KMnO4降解2-溴酚的氧化产物与反应路径
2017-11-23庞素艳姜成春
庞素艳,杨 悦,姜成春,周 扬,江 进,马 军
KMnO4降解2-溴酚的氧化产物与反应路径
庞素艳1,2*,杨 悦2,姜成春3,周 扬4,江 进4,马 军4
(1.吉林建筑大学市政与环境工程学院,吉林长春130118;2.哈尔滨理工大学化学与环境工程学院,黑龙江哈尔滨150040;3.深圳职业技术学院建筑与环境工程学院,广东深圳 518055;4.哈尔滨工业大学城市水资源与水环境国家重点实验室,黑龙江哈尔滨 150090)
为了探讨KMnO4氧化降解溴酚过程中溴代聚合产物的生成机理,利用三重四级杆串联线性离子阱液相-质谱联用仪(LC-MS/MS)对KMnO4氧化降解2-溴酚的产物进行检测分析.结果表明,根据溴的天然同位素特性,建立了LC-MS/MS-PIS(79和81)子找母质谱扫描方法测定溴代有机物,测得KMnO4氧化降解2-溴酚的主要产物为4个质量数相同,341/343(79)和343/345(81),且分子结构中含有2个溴的氧化耦合产物,同位素丰度比为1:1.推测4个溴代聚合产物为同分异构体,由2-溴酚氧自由基通过C-C和C-O耦合产生,其中,C-C耦合的聚合产物先出峰,C-O耦合的聚合产物后出峰.2-溴酚氧自由基发生氧化耦合反应,理论上产生的8个溴代聚合产物并没有全部被检测到,主要是由于酚氧自由基的氧化耦合速率不同,导致聚合产物的形成产率不同.
KMnO4;2-溴酚;聚合产物;氧化耦合;反应路径
溴酚类有机物被广泛应用于助燃剂、木材防腐剂、聚合物等产品的生产过程中,导致地表水中溴代污染物含量增加[1-2].溴酚类污染物自身毒性高且能够危害人类身体健康和水生生态环境[3-6].同时,溴酚也能够引起饮用水的嗅味问题,而且嗅阈值非常低,只有ng/L范围[7].目前,溴酚的处理技术主要有二氧化锰氧化[8]、光催化氧化[9-13]、过硫酸盐催化氧化[14]等,同时,这些研究的产物分析结果证实溴酚的氧化降解产物是一些聚合物,如羟基化多溴联苯醚(OH-PBDEs)和羟基化多溴联苯(OH-PBBs).
高锰酸钾(KMnO4)作为绿色氧化剂,运输、储存、使用方便,且氧化后不易产生有毒有害副产物,能够在水处理过程中进行大规模应用. Jiang等[15]研究了KMnO4降解2,4-二溴酚的氧化产物和反应路径,LC-MS/MS质谱测定结果表明,2,4-二溴酚氧化后产生2个含有4个溴的聚合产物,一个为多溴联苯醚,由2个2,4-二溴酚氧自由基通过邻位C与O耦合产生,另一个是由2个2,4-二溴酚氧自由基通过邻位C与邻位C耦合产生,结构中含有2个羟基,且每个苯环中都含有1个.到目前为止,还没有关于KMnO4氧化降解2-溴酚氧化产物与反应路径的研究.
因此,本文利用液相色谱质谱联用仪(LC-MS/MS)测定KMnO4降解2-溴酚过程中的氧化产物,研究溴代氧化产物的质谱特点及反应路径.
1 实验部分
1.1 材料
2-溴酚(2-BrP)为分析纯,购买于Sigma公司.乙腈为色谱醇,购买于Merck公司,甲酸为色谱纯,购买于Sigma公司.实验中所用其他试剂均为分析纯,购买于国药集团上海化学试剂有限公司.
1.2 试验方法
一系列含10µmol/L 2-溴酚的纯水中(含10%乙腈),加入不同浓度KMnO4起始反应(5~20µmol/L),反应完全后(即KMnO4完全被消耗),用0.45µm的玻璃纤维膜过滤,利用液相色谱质谱联用仪(LC-MS/MS)对过滤后样品进行产物分析测定.
1.3 分析方法
KMnO4降解2-溴酚的氧化产物采用AB SCIEX QTRAP 5500三重四级杆串联线性离子阱质谱与Agilent 1260高效液相联用(LC-MS/ MS)进行分析测定.色谱柱为Agilent Poroshell 120EC-C18(4.6mm×150mm,2.7μm),流动相为乙腈(A)和含1‰甲酸的超纯水(B),流动相梯度为A先从5%开始,保持5min,然后在30min内从5%线性升到50%,保持10min,再在0.1min内降到5%,保持5min,流速为200μL/min,进样量为10μL,柱温为35℃.采用电喷雾离子源负离子模式(ESI-)进行检测,测定方法选择子找母扫描模式(PIS),即在四级杆Q3设定特殊质量数的子离子,然后在四级杆Q1设定质量数扫描范围,寻找能够产生该子离子的母离子.Q1设定扫描范围为50~500Da, Q3设定子离子质量数为79或81Da,扫描速度为1000Da/s,离子源电压和温度分别为-4500V和500℃,氮气(N2)作为气帘气,流速为35L/min,去簇电压(DP)和入口电压(EP)分别为-70V和-10V,碰撞电压(CE)为-30V~-100V.
2 结果与讨论
2.1 溴代有机物质谱测定方法的建立
天然环境中溴(Br)的同位素主要有2个,质量数为79和81(Br79和Br81),且丰度比为1:1.研究中根据溴的这一同位素特性,建立了一种简便、快速,可以选择性检测溴代有机物的质谱检测方法,其原理主要是利用溴代有机物在ESI源负电(ESI-)模式下,通过溴离子的同位素信息,进行三重四级杆的质谱扫描追踪母离子测定,即子找母质谱扫描模式[16-17].
研究中计算了溴代有机物中含溴元素个数与质谱测定信息的关系,见表1[16,18-22].例如,2-溴酚分子结构中含有1个溴,进行质谱全扫描模式测定时,会产生2个1:1的质谱峰,采用79或81子找母质谱扫描模式(79和81)进行测定时,会各产生1个质谱峰.
2.2 KMnO4降解2-溴酚的氧化产物与反应路径
由图1可见,全扫描色谱图中观察不到明显的2-溴酚色谱峰,而在子找母扫描色谱图中能够观察到响应值很高的色谱峰.因此,与全扫描质谱模式相比,子找母质谱扫描模式对溴代有机物的测定更灵敏,响应值更高.2-溴酚的保留时间为24.8min,在嵌入的质谱图中,子找母扫描时质量数为171(79)和173(81),全扫描时质量数为171/173,且质谱峰的溴同位素丰度比为1:1,与表1中含1个溴的总结相一致.
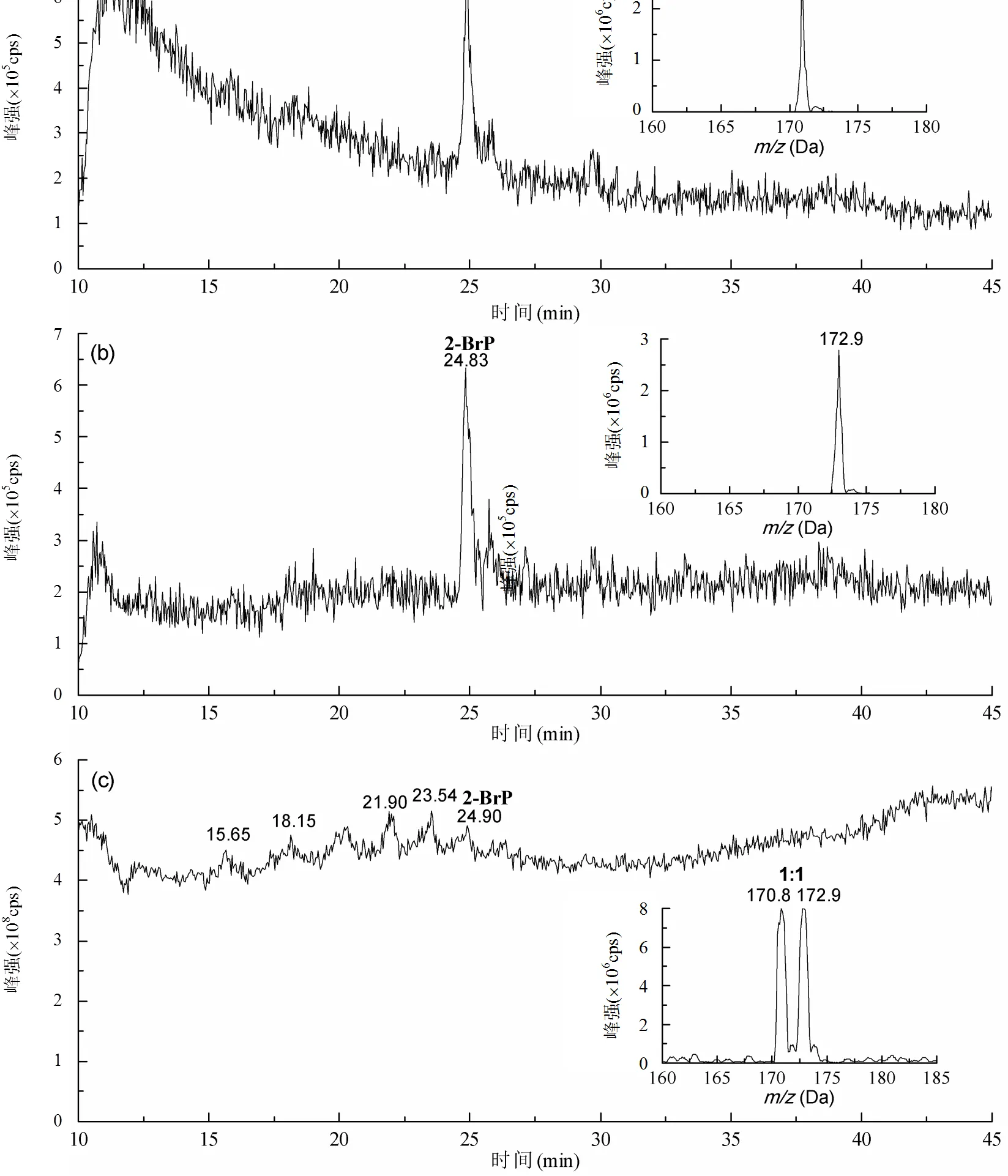
图1 2-溴酚标准样品LC-MS/MS色谱图
(a) PIS79, (b) PIS81, (c) 全扫描
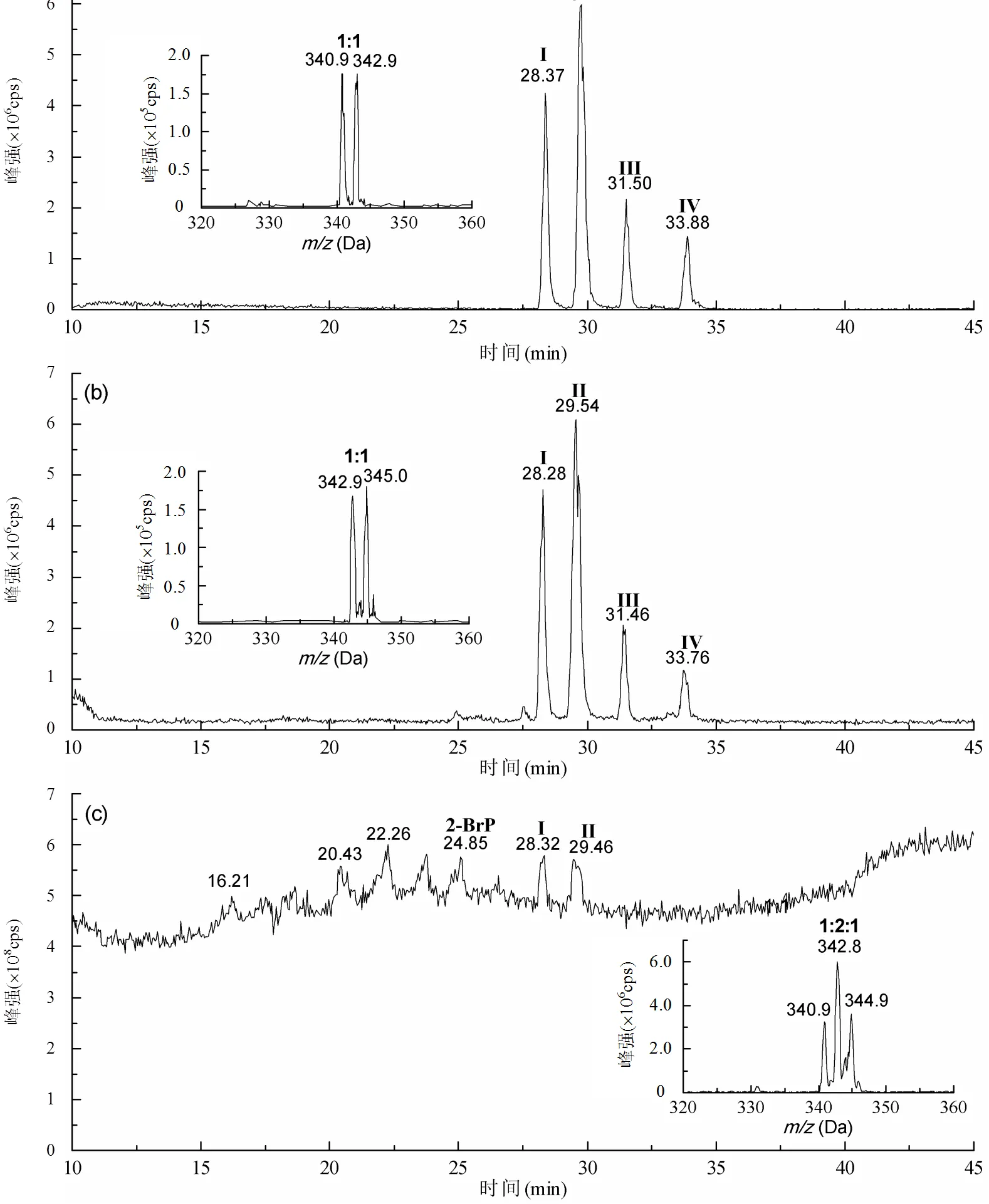
图2 KMnO4氧化2-溴酚的LC-MS/MS色谱图
(a) PIS79, (b) PIS81, (c) 全扫描

图3 2-溴酚氧自由基的所有C-C和C-O耦合反应

表1 溴代有机物在LC-MS/MS全扫描和子找母扫描中理论同位素丰度规律
注:为被检测物质的最小质量数.
从图2可见,与全扫描色谱图相比,采用子找母扫描模式测定KMnO4降解2-溴酚氧化产物的色谱峰更清晰、更灵敏、更全面.与2-溴酚标准色谱图相比,通过子找母质谱扫描模式检测到KMnO4降解2-溴酚产生4个主要产物,分别标记为I、II、III、IV,而在全扫描模式下只测到产物I和II,并且色谱峰非常小.从质谱图可以看出,4个产物进行子找母扫描时质量数相同,为341/343(79)和343/345(81),且2个质谱峰的同位素丰度比为1:1,应该是同分异构体.在全扫描时质量数为341/343/345,且丰度比为1:2:1.根据表1的计算结果,推测产物I-IV中含有2个Br,可能是目标物2-溴酚氧自由基的聚合物.这一测定结果,与Jiang等[15]研究中KMnO4氧化降解2,4-二溴酚的LC-MS/MS质谱检测结果相似.2,4-二溴酚氧化后产生2个含有4个溴的聚合产物,而2-溴酚氧化后产生4个含有2个Br的聚合产物.溴酚中溴离子的个数和位置直接导致其氧化产物和反应路径不同,从而导致LC-MS/MS质谱测定结果不同.
酚氧自由基易发生氧化耦合反应,在耦合过程中会产生各种聚合产物[23-28].理论上,2-溴酚的4个酚氧自由基如果全部参与反应,通过C-O和C-C耦合可能产生8个含溴聚合产物,见图3.这8个溴代聚合产物中有5个聚合产物的质量数为341/343/345,含有2个溴,其中有2个聚合产物是通过C-O耦合生成,另外3个聚合产物是通过C-C耦合生成.但利用LC-MS/MS-PIS测定只检测到4个聚合产物(图2),同时也不能确定这4个产物是5个聚合产物中的哪一个.根据Jiang等[15]的研究结果,只能确定通过C-C耦合,含有2个羟基的聚合产物先出峰,通过C-O耦合,含有1个羟基的聚合产物后出峰.
图3中质量数为263/265的3个聚合产物是2-溴酚氧自由基通过脱1个溴产生,但是在LC-MS/MS-PIS测定过程中并未检测到质量数为263/265的产物.2-溴酚氧自由基发生耦合反应理论上产生的聚合产物并没有全部被检测到,主要是由于酚氧自由基相互耦合的速率不同,从而导致聚合产物的产率有所不同[15,23].
3 结论
3.1 根据溴的天然同位素特性,建立了一种简便、快速LC-MS/MS-PIS (79和81)子找母质谱扫描测定方法.
3.2 LC-MS/MS-PIS子找母质谱扫描方法测得KMnO4氧化2-溴酚的主要产物为4个不脱溴的氧化耦合产物,分子结构中含有2个溴,质量数为341/343(79)和343/345(81),丰度比为1:1.
3.3 KMnO4氧化2-溴酚产生的4个溴代聚合产物推测是由2-溴酚氧自由基通过C-C和C-O耦合产生,其中,C-C耦合的聚合产物先出峰,C-O耦合的聚合产物后出峰.
3.4 2-溴酚氧自由基发生耦合反应理论上产生的8个溴代聚合产物并没有全部被检测到,主要是由于酚氧自由基的氧化耦合速率不同,导致耦合产物的形成产率不同.
[1] Sim W J, Lee I S, Choi S D, et al. Distribution and formation of chlorophenols and bromophenols in marine and riverine environments [J]. Chemosphere, 2009,77:552-558.
[2] Xiong J, Li G, An T, et al. Emission patterns and risk assessment of polybrominated diphenyl ethers and bromophenols in water and sediments from the Beijiang River, South China [J]. Environ. Pollut., 2016,219:596-603.
[3] Hassenklover T, Predehl S, Pilli J, et al. Bromophenols, both present in marine organisms and in industrial flame retardants, disturb cellular Ca2+signaling in neuroendocrine cells (PC12) [J]. Aquat. Toxicol., 2006,76:37-45.
[4] Kammann U, Vobach M, Wosniok W. Toxic effects of brominated iodoles and phenols on zebrafish embryos. Arch [J]. Environ. Contam. Toxicol., 2006,51:97-102.
[5] Bruchajzer E, Szymanska J A, Piotrowski J K. Acute and subacute nephrotoxicity of 2–bromophenol in rats [J]. Toxicol. Letters, 2002,134:245-252.
[6] Yang M, Zhang X. Comparative developmental toxicity of new aromatic halogenated DBPs in a chlorinated saline sewage effluent to the marine polychaete platynereis dumerilii [J]. Environ. Sci. Technol., 2013,47:10868-10876.
[7] Acero J L, Piriou P, von Gunten U. Kinetics and mechanisms of formation of bromophenols during drinking water chlorination: Assessment of taste and odor development [J]. Water Res., 2005,39:2979-2993.
[8] Lin K, Yan C, Gan J. Production of hydroxylated polybrominated diphenyl ethers (OH-PEDEs) from bromophenols by manganese dioxide [J]. Environ. Sci. Technol., 2014,48:263-271.
[9] Eriksson J, Rahm S, Green N, et al. Photochemical transformations of tetrabromobisphenol A and related phenols in water [J]. Chemosphere, 2004,54:117-126.
[10] Liu H, Zhao H, Quan X, et al. Formation of 2’-hydroxy-2, 3’,4,5’-tetrabromodipheyl ether (2’-HO-BDE68) from 2,4- dibromophenol in aqueous solution under simulated sunlight irradiation [J]. Chemosphere, 2011,84:512-518.
[11] Saeed A, Altarawneh M, Dlugogorski B Z. Photodecomposition of bromophenols [J]. Chemosphere, 2016,150:749-758.
[12] Zhu Q, Igarashi M, Sasaki M, et al. Degradation and debromination of bromophenols using a free-base porphyrin and metalloporphyrins as photosensitizers under conditions of visible light irradiation in the absence and presence of humic substances [J]. Appl. Catal. B- Environ.,2016,183:61-68.
[13] Xie B, Li X, Huang X, et al. Enhanced debromination of 4-bromophenol by the UV/sulfite process: Efficiency and mechanism [J]. J. Environ. Sci., 2017,54:231-238.
[14] Guan C, Jiang Jin, Luo C, et al. Oxidation kinetics of bromophenols by nonradical activation of peroxydisulfate in the presence of carbon nanotube and formation of brominated polymeric products. Environ. Sci. Technol., 2017,51:10718-10728.
[15] Jiang J, Gao Y, Pang S Y, et al. Oxidation of bromophenols and formation of brominated polymeric products of concern during water treatment with potassium permanganate [J]. Environ. Sci. Technol., 2014,48:10850-10858.
[16] Zhang X, Talley J W, Boggess B, et al. Fast selective detection of polar brominated disinfection byproducts in drinking water using precursor ion scans [J]. Environ. Sci. Technol., 2008,42:6598-6603.
[17] 罗从伟,马 军,江 进,等.UV/H2O2降解2,4,6-三氯苯甲醚动力学及产物研究[J].中国环境科学, 2017,37(5):1831-1837.
[18] Zhai H, Zhang X. Formation and decomposition of new and unknown polar brominated disinfection byproducts during chlorination [J]. Environ. Sci. Technol., 2011,45:2194-2201.
[19] Pan Y, Zhang X. Four groups of new aromatic halogenated disinfection byproducts: Effect of bromide concentration on their formation and speciation in chlorinated drinking water [J]. Environ. Sci. Technol., 2013,47:1265-1273.
[20] Xiao F, Zhang X, Zhai H, et al. New halogenated disinfection byproducts in swimming pool water and their permeability across skin [J]. Environ. Sci. Technol., 2012,46:7112-7119.
[21] Ding G, Zhang X, Yang M, et al. Formation of new brominated disinfection byproducts during chlorination of saline sewage effluents [J]. Water Res., 2013,47:2710-2718.
[22] Pang S Y, Jiang J, Gao Y, et al. Oxidation of flame retardant tetrabromobisphenol A by aqueous permanganate: Reaction kinetics, brominated products, and pathways [J]. Environ. Sci. Technol., 2014,48:615-623.
[23] Dec J, Haider K, Bollag J M. Release of substituents from phenolic compounds during oxidative coupling reactions [J]. Chemosphere, 2003,52:549-556.
[24] Poerschmann J, Trommler U. Pathways of advanced oxidation of phenol by Fenton's reagent-Identification of oxidative coupling intermediates by extractive acetylation [J]. J. Chromatogr. A, 2009,1216:5570-5579.
[25] Castillo I, Pérez V, Monsalvo I, et al. Copper (II) complexes of piperazine-derived tetradentate ligands and their chiral diazabicyclic analogues for catalytic phenol oxidative C–C coupling [J]. Inorg. Chem. Commun., 2013,38:1-4.
[26] Gao R, Xu F, Li S, et al. Formation of bromophenoxy radicals from complete series reactions of bromophenols with H and OH radicals [J]. Chemosphere, 2013,92:382-390.
[27] Lin K, Zhou S, Chen X, et al. Formation of hydroxylated polybrominated diphenyl ethers from laccase-catalyzed oxidation of bromophenols [J]. Chemosphere, 2015,138:806-813.
[28] Huguet M, Deborde M, Papot S, et al. Oxidative decarboxylation of diclofenac by manganese oxide bed filter [J]. Water Res., 2013,47:5400-5408.
Products and pathways of 2-bromophenol oxidation by potassium permanganate.
PANG Su-yan1,2*, YANG Yue2, JIANG Cheng-chun3, ZHOU Yang4, JIANG Jin4, MA Jun4
(1.School of Municipal and Environmental Engineering, Jilin Jianzhu University, Changchun 130118, China;2.College of Chemical and Environmental Engineering, Harbin University of Science and Technology, Harbin 150040, China;3.School of Civil and Environmental Engineering, Shenzhen Polytechnic, Shenzhen 518055, China;4.State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, China)., 2017,37(11):4159~4165
The purpose of this article was to investigate the mechanism responsible for the formation of brominated polymeric products from oxidation of bromophenols by aqueous potassium permanganate. Experiments were conducted to determine brominated oxidation products of 2-bromophenol by aqueous potassium permanganate using liquid chromatography-triple quadrupole mass spectrometry (LC-MS/MS). The results showed that four polymeric products of341/343 (at79) and 343/345 (at81) containing two bromine atoms were detected by the precursor ion scan (PIS) approach at79 and 81, respectively, and their abundance was about 1:1, consistent with the natural isotope of bromine atom. The four polymeric products were isomers, and they were formed by the C-O and C-C coupling of 2-bromophenoxy radicals, where the C-C coupling products eluted faster than the C-O coupling ones in LC-MS/MS. According to phenolic coupling theory, there would be eight brominated polymeric products. However, they were partially detected, probably due to the difference in coupling rates of phenoxy radicals.
potassium permanganate;2-Bromophenol;polymeric product;oxidative coupling;reaction pathways
X703.5
A
1000-6923(2017)11-4159-07
庞素艳(1978-),女,吉林辽源人,教授,博士,主要从事水质物化处理技术与理论.发表论文30余篇.
2017-05-03
国家自然科学基金资助项目(51578203, 51378316)
* 责任作者, 教授, psyhit@126.com
