29(5):270-274.
[14]Wojcik MH, Hawthorne JS.Sensitivity of Commercial Ethyl Glucuronide(ETG)Testing in Screening for Alcohol Abstinence[J].Alcohol Alcohol, 2007,42(4):317-320.
[15]Høiseth G,Bernard JP,Karinen R,et al.A Pharmacokinetic Study of Ethyl Glucuronide in Blood and Urine:Applications to Forensic Toxicology[J].Forensic Science International, 2007,172(2):119-124.
[16]Helander A,Beck O.Mass Spectrometric Identification of Ethyl Sulfate as an Ethanol Metabolite in Humans[J].Clinical Chemistry, 2004,50(5):936-937.
[17]Martin WF,Skipper GE,Wolfgang W.Ethyl Glucuronide—The Direct Ethanol Metabolite on the Threshold from Science to Routine Use[J].Addiction, 2003,98(2):51-61.
[18]Wurst FM, Dresen S, Allen JP, et al.Ethyl Sulphate:A Direct Ethanol Metabolite Reflecting Recent Alcohol Consumption[J].Addiction, 2006,101(2):204-211.
[19]Kummer N, Wille S, Di FV, et al.A Fully Validated Method for the Quantification of Ethyl Glucuronide and Ethyl Sulphate in Urine by UPLC-ESI-MS/MS Applied in a Prospective Alcohol Self-monitoring Study[J].Journal of Chromatography B, 2013,929(7):149-154.
[20]Albermann ME,Musshoff F,DoberentzO E,et al.Preliminary Investigations on Ethyl Glucuronide and Ethyl Sulfate Cutoffs for Detecting Alcohol Consumption on the Basis of an Ingestion Experiment and on Data from Withdrawal Treatment[J].International Journal of Legal Medicine,2012,126(5):757-764.
[21]Janda I, Alt A.Improvement of Ethyl Glucuronide Determination in Human Urine and Serum Samples by Solidphase Extraction[J].Journal of Chromatography B Biomedical Sciences&Applications, 2001,758(2):229-234.
[22]Beyer J, Tu NV, Gerostamoulos D, et al.Validated Method for the Determination of Ethylglucuronide and Ethylsulfate in Human Urine[J].Analytical&Bioanalytical Chemistry, 2011,400(1):189-196.
[23]Halter CC, Dresen S, Auwaerter V, et al.Kinetics in Serum and Urinary Excretion of Ethyl Sulfate and Ethyl Glucuronide after Medium Dose Ethanol Intake[J].International Journal of Legal Medicine, 2008,122(2):123-128.
[24]Høiseth G,Morini L,Polettini A,et al.Blood Kinetics of Ethyl Glucuronide and Ethyl Sulphate in Heavy Drinkers during Alcohol Detoxification[J].Forensic Science International, 2009,188(1):52-56.
[25]Lostia AM,Vicente JL,Cowan DA.Measurement of Ethyl Glucuronide,Ethyl Sulphate and Their Ratio in the Urine and Serum of Healthy Volunteers after Two Doses of Alcohol[J].Alcohol&Alcoholism, 2013,48(1):74-82.
[26]Høiseth G, Yttredal B, Karinen R, et al.Ethyl Glucuronide Concentrations in Oral Fluid, Blood, and Urine after Volunteers Drank 0.5 and 1.0 g/kg Doses of Ethanol[J].Journal of Analytical Toxicology, 2010,34(6):319-324.
[27]Janda I,Weinmann W,Kuehnle T,et al.Determination of Ethyl Glucuronide in Human Hair by SPE and LC-MS/MS[J].Forensic Science International, 2002,128(1-2):59-65.
[28]Kerekes I,Yegles M,Grimm U,et al.Ethyl Glucuronide Determination:Head Hair Versus Non-head Hair[J].Alcohol&Alcoholism, 2009,44 (1):62-66.
[29]Kintz P.2014 Consensus for the Use of Alcohol Markers in Hair for Assessment of Both Abstinence and Chronic Excessive Alcohol Consumption[J].Forensic Science International, 2015,249:A1-A2.
[30]Kharbouche H,Faouzi M,Sanchez N,et al.Diagnostic Performance of Ethyl Glucuronide in Hair for the Investigation of Alcohol drinking Behavior:A Comparison with Traditional Biomarkers[J].International Journal of Legal Medicine, 2012,126(2):243-250.
[31]Politi L, Morini, L., Leone, F., Polettini, A.Ethyl Glucuronide in Hair:Is It A Reliable Marker of Chronic High Levels of Alcohol Consumption?[J].Addiction, 2006,101:1408-1412.
[32]Stewart SH,Koch DG,Willner IR,et al.Hair Ethyl Glu-curonide is Highly Sensitive and Specific for Detecting Moderate-to-Heavy Drinking in Patients with Liver Disease[J].Alcohol&Alcoholism, 2013,48(1):83-87.
[33]Crunelle C L,Yegles M,De D M,et al.Hair Ethyl Glucuronide Concentrations in Teetotalers:Should We Reevaluate the Lower Cut-off[J].Forensic Science International, 2017,274:107-108.
[34]Crunelle C L,Cappelle D,Yegles M,et al.Ethyl Glucuronide Concentrations in Hair:A Controlled Alcoholdosing Study in Healthy Volunteers[J].Analytical&Bioanalytical Chemistry, 2015,408(8):2019-2025.
[35]Morini L,Colucci M,Ruberto M G,et al.Determination of Ethyl Glucuronide in Nails by Liquid Chromatography Tandem Mass Spectrometry as A Potential New Biomarker for Chronic Alcohol Abuse and Binge Drinking Behavior[J].Analytical&Bioanalytical Chemistry, 2012,402 (5):1865-1870.
[36]Berger L,Fendrich M,Jones J,et al.Ethyl Glucuronide in Hair and Fingernails as A Long-term Alcohol Biomarker[J].Addiction, 2014,109(3):425-431.
[37]Goecke T W,Burger P,Fasching P A,et al.Meconium Indicators of Maternal Alcohol Abuse during Pregnancy and Association with Patient Characteristics[J].Biomed Research International, 2014,2014(1):7028-7048.
[38]Morini L,Falc N M,Pichini S,et al.Ethyl-glucuronide and Ethyl-sulfate in Placental and Fetal Tissues by Liquid Chromatography Coupled with Tandem Mass Spectrometry[J].Analytical Biochemistry, 2011,418(1):30-36.
[39]Thierauf A, Musshoff F, Madea B.Post-mortem Biochemical Investigations of Vitreous Humor[J].Forensic Science International, 2009,192(1-3):78-82.
[40]Thierauf A,Kempf J,Perdekamp M G,et al.Ethyl Sulphate and Ethyl Glucuronide in Vitreous Humor as Postmortem Evidence Marker for Ethanol Consumption prior to Death[J].Forensic Science International, 2011,210(1):63-68.
[41]Musshoff F, Albermann E, Madea B.Ethyl Glucuronide and Ethyl Sulfate in Urine after Consumption of Various Beverages and Foods--misleading Results?[J].International Journal of Legal Medicine, 2010,124(6):623-630.
[42]Al-Asmari A I, Anderson R A, Appelblad P.Direct Determination of Ethyl Glucuronide and Ethyl Sulfate in Postmortem Urine Specimens Using Hydrophilic Interaction Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry[J].Journal of Analytical Toxicology,2010,34(5):261-272.
[43]Freire Iá, Barrera AMB, Silva PC, et al.Microwave Assisted Extraction for the Determination of Ethyl Glucuronide in Urine by Gas Chromatography-Mass Spectrometry[J].Journal of Applied Toxicology, 2008,28(6):773-778.
[44]álvarez I, Bermejo AM, Tabernero MJ, et al.Microwaveassisted Extraction:A Simpler and Faster Method for the Determination of Ethyl Glucuronide in Hair by Gas Chromatography–Mass Spectrometry[J].Analytical&Bioanalytical Chemistry, 2009,393(4):1345-1350.
[45]Cabarcos P,Hassan HM,Tabernero MJ,et al.Analysis of Ethyl Glucuronide in Hair Samples by Liquid Chromatography-Elecrospray Ionization–Tandem Mass Spectrometry (LC-ESI-MS/MS)[J].Journal of Applied Toxicology Jat, 2013,33(7):638-643.
[46]Paul R, Tsanaclis L, Kingston R, et al.Simultaneous Determination of GHB and EtG in Hair Using GCMS/MS[J].Drug Testing&Analysis, 2011,3(4):201-205.
[47]Morini L, Politi L, Groppi A, et al.Determination of Ethyl Glucuronide in Hair Samples by Liquid Chromatography/Electrospray Tandem Mass Spectrometry.J Mass Spectrom[J].Journal of Mass Spectrometry, 2010,41(1):34-42.
[48]Politi L,Zucchella A,Morini L,et al.Markers of Chronic Alcohol Use in Hair:Comparison of Ethyl Glucuronide and Cocaethylene in Cocaine Users[J].Forensic Science International, 2007,172(1):23-27.
[49]Albermann ME,Musshoff F,Aengenheister L,et al.Investigations on the Influence of Different Grinding Procedures on Measured Ethyl Glucuronide Concentrations in Hair Determined with An Optimized and Validated LCMS/MS Method[J].Analytical&Bioanalytical Chemistry,2012,403(3):769-776.
[50]Appenzeller BMR,Agirman R,Neuberg P,et al.Segmental Determination of Ethyl Glucuronide in Hair:A Pilot Study[J].Forensic Science International, 2007,173 (2):87-92.
[51]Kintz P, Villain M, Vallet E, et al.Ethyl Glucuronide:Unusual Distribution Between Head Hair and Pubic Hair[J].Forensic Science International, 2008,176(1):87-90.
[52]Kharbouche H,Sporkert F,Troxler S,et al.Development and Validation of A Gas Chromatography-Negative Chemical Ionization Tandem Mass Spectrometry Method for the Determination of Ethyl Glucuronide in Hair and Its Application to Forensic Toxicology[J].Journal of Chromatography B, 2009,877(23):2337-2343.
[53]Agius R,Ferreira LM,Yegles M.Can Ethyl Glucuronide in Hair be Determined Only in 3 cm Hair Strands?[J].Forensic Science International, 2012,218(1-3):3-9.
[54]Agius R,Nadulski T,Kahl H G,et al.Ethyl Glucuronide in Hair-A Highly Effective Test for the Monitoring of Alcohol Consumption[J].Forensic Science International,2012,218(1-3):10-14.
[55]Agius RNT, Kahl HG, Schrader J, et al.Validation of A Headspace Solid-phase Microextraction-GC-MS/MS for the Determination of Ethyl Glucuronide in Hair According to Forensic Guidelines[J].Forensic Science International 2010,196(1-3):3-9.
[56]Fosen JT,Morini L,Sempio C,et al.Ethyl Glucuronide Elimination Kinetics in Fingernails and Comparison to Levels in Hair[J].Alcohol&Alcoholism, 2017,52(5):580-586.
[57]Jones J,Jones M,Plate C,et al.Liquid Chromatography-Tandem Mass Spectrometry Assay to Detect Ethyl Glucuronide in Human Fingernail:Comparison to Hair and Gender Differences[J].American Journal of Analytical Chemistry, 2012,3(1):83-91.
[58]Valano F, Favretto D, Palumbo D, et al.A Novel, Simultaneous Extraction of FAEE and EtG from Meconium and Analysis by LC-MS/MS[J].Analytical&Bioanalytical Chemistry, 2016,408 (10):1-8.
[59]Sharma P, Bharat V, Murthy P.Quantitation of Ethyl Glucuronide in Serum&Urine by Gas Chromatography-Mass Spectrometry[J].Indian Journal of Medical Research,2015,141(1):75-80.
[60]Mnch B, Becker R,Jung C,et al.The Homogeneity Testing of EtG in Hair Reference Materials:A Highthroughput Procedure Using GC-NCI-MS[J].Forensic Science International, 2013,226(1-3):202-207.
[61]Favretto D,Nalesso A,Frison G,et al.A Novel and An Effective Analytical Approach for the LC-MS Determination of Ethyl Glucuronide and Ethyl Sulfate in Urine[J].International Journal of Legal Medicine, 2010,124 (2):161-164.
[62]Vezzoli S, Bernini M, De F F.Ethyl Glucuronide in Vitreous Humor and Blood Postmortem Specimens:Analysis by Liquid Chromatography-Electrospray Tandem Mass Spectrometry and Interpreting Results of Neo-formation of Ethanol[J].Annali Dellistituto Superiore Di Sanità, 2015,51(1):19-27.
[63]Bicker W,L Mmerhofer M,Keller T,et al.Validated Method for the Determination of the Ethanol Consumption Markers Ethyl Glucuronide, Ethyl Phosphate, and Ethyl Sulfate in Human Urine by Reversed-phase/Weak Anion Exchange Liquid Chromatography-Tandem Mass Spectrometry[J].Analytical Chemistry, 2006,78(16):5884-5892.
[64]赵晖,卓先义,严慧,等.液相色谱-串联质谱法测定血液、尿液中乙基葡萄糖醛酸苷[J].法医学杂志,2010,26(4):269-272.
[65]Cappelle D, Crunelle C L,Nuijs ALNV, et al.P1:GCMS or GC-MS/MS for the determination of ethyl glucuronide in hair?[J].Toxicologie Analytique Et Clinique,2014,26(2):31.
[66]Delphine C,Hugo N,Michel Y,et al.Gas Chromatographic Determination of Ethyl Glucuronide in Hair:Comparison between Tandem Mass Spectrometry and Single Quadrupole Mass Spectrometry[J].Forensic Science International, 2015,249:20-24.
[67]沈保华,严慧,赵晖,等.气相色谱-串联质谱法测定血液中乙基葡萄糖醛酸苷[J].法医学杂志,2009,25(6):431-433.
[68]Dresent S,Weinmann W,Wurst FM.Forensic Confirmatory Analysis of Ethyl Sulfate—A New Marker for Alcohol Consumption—by Liquid-Chromatography/Electrospray Ionization/Tandem Mass Spectrometry[J].Journal of the American Society for Mass Spectrometry, 2004,15(11):1644-1648.
[69]Morini L,Politi L,Zucchella A,et al.Ethyl Glucuronide and Ethyl Sulphate Determination in Serum by Liquid Chromatography–Electrospray Tandem Mass Spectrometry[J].Clinica Chimica Acta, 2007,376(1-2):213-219.
[70]Favretto D,Nalesso A,Frison G,et al.No-discharge Atmospheric Pressure Chemical Ionization Mass Spectrometry of Ethyl Glucuronide and Ethyl Sulfate[J].Journal of Mass Spectrometry Jms, 2010,45(1):121-124.
[71]Hegstad S,Helland A,Hagemann C,et al.EtG/EtS in Serum by UHPLC-MS-MS in Suspected Sexual Assault Cases[J].Journal of Analytical Toxicology, 2017,41(7):618-622.
[72]Pichini S,Morini L,Marchei E,et al.Ethylglucuronide and Ethylsulfate in Meconium to Assess Gestational Ethanol Exposure:Preliminary Results in Two Mediterranean Cohorts[J].Can J Clin Pharmacol, 2009,16(2):370-375.
[73]Matlow JN,Aleksa K,Lubetsky A,et al.The Detection and Quantification of Ethyl Glucuronide in Placental Tissue and Placental Perfusate by Headspace Solid-phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry[J].Journal of Population Therapeutics and Clinical Pharmacology=Journal de la Therapeutique des Populations et de la Pharamcologie Clinique, 2012,19(3):473-482.
[74]Pragst F, Auwaerter V, Sporkert F, et al.Analysis of Fatty Acid Ethyl Esters in Hair as Possible Markers of Chronically Elevated Alcohol Consumption by Headspace Solid-phase Microextraction (HS-SPME) and Gas Chromatography-Mass Spectrometry (GC-MS)[J].Forensic Science International, 2001,121(1-2):76-88.
[75]Giovanni ND,Donadio G,Chiarotti M.The Reliability of Fatty Acid Ethyl Esters(FAEE) as Biological Markers for the Diagnosis of Alcohol Abuse[J].Journal of Analytical Toxicology, 2007,31(2):93-97.
[76]De Giovanni N,Donadio G,Chiarotti M.Ethanol Contamination Leads to Fatty Acid Ethyl Esters in Hair Samples[J].Journal of Analytical Toxicology, 2008,32 (2):156-159.
[77]Elisabetta B,Ester DB,Fabio V,et al.Fatty Acid Ethyl Esters in Hair:Correlation with Self-reported Ethanol Intake in 160 Subjects and Influence of Estroprogestin Therapy[J].Drug Testing&Analysis, 2014,6(9):930-935.
[78]Pragst F,Rothe M,Moench B,et al.Combined Use of Fatty Acid Ethyl Esters and Ethyl Glucuronide in Hair for Diagnosis of Alcohol Abuse:Interpretation and Advantages[J].Forensic Science International, 2010,196 (1):101-110.
[79]Susse S,Selavka CM,Mieczkowski T,et al.Fatty Acid Ethyl Ester Concentrations in Hair and Self-reported Alcohol Consumption in 644 Cases from Different Origin[J].Forensic Science International, 2010,196(1):111-117.
[80]Viviankulaga, Danielacaprara, Umariqbal, et al.Fatty acid ethyl esters (FAEE);comparative accumulation in human and guinea pig hair as a biomarker for prenatal alcohol exposure[J].Alcohol Alcohol, 2006,41(5):534-539.
[81]Bakdash A,Burger P,Goecke TW,et al.Quantification of Fatty Acid Ethyl Esters(FAEE) and Ethyl Glucuronide(EtG)in Meconium from Newborns for Detection of Alcohol Abuse in A Maternal Health Evaluation Study[J].Analytical&BioanalyticalChemistry, 2010,396(7):2469-2477.
[82]Pichini S, Pellegrini M, Gareri J, et al.Liquid Chromatography-Tandem Mass Spectrometry for Fatty Acid ethyl Esters in Meconium:Assessment of Prenatal Exposure to Alcohol in Two European Cohorts[J].Journal of Pharmaceutical&Biomedical Analysis, 2008,48(3):927-933.
[83]Hastedt M,Herre S,Pragst F,et al.Workplace Alcohol Testing Program by Combined Use of Ethyl Glucuronide and Fatty Acid Ethyl Esters in Hair[J].Alcohol&Alcoholism, 2012, 47 (2):127-132.
[84]Chan D,Baroz B,PellerinELLERIN B,et al.Population Baseline of Meconium Fatty Acid Ethyl Esters among Infants of Nondrinking Women in Jerusalem and Toronto[J].Therapeutic Drug Monitoring, 2003,25(3):271-278.
[85]Garcia-algar O, Kulaga VJ, Koren G, et al.Alarming Prevalence of Fetal Alcohol Exposure in A Mediterranean City[J].Therapeutic Drug Monitoring, 2008,30(2):249-254.
[86]Klein J, Karaskov T, Korent G.Fatty Acid Ethyl Esters:A Novel Biologic Marker for Heavy in Utero Ethanol Exposure:A Case Report[J].Therapeutic Drug Monitoring,1999,21(6):644-646.
[87]Bearer CF,Jacobson JL,Jacobson SW,et al.Validation of A New Biomarker of Fetal Exposure to Alcohol[J].Journal of Pediatrics, 2003,143(4):463-469.
[88]Moore CM,Lewis D.Fatty Acid Ethyl Esters in Meconium:Biomarkers for the Detection of Alcohol Exposure in Neonates[J].Clinica Chimica Acta, 2001,312(1-2):235-237.
[89]Bearer CF,Santiago LM,Oriordan MA,et al.Fatty Acid Ethyl Esters:Quantitative Biomarkers for Maternal Alcohol Consumption[J].Journal of Pediatrics, 2005,146(6):824-830.
[90]Hutson JR,Rao C,Fulga N,et al.An Improved Method for Rapidly Quantifying Fatty Acid Ethyl Esters in Meconium Suitable for Prenatal Alcohol Screening[J].Alcohol,2011,45(2):193-199.
[91]Chan D, Klein J, Karaskov T, et al.Fetal Exposure to Alcohol as Evidenced by Fatty Acid Ethyl Esters in Meconium in the Absence of Maternal Drinking History in Pregnancy[J].Therapeutic Drug Monitoring, 2004,26 (5):474-481.
[92]Moore C, Jones J, Lewis D, et al.Prevalence of Fatty Acid Ethyl Esters in Meconium Specimens[J].Clinical Chemistry, 2003,49(1):133-136.
[93]Vaiano F, Favretto D, Palumbo D, et al.A Novel, Simultaneous Extraction of FAEE and EtG from Meconium and Analysis by LC-MS/MS[J].Analytical&Bioanalytical Chemistry, 2016, 408 (10):2587-2594.
[94]Kwak HS,Kang YS,Han KO,et al.Quantitation of Fatty Acid Ethyl Esters in Human Meconium by An Improved Liquid Chromatography/Tandem MassSpectrometry[J].Journal of Chromatography B Analytical Technologies in the Biomedical&Life Sciences, 2010,878(21):1871-1874.
[95]Politi L, Mari F, Furlanetto S, et al.Determination of Fatty Acid Ethyl Esters in Hair by GC-MS and Application in A Population of Cocaine Users[J].Journal of Pharmaceutical&Biomedical Analysis, 2011,54(5):1192-1195.
[96]Kwak HS, Han JY, Choi J S, et al.Dose-response and Time-response Analysis of Total Fatty Acid Ethyl Esters in Meconium as A Biomarker of Prenatal Alcohol Exposure[J].Prenatal Diagnosis, 2014,34(9):831-838.
[97]Zelner I,Kenna K,Brien JF,et al.Meconium Fatty Acid Ethyl Esters as Biomarkers of Late Gestational Ethanol Exposure and Indicator of Ethanol-induced Multi-organ Injury in Fetal Sheep[J].Plos One, 2013,8(3):e59168.
[98] álvarez I.Analysis of Fatty Acid Ethyl Esters in Hair by Headspace Solid-Phase Microextraction (HS-SPME) and Gas Chromatography-Mass Spectrometry (GC-MS)[J].Analytical Letters, 2009,42(18):2962-2977.
[99]Zelner I, Shor S, Lynn H, et al.Clinical Use of Meconium Fatty Acid Ethyl Esters for Identifying Children at Risk for Alcohol-Related Disabilities:the First Reported Case.J Popul Ther Clin Pharmacol[J].The Canadian Journal of Clinical Pharmacology=Journal Canadien de Pharmacologie Clinique, 2012,19(1):26-31.
[100]Raepsaet-charlier MT.Determination of Eight Fatty Acid Ethyl Esters in Meconium Samples by Headspace Solidphase Microextraction and Gas Chromatography-Mass Spectrometry[J].Journal of Separation Science, 2015,33(14):2115-2122.
[101]Cabarcos P, Tabernero MJ, Otero JL, et al.Quantification of Fatty Acid Ethyl Esters(FAEE) and Ethyl Glucuronide (EtG) in Meconium for Detection of Alcohol Abuse during Pregnancy:Correlation Study between Both Biomarkers[J].Journal of Pharmaceutical&Biomedical Analysis, 2014,100(21):74-78.
[102]Cabarcos P, Tabernero MJ, Alvarez I, et al.A New Method for Quantifying Prenatal Exposure to Ethanol by Microwave-Assisted Extraction (MAE) of Meconium Followed by Gas Chromatography-Mass Spectrometry(GC-MS) [J].Analytical& Bioanalytical Chemistry,2012,404(1):147-155.
[103]Gonzalez-lllan F,Ojeda-Torres G,Dazv-Vazquez LM,et al.Detection of Fatty Acid Ethyl Esters in Skin Surface Lipids as Biomarkers of Ethanol Consumption in Alcoholics, Social Drinkers, Light Drinkers, and Teetotalers Using a Methodology Based on Microwave-Assisted Extraction Followed by Solid-Phase Microextract[J].Journal of Analytical Toxicology, 2011,35(4):232-237.
[104]Gareri J,Lynn H,Handley M,et al.Prevalence of Fetal Ethanol Exposure in A Regional Population-based Sample by Meconium Analysis of Fatty Acid Ethyl Esters[J].Therapeutic Drug Monitoring, 2008,30(2):239-245.
[105]Hutson JR,Gareri MJ N,Koren G.The Incidence of Prenatal Alcohol Exposure in Montevideo Uruguay As Determined by Meconium Analysis[J].Therapeutic Drug Monitoring, 2010,32(3):311-317.
[106]Albermann ME,Madea B,Musshoff F.A SPME-GC/MS Procedure for the Determination of Fatty Acid Ethyl Esters in Hair for Confirmation of Abstinence Test Results[J].Journal of Chromatographic Science, 2014,52(9):955-960.
[107]Zimmermann CM.Gas Chromatography Tandem Mass Spectrometry for Biomarkers of Alcohol Abuse in Human Hair[J].Therapeutic Drug Monitoring, 2010,32(2):216-223.
[108]Pichini S,Marchei E,Vagnarelli F,et al.Assessment of Prenatal Exposure to Ethanol by Meconium Analysis:Results of An Italian Multicenter Study[J].Alcoholism Clinical&Experimental Research, 2012,36(3):417-424.
[109]Pragst F.Interpretation Problems in A Forensic Case of AbstinenceDetermination UsingAlcoholMarkersin Hair[J].Forensic Science International, 2012,217(1-3):4-7.
[110]Sterneck M,Yegles M,Rothkirch VG,et al.Determination of Ethyl Glucuronide in Hair Improves Evaluation of Long-term Alcohol Abstention in Liver Transplant Candidates[J].Liver International Official Journal of the International Association for the Study of the Liver, 2014,34(3):469–476.
[111]Kummer N,Wille SM,Poll A,et al.Quantification of EtG in Hair,EtG and EtS in Urine and PEth Species in Capillary Dried Blood Spots to Assess the Alcohol Consumption in Driver's Licence Regranting Cases[J].Drug&Alcohol Dependence, 2016, 165:191-197.
[112]Høiseth G,Karinen R,Christophersen A,et al.Practical Use of Ethyl Glucuronide and Ethyl Sulfate in Postmortem Cases as Markers of Antemortem Alcohol Ingestion[J].International Journal of Legal Medicine, 2010,124(2):143-148.
[113]Arndt T, Schröfel S, G Ssregen B, et al.Inhalation but not Transdermal Resorption of Hand Sanitizer Ethanol Causes Positive Ethyl Glucuronide Findings in Urine[J].Forensic Science International, 2014,237(3):126-130.
[114]Høiseth G,Yttredal B,Karinen R,et al.Levels of Ethyl Glucuronide and Ethyl Sulfate in Oral Fluid,Blood, and Urine after Use of Mouthwash and Ingestion of Nonalcoholic Wine[J].Journal of Analytical Toxicology, 2010,34(2):84-88.
[115]Arndt T, Schröfel S, Stemmerich K.Ethyl Glucuronide Identified in Commercial Hair Tonics[J].Forensic Science International, 2013,231(1-3):195-198.
[116]Kerekes I, Yegles M.Coloring, Bleaching, and Perming:Influence on EtG Content in Hair[J].Therapeutic Drug Monitoring, 2013,35(4):527-529.
Review on the Analysis and Application of Alcohol Biomarkers
FENG Xue-yi1,2,SHEN Min1
(1.Shanghai Key Laboratory of Forensic Medicine,Shanghai Forensic Platform,Academy of Forensic Science,Shanghai 200063,China;2.Institute of Criminal Science and Technology,Jing'an Branch of Shanghai Public Security Bureau,Shanghai 200070,China)
Alcohol abuse,a public health problem of great importance,is the third leading cause of death and disability in the word according to the World Health Organization.Determination and evaluation of ethanol in vivo has been the main task of forensic toxicological analysis in the cases of traffic accident,alcohol or drug abuse,occupational health and poison intoxication.The biomarkers of ethanol present more advantages over ethanol itself in terms of sensitivity,specificity,detection window,identification ability and application areas.This review summarized the analytical procedures and applications of direct alcohol biomarkers of ethyl glucuronide,ethyl sulfate and fatty acid ethyl esters,in an attempt to promote the progress of forensic toxicological analysis and provide lawsuit and social identification activity with more scientific,reliable and efficient information.
alcohol biomarker;alcohol abuse;ethyl glucuronide;ethyl sulfate;fatty acid ethyl esters
DF795.4
A
10.3969/j.issn.1671-2072.2017.06.007
1671-2072-(2017)06-0036-14
2017-05-17
国家自然科学基金(81772022);上海市科技攻关项目(15DZ1207500);上海市法医学重点实验室资助项目(17DZ2273200);上海市司法鉴定专业技术服务平台资助项目(16DZ2290900)
冯雪伊(1991—),女,硕士研究生,主要从事分析化学研究。 E-mail:milk_juice@sina.com。
沈敏(1955—),女,研究员,博士研究生导师,主要从事法医毒物鉴定研究和司法鉴定管理工作。E-mail:shenm@ssfjd.cn。
(本文编辑:严 慧)
