Abnormal Concentration of GABA and Glutamate in The Prefrontal Cortex in Schizophrenia.-An in Vivo 1H-MRS Study
2017-11-29TianyiCHENYingchanWANGJianyeZHANGZuoweiWANGJialeXUYaoLIZhileiYANGDengtangLIU
Tianyi CHEN , Yingchan WANG, Jianye ZHANG, Zuowei WANG, Jiale XU, Yao LI, Zhilei YANG,Dengtang LIU,*
Abnormal Concentration of GABA and Glutamate in The Prefrontal Cortex in Schizophrenia.-An in Vivo 1H-MRS Study
Tianyi CHEN1,2, Yingchan WANG1, Jianye ZHANG1, Zuowei WANG2, Jiale XU3, Yao LI3, Zhilei YANG4,Dengtang LIU1,*
schizophrenia; magnetic resonance spectroscopy; γ-aminobutyric acid; glutamate
1. Background
The etiology and pathomechanism of schizophrenia are unknown. The traditional Dopamine (DA) hypothesis also has a certain degree of limitation and is not able to fully explain the psychopathology and therapeutics of schizophrenia.[1]The glutamate (Glu) and γ-aminobutyric acid (GABA) hypotheses are important etiological hypotheses for schizophrenia, which posit that Glu or GABA concentrations are abnormal in the brains of those with schizophrenia.[2]Magnetic resonance spectroscopy (MRS) techniques can measure the in vivo metabolite concentration in the brain.Most studies have found that levels of glutamate in schizophrenia increase in the ventromedial prefrontal cortex (including the anterior cingulate cortex),however, there were no significant differences in other brain regions such as the parietal-occipital lobe,temporal lobe, hippocampus, and thalamus found,when compared with to those without schizophrenia.[3]In recent years, determination of the in vivo brain GABA concentration has become possible with the development of high-field-strength MRI technology and data analysis methods. Studies from other countries have revealed abnormal GABA concentration in the medial prefrontal lobe (cingulate gyrus or ventromedial prefrontal cortex) in schizophrenia.[4,5]However, there are at least two limitations in the current GABA studies of schizophrenia: 1) the impact of antipsychotic drugs on the study outcome has not been completely excluded;[6,7]2) the positioning of the medial prefrontal lobe is often very vague or inaccurate.[6,8-9]The ventromedial prefrontal cortex and anterior cingulate cortex are the critical brain areas of schizophrenia. However, the two areas have not been clearly differentiated in previous studies. This study measured the concentration of glutamate and GABA in the dorsolateral prefrontal cortex, ventromedial prefrontal cortex and anterior cingulated cortex in individuals with schizophrenia using high-field- strength MRS. The correlation between results and clinical symptoms was also studied.
2. Methods
2.1 Participants
2.1.1 Schizophrenia group
All participants in this study were outpatients or inpatients at the Shanghai Mental Health Center(Shanghai, China). Of these participants 50 did not take medication, 18 cases declined to participate in this study, and 4 cases met exclusion criteria upon collection of demographic data. In the end, 28 patients were enrolled and underwent magnetic resonance imaging. There were 3 cases that withdrew from the study due to inability to complete the magnetic resonance imaging and 1 case that was excluded because of excessive spontaneous head movements.The remaining 24 cases met the following criteria: 1)meet the diagnostic criteria for schizophrenia according to DSM-IV; 2) had not taken antipsychotic medication for at least two weeks; 3) aged 18 to 45 years; and 4) written informed consent provided by the patient and patient's family members. The exclusion criteria were the following: 1) severe physiological diseases,psychoactive substance or alcohol dependence, or mental retardation; 2) patients receiving MECT; 3)women who were pregnant or lactating; and 4) those who had abnormal brain structures as found by conventional MRI. There were 10 male cases and 14 female cases with a mean(sd) age of 28.8 (8.3) years and mean (sd) years of education of 13.5 (3.9) years.The number of episodes in patients was 1.58 (0.5), and the median (QR25, QR75) course of disease was 24(2, 57) months. The Positive and Negative Syndrome Scale (PANSS) and the Clinical Global Impression Scale(CGI) were used to assess the mental symptoms and severity of the disease. The mean (sd) total PANSS score was 82.8 (11.6); the mean (sd) positive symptom score was 22.7 (5.8); the mean (sd) negative symptom score was 19.9 (5.6). The mean (sd) score of general psychopathology was 40.5 (5.6). The mean (sd) score for the severity of CGI disease was 4.8 (0.9).
2.1.2 Healthy control group
30 healthy volunteers were recruited from the community. Several persons were excluded for the following reasons: 3 persons were younger than 18 or older than 45, 2 persons had a history of substance abuse, and 1 person had a family history of psychosis.None of the remaining 24 cases met the diagnostic criteria for any mental disorder as assessed by the Mini-International Neuropsychiatric Interview (MINI6.0).Patients with a family history of psychosis and severe physiological diseases, pregnant or lactating women,and those with abnormal brain structures as found by conventional MRI were also excluded. All participants gave consent to take part in this study. In this group there were 10 males and 14 females with a mean(sd) age of 26.6 (4.7) years and a mean(sd) years of education of 14.1 (2.6) years. The differences of age,educational level, and gender between the two groups were not statistically significant.
2.2 Assessment
2.2.1 Scale assessment
PANSS[10,11]and CGI[12]were used to assess the symptom severity of the patients with schizophrenia.The MINI.6.0[13]was used to interview and screen all participants. All scale assessments were conducted by a psychiatrist who had been trained in use of these scales.
2.2.2 MRI scan
Siemens MRI 3.0 (Magnetom Verio syngo MR B17)32-channel coil was used to conduct the MRI. First,the magnetization-prepared rapid gradient-echo(MPRAGE) sequence was used to acquire the T1 structural image of the subjects. Subsequently, the MEshcher-GArwood Point RESolved Spectroscopy sequence (MEGA-PRESS) was used to collect the MRS data of the subjects.[8,14]The areas of interest included: the left dorsolateral prefrontal cortex(lDLPFC), ventromedial prefrontal cortex, and anterior cingulate cortex. The sizes of the corresponding areas of interest were 35mmX25mmX30mm of the left dorsolateral prefrontal cortex (lDLPFC),20mmX40mmX30mm of the ventromedial prefrontal cortex, and 40mmX20mmX20mm of the anterior cingulate cortex. Figures 2 to 4 show the location and sizes of the areas of interest. The dorsolateral prefrontal cortex voxel frame makes the upper edge be roughly parallel to the skull in coronal position, include the cerebral cortex, but avoid overlapping with the skull, in addition to coinciding with the upper inner edge and the superior frontal sulcus. Voxels included the middle frontal gyrus. The ventromedial prefrontal voxel frame on the horizontal axis took the longitudinal fissure of the brain as the midpoint extending 20mm to both sides and its trailing edge was located in the anterior cingulate cortex of the genu of corpus callosum in the sagittal plane, while trying not to include the anterior cingulate cortex. The lower edge was located in the upper part of the orbital gyrus. The anterior cingulate cortex: the voxel frame was symmetrical on the right and left sides taking the longitudinal fissure of the brain as the section;the anterior margin arose from the upper part of the genu of the corpus callosum; the lower edge along the upper region of the corpus callosum was out of shape;the upper and lower edges were approximately parallel to the corpus callosum. The MRS scan parameter was:TR=1500ms; TE=68ms; the number of acquisition was 164 times; the acquisition time of each area of interest was about 6 minutes. The MRI scan was calibrated before running. Manual shimming made the full width at half maximum of the water peak lower than 15 Hz.[15,16]At the same time, the chemical shift imaging stimulated by the radio-frequency pulse (PF pulse) underwent water content suppression. The water suppression rate(WS rate) was larger than 98%. Afterwards, the water suppression rates were collected with the same voxel at the same location (4 acquisition times).
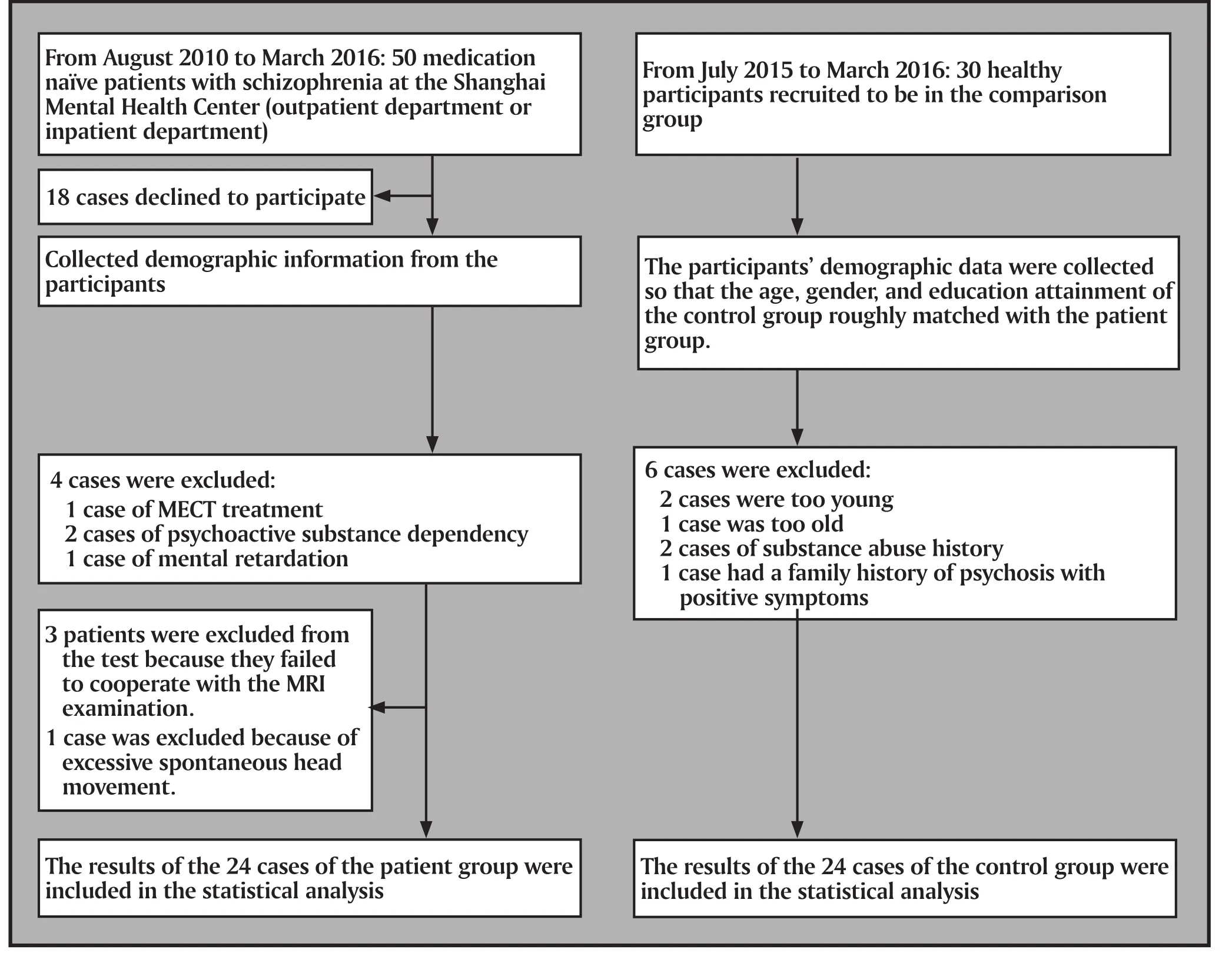
Figure 1. Flowchart of the study

Figure 2. Size and location of the region of interest of the left dorsolateral prefrontal cortex (lDLPFC); the volume is 35mm X 25mm X 30mm

Figure 3. Size and location of the region of interest of the ventromedial prefrontal cortex (vmPFC); the volume is 20mm X 40mm X 30mm
2.2.3 Image post-processing and data collection
The LCModel software from the Shanghai Jiao Tong University School of Biomedical Engineering workstation was used to process data. The editing frequency of the spectrum was 1.9ppm; the delta frequency was -1.7ppm; the editing bandwidth was 45Hz.Water was used as the internal reference to separately calculate the concentrations of the γ-aminobutyric acid(GABA), glutamate (Glu), glutamine (Gln), N-acetylaspartate(NAA), and N-acetylaspartylglutamate (NAAG) in the areas of interest and the ratios of GABA, Glu, and Gln to(NAA+NAAG).
2.3 Statistical processing
SPSS 19.0 statistical software package was used for statistical analysis. The metabolite concentration was expressed using mean (standard deviation). The data from the non-normal distribution were expressed using median (quartiles). The between-group differences of the schizophrenia and healthy comparison groups were analyzed by two independent samples t-tests.T-test was used when the variance was heterogenous.Non-normal distribution data were checked using the Mann-Whitney U test. The age structure ratio in the general data was analyzed using chi-squared. Pearson correlation analysis was used to examine correlations.Spearman correlation analysis was used to analyze nonnormal distribution data.

Figure 4. Size and location of the region of interest of the anterior cingulate cortex (ACC); the volume is 40mm X 20mm X 20mm
3. Results
3.1 Comparison of the GABA and glutamate concentrations
Table 1 lists the comparisons of the metabolite concentrations such as GABA and glutamate in the three regions of interest.
3.1.1 Left dorsolateral prefrontal cortex
Table 1 shows that the between-group differences of the GABA, Glu, and Gln concentrations and the GABA/(NAA+NAAG), Glu/ (NAA+NAAG), Gln/ (NAA+NAAG)concentration ratios between the schizophrenia group and comparsion group were not statistically significant.
3.1.2 Ventromedial prefrontal cortex
The mean (sd) concentrations of GABA of the schizophrenia group and the comparison group were 2.50 (1.53) and 1.83 (0.63) respectively; the mean (sd)concentrations of Glu were 5.13 (2.44) and 3.49 (1.91);the mean (sd) concentrations of Gln were 2.46 (1.66)and 4.36 (1.90). The between-group differences for Glu (t=2.60, p=0.013) and Gln (t=-3.68, p=0.001)were statistically significant, suggesting an elevated Glu concentration and a reduced Gln concentration in schizophrenia. The Glu and Gln concentrations were negatively correlated (R=-0.86, p<0.001). The betweengroup differences of the GABA concentrations was not statistically significant (t= 1.97, p=0.058). Further ratio analysis suggested that the between-group difference of Glu/ (NAA+NAAG) was statistically significant (z=-2.18, p=0.030) and the between-group difference of Gln/ (NAA+NAAG) was statistically significant (z=-3.01, p=0.004).
3.1.3 Anterior cingulated cortex
Since the GABA concentrations of the schizophrenia group and the comparison group were non-normally distributed, and were expressed as medians 1.90(Q1=1.55, Q3=2.09) and 2.16 (Q1=1.87, Q3=2.59)respectively; the medians of Gln concentrations were 0.36 (Q1=0.00, Q3=0.74) and 0.29 (Q1=0.00,Q3=0.59); the mean Glu concentrations were 6.07(2.48) and 6.54 (1.99); the between-group difference of the GABA concentrations was statistically significant(z=-2.95, p=0.003); the between-group difference of Glu and Gln was not statistically significant; further ratio analysis suggested that the between-group difference of the GABA/(NAA+NAAG) was statistically significant (z=-2.72, p=0.012).
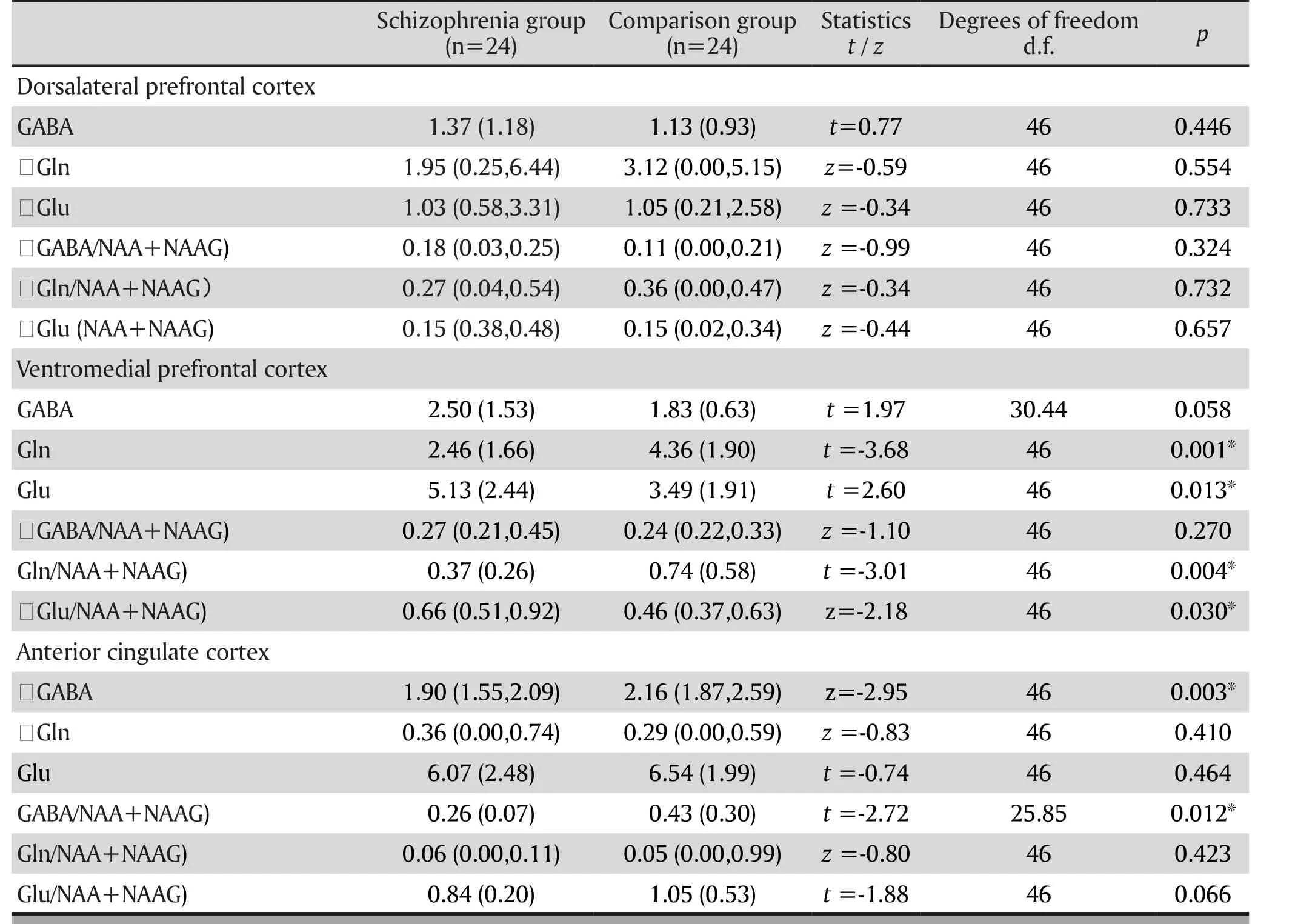
Table 1. Comparison of the metabolite concentrations such as GABA and glutamine
3.2 Correlation of GABA and glutamate concentration with the demographic characteristics and clinical symptoms
The correlation between education level and duration of disease and GABA, glutamate, and the ratio of each area of interest was not statistically significant. Table 2 shows that age was negatively correlated with the concentration of GABA in the anterior cingulate cortex(r=-0.494, p=0.014); age was negatively correlated with the GABA/ (NAA+NAAG) in the anterior cingulated cortex(r=-0.473, p=0.020) and was negatively correlated with Glu/ (NAA+NAAG) of the anterior cingulated cortex (r=-0.459, p=0.024). However, only the negative correlation of age and the GABA concentration in the anterior cingulate cortex was statistically significant after the Bonferroni correction was used (p value should than 0.017). In the comparison group, there was no such correlation. The positive symptom scores of the PANSS scale were negatively correlated with the Gln concentration in the anterior cingulate cortex (r=-0.406, p=0.049) and was negatively correlated with Gln/(NAA+NAAG) in the same area (r=-0.419, p=0.042).The negative symptom scores of the PANSS scale were positively correlated with the GABA in the anterior cingulate cortex (r=-0.417, p=0.043). None of the correlations above was statistically significant after the Bonferroni correction was applied (p value should be less than 0.017). There was a lack of correlation between the GABA concentrations and glutamate in other areas of interest and clinical symptoms.
4. Discussion
4.1 Main findings
Regarding glutamates in the prefrontal cortex, our study was consistent with most of the results from previous studies that there was no change in the left dorsolateral prefrontal cortex and an increase of Glu concentration in the ventromedial prefrontal cortex.The concentration of GABA in the prefrontal cortex corresponded to the concentration of Glu; there was no change in the left dorsolateral prefrontal cortex. There was a rising trend in the ventromedial prefrontal cortex.The results of studies from Kegeles and colleagues(2012), Fuente-Sandoval and colleagues (2015), and Zhilei Yang and colleagues (2015)[4,14,17]tended to show that: the GABA concentration in the ventromedial prefrontal cortex was elevated in the early stage of the disease. The ketamine challenge experiment found that ketamine selectively acted on the NMDA receptor that caused an elevation of Glu in the vmPFC resulting in positive symptoms, negative symptoms, and cognitive impairment in healthy people. In addition, they found that glutamine was converted back to glutamate as the release of glutamate increased. This was completely in line with results we saw from patients in this study.
The anterior cingulate cortex is an important component of the limbic system, and is related to cognitive attention. Animal and neurobiology experiments have shown that schizophrenia is associated with genetic defects that involve changes of GABA-ergic neurons in the early stages of ontogeny.[18]Yu Zhe and colleagues (2013) found that, in a schizophrenia animal model of GABA transporter 1 deficiency caused by GAT1, knockout rats with a decline in GABA showed would show a variety of schizophrenia-like symptoms such as positive symptoms, negative symptoms, and cognitive deficits.[19]The neurons of the cingulate gyrus were closely related to the prefrontal cortex. The pyramidal neurons of the cerebral cortex and hippocampus were densely distributed with the anterior synapses of GABA neurons. There were feed-forward and feedback loops of neurons between the GABA neurons and Glu pyramidal neurons.[20]Our study found a decrease in GABA concentration in the anterior cingulate cortex;therefore, the GABA- glutamate hypothesis speculated that Fast-spiking parvalbumin positive interneurons released less GABA in the course of schizophrenia. The projection to the prefrontal cortex could free Glu from inhibited release, in which the increased Glu could give feedback inducing GABA to go up again.
Previous studies have shown that NAA increased in the caudate nucleus and cerebellum in patients with first-episode schizophrenia.[21]Additionally,studies have found that NAAG was higher in younger patients with schizophrenia than in healthy controls.[9]Other studies have found progressive loss of brain tissues in schizophrenia and reduction of neuronal density decreased in the prefrontal cortex, as well as(NAA+NAAG) representative neuronal density and activity gradually decreased with the development of disease.[22,23]Due to the production of NAGG from the combination of NAA and Glu, this may be one of the compensatory regulation mechanisms for the change of Glu levels in patients with schizophrenia. This experiment concurrently compared the concentrations of various metabolites, and their ratio to (NAA+NAAG).The ratio was corrected for the differences of the neuronal density in individual brain tissues and deducted the part of compensation that could better reflect the Glu and GABA function of the patients with schizophrenia, not the structural abnormality. In this study, the between-group difference of the GABA concentration in the ventromedial prefrontal cortex between the schizophrenia group and comparison group neared statistical significance. Yet, its ratio with(NAA+NAAG) has no between-group difference and may possibly reflect the change of GABA concentration as a kind of compensatory result.
The reason for the abnormality of Glu among patients with schizophrenia is not certain. Genetics suggests the possibility of an allelic variation in GRM3 that affected the Gln-Glu cycle, resulting in Glu translocation disorder.[23]Gln is converted to Glu by the glutaminase in presynaptic neurons. Glu, released into the synaptic gap, is reabsorbed by astrocytes and regenerated into Gln by glutamine synthetase. This study observed an increase of Glu concentrations in the same area of interest and a decrease of the Gln level and Gln/ (NAA+NAAG). Moreover, the higher the Glu concentration, the lower the Gln concentration was.This may be due to an increase in the release of Glu that was freed from inhibition, resulting in an increase of Gln consumption. It may also be due to the metabolic impairment of Glu, resulting in reduced Gln productionwithout colloid cells resorption. Or it could be the result of the interaction between these two reasons.[24]
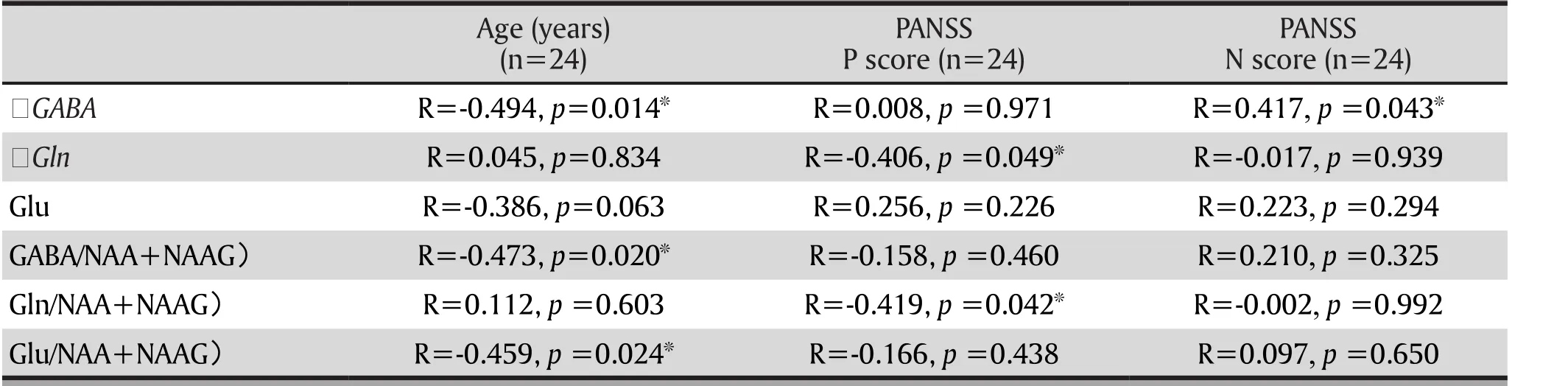
Table 2. The correlation of GABA in the anterior cingulate cortex and glutamine in schizophrenia with other clinical data
In healthy human brains, Glu and GABA decrease with age.[25]In this study, GABA of the patients with schizophrenia decreased with age. Nevertheless, there was no similar trend in the comparison group. This may be related to the general lack of elderly persons in this study. Rowland and colleagues (2016) also found that GABA was significantly reduced in the older segment of a schizophrenia group (over 35 years) compared to the younger segment (under 35 years). However, there is no such difference in the control group.[26]This resembles the results of this study. These results reflect a gradual decline of GABA in the anterior cingulate cortex in patients with schizophrenia, that is earlier and more pronounced compared with the normal population.
Egerton and colleagues (2012) suggest that glutamate /Cr in the anterior cingulate cortex is positively related to negative symptoms.[27]Reid and colleagues (2012) found that the severity of negative symptoms was negatively related to the Glx level.[22]However, Poels and colleagues (2013) mentioned in a systematic review, most studies have not found the correlation between the metabolites and clinical symptoms.[3]The underlying cause of these different results deserves further investigation. Most previous studies coherently agreed that presynaptic DA in striatum and prefrontal cortex increased, triggering psychotic positive symptoms.[28]The Glu system and the DA system have the intersection point. Therefore,we hypothesized that the concentration of Glu/Gln and the positive symptoms are correlated. Moreover,Stone and colleagues (2012) have shown in the normal population that the elevated Glu concentration in the anterior cingulate cortex and the degree of positive symptoms induced by ketamine were related to each other.[29]However, this study found that the correlation between metabolite concentration after calibration and symptoms was not statistically significant. Therefore,a study with a larger sample size is needed to further validate this hypothesis.
4.2 Limitations
This study is cross sectional in nature, therefore we did not conduct any follow up regarding changes in metabolite concentration and changes in clinical status.The sample size of this study was relatively small.This could lead to a false positive correlation between some metabolites and the patients' demographics and their degree of clinical symptoms such as cognitive impairment and so on. In this experiment, the metabolite concentration calculated by LCModel was not further corrected by partial volume correction.The proportion of gray matter, white matter, and cerebrospinal fluid was not compared between groups. Therefore, the tissue volume difference of the individuals and the effects of tissue components on each metabolite concentration cannot be confirmed.
4.3 Implications
This study differentiated the ventromedial prefrontal cortex from the anterior cingulate cortex. Excluding the effects of drugs, the metabolite concentrations were detected respectively by the MEGA-PRESS sequence and LCModel software using the 1H-MRS method. It was found that there were different changes in metabolite concentration in different regions of interest. The results supported the hypothesis of GABA- glutamate abnormalities in schizophrenia and found that the GABA in the anterior cingulate cortex in the patients with schizophrenia had a tendency to accelerate depletion.For future studies, we can start by increasing the sample size. Moreover, a comparative observation can be made before and after the intervention to the areas of interest with significant differences. Additionally, we can analyze the relationship between the metabolites and positive symptoms, negative symptoms, cognitive impairment and so forth, as well as further exploring the predictive effects of brain metabolites such as GABA and Glu on disease development and prognosis.
Funding statement
The National Natural Science Foundation of China(project code: 81371479); Shanghai Science and Technology Commission Guidance project (project code: 15411964400); "Joint Key Project Tackling Important Diseases" of Health Department of Shanghai Municipal Government (project code: 2014ZYJB0002);Appropriate Technique of Municipal Hospital in Shanghai; Shanghai Mental Disease Clinical Medical Center (2014); Department of Early Psychiatric Disorders of Shanghai Mental Health Center (2013-YJTSZK-05);Project of Shanghai Municipal Health and Family Planning Commission (20144Y0054); The Shanghai Natural Science Foundation of China (16ZR1430500)
Conflict of interest statement
The authors declare no conflict of interest related to this manuscript.
Informed consent
All the participants provided written informed consent.
Ethical approval
This study was approved by the ethics committee of Shanghai Mental Health Center
Authors' contributions
Study design: Dengtang Liu, Tianyi Chen
Data collection: Tianyi Chen, Yingchan Wang, Zhilei Yang
Acquisition of image data: Jianye Zhang
Quality control and clinical guidance: Zuowei Wang
Experimental data analysis: Tianyi Chen, Jiale Xu
Provision of the tools for analysis: Yao Li
1. Bigliani V, Mulligan RS, Acton PD, Visvikis D, Ell PJ,Stephenson C, et al. In vivooccupancy of striatal and temporal cortical D2/D3 dopamine receptors by typical antipsychotic drugs. [123I]epidepride single photon emission tomography (SPET) study. Br J Psychiatry. 1999;175: 231-238. doi: https://doi.org/10.1192/bjp.175.3.231
2. Liu XH. [Oxford psychiatric textbooks. Fifth Edition. Chinese version]. Sichuan: Sichuan University Press; 2010. p. 324-325. Chinese
3. Poels EM, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC,Lieberman JA, et al. Imaging glutamate in schizophrenia:review of findings and implications for drug discovery. Mol Psychiatry. 2013; 19(1): 20-29. doi: https://doi.org/10.1038/mp.2013.136
4. Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X,et al . Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012; 69(5): 449. doi: https://doi.org/10.1001/archgenpsychiatry.2011.1519
5. Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A, et al. Reduction of brain γ-aminobutyric acid(GABA) concentrations in early-stage schizophreniapatients:3T Proton MRS study. Schizophr Res. 2009. 112(1-3): 192-193. doi: https://doi.org/10.1016/j.schres.2009.04.026
6. Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: A proton ma6netic resonance spectroscopy study. Schizophr Res. 2010; 117(1): 83-91. doi: https://doi.org/10.1016/j.schres.2009.11.011
7. Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci.2010; 30(10): 3777-3781. doi: https://doi.org/10.1523/JNEUROSCI.6158-09.2010
8. Ongür D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF,et al. Elevated gamma- aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010; 68(7): 667-670. doi:https://doi.org/10.1016/j.biopsych.2010.05.016
9. Rowland LM, Kontson K, West J, Edden RA, Zhu H,Wijtenburg SA, et al. In vivo measurements of glutamate,gaba, and naag in schizophrenia. Schizophr Bull. 2013; 39(5):1096-1104. doi: https://doi.org/10.1093/schbul/sbs092
10. Kay S R, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull.1987; 13(2): 261-276. doi: https://doi.org/10.1093/schbul/13.2.261
11. Si TM, Yang JZ, Shu L, Wang XL, Kong QM, Zhou M, et al. [The Reliability,Validity of PANSS and its Implication]. Zhongguo Xin Li Wei Sheng Za Zhi. 2004; 18(1): 45-47. Chinese. doi:http://dx.chinadoi.cn/10.3321/j.issn:1000-6729.2004.01.016.
12. Wu WY, Zhang MY. [Clinical Global Impression (CGI)].Shanghai Arch Psychiatry. 1984; 2(1): 76-77. Chinese
13. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E. The Mini- International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998; 59 (Suppl 20): 22-33
14. Fuente-Sandoval CDL, Reyesmadrigal F, Mao X, León-Ortiz P, Rodríguez-Mayoral O, Solís-Vivanco R, et al. Cortico-Striatal GABAergic and Glutamatergic Dysregulations in Subjects at Ultra-High Risk for Psychosis Investigated with Proton Magnetic Resonance Spectroscopy. Int J Neuropsychopharmacol. 2015; 19(3): pyv105. doi: https://doi.org/10.1093/ijnp/pyv105
15. Bartha R. Effect of signal-to-noise ratio and spectral linewidth on metabolite quantification at 4 T. NMR Biomed.2007; 20(5): 512-521. doi: https://doi.org/10.1002/nbm.1122
16. Tan SH, Liang CH, Zheng JH, Xu L. [Effect of shimming on water suppression and metabolites concentrations of 3.0T proton spectrum]. Zhongguo Yi Xue Ying Xiang Ji Shu. 2010;26(2): 369-371. doi: http://dx.chinadoi.cn/10.13929/j.1003-3289.2010.02.009. Chinese
17. Yang ZL, Zhu YQ, Song ZH, Mei L, Zhang JY, Chen TY, et al.[Comparison of the density ofgamma-aminobutyric acid in the ventromedial prefrontal cortex of paitents with ifrst-episode psychosis and healthy controls]. Shanghai Arch Psychiatry. 2015; 27(6): 341-347. doi: https://doi.org/10.11919/j.issn.1002-0829.215130
18. Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, et al .Interplay between DISC1 and GABA Signaling Regulates Neurogenesis in Mice and Risk for Schizophrenia. Cell.2012; 148(5): 1051-1064. doi: https://doi.org/10.1016/j.cell.2011.12.037
19. Yu Z, Fang Q, Xiao X, Wang YZ, Cai YQ, Cao H, et al. GABA Transporter-1Deficiency Confers Schizophrenia-Like Behavioral Phenotypes. PLoS ONE. 2013; 8(7): e69883. doi:https://doi.org/10.1371/journal.pone.0069883
20. Benes FM, Berretta S. GABAergic Interneurons: Implications for Understanding Schizophrenia and Bipolar Disorder.Neuropsychopharmacology. 2001; 25(1): 1-27. doi: https://doi.org/10.1016/S0893-133X(01)00225-1
21. de la Fuente-Sandoval C, León-Ortiz P, Favila R, Stephano S, Mamo D, Ramírez- Bermúdez J, et al . Higher Levels of Glutamate in the Associative-Striatum of Subjects with Prodromal Symptoms of Schizophrenia and Patients with First-Episode Psychosis. Neuropsychopharmacology. 2011;36(9): 1781-1791. doi: https://doi.org/10.1038/npp.2011.65
22. Kraguljac NV, Reid M, White D, Jones R, den Hollander J,Lowman D, et al. Neurometabolites in schizophrenia and bipolar disorder - A systematic review and meta-analysis.Psychiatry Research: Neuroimaging. 2012; 203(2-3): 111-125.doi: https://doi.org/10.1016/j.pscychresns.2012.02.003
23. Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE, et al. Glutamate in Schizophrenia: A Focused Review and Meta-Analysis of 1H-MRS Studies.Schizophr Bull. 2012; 39(1): 120-129. doi: https://doi.org/10.1093/schbul/sbr069
24. Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM.N-Acetylaspartate in the CNS: From Neurodiagnostics to Neurobiology. Progress in Neurobiology. 2007; 81(2): 89-131.doi: https://doi.org/10.1016/j.pneurobio.2006.12.003
25. Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, et al . Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013; 78(9): 75–82. doi: https://doi.org/10.1016/j.neuroimage.2013.04.012
26. Rowland LM, Krause BW, Wijtenburg SA,McMahon RP,Chiappelli J, Nugent KL, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Molecular Psychiatry. 2015; 21(2): 198-204. doi: https://doi.org/10.1038/mp.2015.34
27. Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ,McGuire PK, et al . Anterior Cingulate Glutamate Levels Related to Clinical Status Following Treatment in First-Episode Schizophrenia. Neuropsychopharmacology.2012; 37(11): 2515-2521. doi: https://doi.org/10.1038/npp.2012.113
28. Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000; 97(14): 8104-8109. doi: https://doi.org/10.1073/pnas.97.14.8104
29. Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamineinduced psychopathology. Mol Psychiatry. 2012; 17(7): 664-665. doi: https://doi.org/10.1038/mp. 2011.171
精神分裂症患者前额叶的GABA及谷氨酸浓度异常--活体1H-MRS研究
陈天意, 王颖婵, 张建业, 汪作为, 许嘉乐, 李瑶, 杨志磊, 刘登堂
精神分裂症;磁共振波谱;γ-氨基丁酸;谷氨酸
Background:The etiology and pathomechanism of schizophrenia are unknown. The traditional dopamine(DA) hypothesis is unable to fully explain its pathology and therapeutics. The glutamate (Glu) and γ-aminobutyric acid (GABA) hypotheses suggest Glu or GABA concentrations are abnormal in the brains of patients with schizophrenia. Magnetic resonance spectroscopy (MRS) show glutamate level increases in the ventromedial prefrontal cortex (vmPFC) including the anterior cingulated cortex (ACC) in those with schizophrenia.
Aims:To investigate the function of the glutamate system (glutamate and γ-aminobutyric acid) in the etiology and pathomechanism of schizophrenia.
Methods:24 drug naïve patients with schizophrenia and 24 healthy volunteers were matched by gender,age, and educational level. The Siemens 3T MRI system was used to collect the magnetic resonance spectroscopy (MRS) data of the subjects. The regions of interest included the left dorsolateral prefrontal cortex (lDLPFC), ventromedial prefrontal cortex (vmPFC), and anterior cingulate cortex (ACC). LCModel software was used to analyze the concentrations of γ-aminobutyric acid (GABA), glutamate (Glu), glutamine(Gln), N-acetylaspartate (NAA), and N-acetylaspartylglutamate (NAAG) in the region of interest. Meanwhile,the Positive and Negative Syndrome Scale (PANSS) and the Clinical Global Impression Scale (CGI) were used to assess the mental symptoms and severity of the disease.
Results:The median GABA concentrations in the anterior cingulate cortex of the schizophrenia group and the healthy control group were 1.90 (Q1=1.55, Q3=2.09) and 2.16 (Q1=1.87, Q3=2.59) respectively; the mean(sd) Glu concentrations were 6.07 (2.48) and 6.54 (1.99); the median Gln concentrations were 0.36 (Q1=0.00,Q3=0.74) and 0.29 (Q1=0.00, Q3=0.59); the between-group difference of the GABA concentrations was statistically significant (Z=-2.95, p=0.003); the between-group difference of the GABA/(NAA+NAAG) was statistically significant (Z=-2.72, p=0.012); the between-group difference of Glu and Gln was not statistically significant. The age of the schizophrenia group was negatively correlated with the GABA concentration in the anterior cingulate (R=-0.494, p=0.014), and negatively correlated with GABA/ (NAA+NAAG) (R=-0.473,p=0.020). Yet there was no such correlation in the control group. After calibration, no significant correlation was found between the clinical symptoms and the concentrations of the metabolites.
Conclusions:The concentration of glutamate in the vemtromedial prefrontal cortex of patients with schizophrenia was abnormal, whereas the concentration of GABA in the anterior cingulate cortex decreased,supporting the hypothesis of abnormal glutamate -GABA in the brains of those individuals with schizophrenia.In patients with schizophrenia, the GABA in the anterior cingulate cortex had an accelerated decline with age.The clinical symptoms may be correlated to the metabolite concentration of the anterior cingulate cortex.
[Shanghai Arch Psychiatry. 2017; 29(5): 277-286.
http://dx.doi.org/10.11919/j.issn.1002-0829.217004]
1Division of Psychiatric Disorders, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
2Shanghai Hongkou Mental Health Center, Shanghai, China
3College of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
4Jiading District Mental Health Center, Shanghai, China
*correspondence: Dr. Dengtang Liu. Mailing address: 600 South Wanping RD, Shanghai, China. Postcode: 200030; E-Mail: erliu110@126.com
背景:精神分裂症的病因及病理机制不明,传统的多巴胺(Dopamine,DA)假说不能完全解释其病理学及治疗学.谷氨酸(glutamate,Glu)及γ-氨基丁酸(γ-aminobutyric acid,GABA)假说认为精神分裂症患者脑内的Glu或GABA浓度异常.磁共振波谱(magnetic resonance spectroscopy,MRS)技术发现精神分裂症谷氨酸类物质水平在前额叶腹内侧(ventromedial prefrontal cortex,vmPFC)(包括前扣带回)(anterior cingulate cortex,ACC)升高.
目的:探讨谷氨酸系统(谷氨酸及γ-氨基丁酸)在精神分裂症原因学及病理机制中的作用.
方法:24例未服药精神分裂症患者及24例性别、年龄及教育程度相配的健康志愿者入组,应用西门子MRI 3T采集被试的磁共振波谱(magnetic resonance spectroscopy,MRS)数据,感兴趣区包括左侧前额叶背外侧(left dorsolateral prefrontal cortex,lDLPFC)、前额叶腹内侧(ventromedial prefrontal cortex,vmPFC)及前扣带回(anterior cingulate cortex,ACC),运用LCModel软件分析了感兴趣区的γ-氨基丁酸(γ-aminobutyric acid,GABA)、谷氨酸(glutamate,Glu)、谷氨酰胺(glutamine,Gln)、氮乙酰天冬氨酸(N-acetylaspartate,NAA)及氮乙酰天冬氨酸谷氨酸(N-acetylaspartylglutamate,NAAG)等物质浓度.同时应用阳性与阴性症状量表(positive and negativeSyndrome scale,PANSS)及临床总体印象量表(Clinical Global Impression Scale,CGI)评估患者的精神症状和疾病严重程度.
结果:精神分裂症组和健康对照组前扣带回的GABA浓度分别为1.90(Q1=1.55,Q3=2.09)及2.16(Q1=1.87,Q3=2.59)、Glu浓度分别为6.07(2.48)及6.54(1.99)、Gln浓度分别为0.36(Q1=0.00,Q3=0.74)及0.29(Q1=0.00,Q3=0.59),GABA浓度的组间差异有统计学意义(Z=-2.95,p =0.003),GABA/(NAA+NAAG)的组间差异有统计学意义(t=-2.72,p =0.012),而Glu及Gln的组间差异均无统计学意义.精神分裂症患者组的年龄与前扣带回的GABA浓度呈负相关(R=-0.494,p=0.014),与GABA/(NAA+NAAG)呈负相关(R=-0.473,p =0.020),但对照组无此相关性.经过FDR方法校正后,未发现临床症状与各代谢产物浓度无显著相关性.
结论:精神分裂症患者前额叶腹内侧的谷氨酸类物质浓度异常,而前扣带回的GABA浓度降低,支持精神分裂症脑内谷氨酸-GABA异常假说.精神分裂症患者前扣带回的 GABA随年龄增长加速减退.临床症状与前扣带回的代谢物浓度可能相关.

Tianyi Chen graduated from the Department of Clinical Medicine in Xiangya School of Medicine Central South University with a major in Mental Health. She is currently studying as a working clinical medicine master's student at Shanghai Jiaotong University School of Medicine. Since 2014 she has been working in the Shanghai Hongkou Mental Health Center where she is an attending physician on a psychiatric unit. Her research interests are early intervention and cognitive training for schizophrenia.
猜你喜欢
杂志排行
上海精神医学的其它文章
- Mismatch Negativity in Han Chinese Patients with Schizophrenia: A Meta-Analysis
- Pleasure Experience and Emotion Expression in Patients with Schizophrenia
- Pretreatment Serum MCP-1 Level Predicts Response to Risperidone in Schizophrenia
- A Cross-Sectional Study on the Characteristics of Tardive Dyskinesia in Patients with Chronic Schizophrenia
- Multidimensional Approaches for A Case of Severe Adult Obsessive - Compulsive Disorder
- Psychiatry and Cinema: What Can We Learn from the Magical Screen?
