Fe2+过硫酸钠体系降解盐酸四环素的研究*
2017-11-07张良波许春红祝慧娜张宝忠
张良波 许春红 祝慧娜 李 莹 张宝忠
(河南工业大学化学化工与环境学院,河南 郑州 450001)
Fe2+过硫酸钠体系降解盐酸四环素的研究*
张良波 许春红 祝慧娜 李 莹 张宝忠
(河南工业大学化学化工与环境学院,河南 郑州 450001)
采用Fe2+/过硫酸钠体系降解水溶液中的盐酸四环素,探讨了盐酸四环素初始浓度、Fe2+浓度、过硫酸钠浓度、温度、pH等因素对降解效果的影响。单因素实验结果表明,盐酸四环素去除率随着盐酸四环素的初始浓度、过硫酸钠浓度、温度的增大而增大;随着Fe2+浓度的增加,盐酸四环素去除率先增大后减小;酸性条件有利于Fe2+/过硫酸钠体系对盐酸四环素的降解。当盐酸四环素初始质量浓度为50mg/L、Fe2+摩尔浓度为0.10mmol/L、过硫酸钠摩尔浓度为2.0mmol/L、反应温度为30 ℃、pH=3.0时,反应90min后盐酸四环素去除率可达87.6%。Fe2+/过硫酸钠体系对盐酸四环素的降解用一级反应动力学方程进行拟合,得到该反应体系下盐酸四环素降解的活化能为5.173kJ/mol。
高级氧化技术Fe2+硫酸根自由基 盐酸四环素
Abstract: The degradation of tetracycline hydrochloride by Fe2+/sodium persulfate system was studied in this paper. The influence of tetracycline hydrochloride initial concentration,Fe2+concentration and sodium persulfate concentration,reaction temperature and pH on tetracycline hydrochloride degradation efficiency was investigated. The results showed that the degradation efficiency of tetracycline hydrochloride increased with the increasing of the initial concentration of tetracycline hydrochloride,the concentration of sodium persulfate and the reaction temperature. The degradation efficiency of tetracycline hydrochloride increased first and then decreased with the increasing of the Fe2+concentration. Acidic condition was favorable for the degradation of tetracycline hydrochloride by Fe2+/sodium persulfate system. The degradation efficiency of tetracycline hydrochloride could reach 87.6% under the condition of the initial mass concentration of tetracycline hydrochloride 50 mg/L,the molar concentration of Fe2+0.10 mmol/L,the molar concentration of sodium persulfate 2.0 mmol/L,the reaction temperature 30 ℃,the pH 3.0 and the reaction time 90 min. The degradation of tetracycline hydrochloride by Fe2+/sodium persulfate system followed the first order reaction kinetic equation. The activation energy of tetracycline hydrochloride degradation was 5.173 kJ/mol in this reaction system.
Keywords: advanced oxidation technology; Fe2+; sulfate radical; tetracycline hydrochloride
盐酸四环素是一种常用的四环素类抗生素,由于其具有明显的致突变、致畸作用而受到人们的广泛关注[1]。盐酸四环素对生物过程有较强的抑制作用,常规的生物处理工艺不能对其有效去除[2]。常规的物理方法如吸附、反渗透和纳滤等可以去除一些水中的盐酸四环素,但不能对其进行有效降解[3]。液氯氧化等化学氧化法能降解盐酸四环素,但可产生致癌的二次污染物。

1 材料与方法
1.1 材料与仪器
盐酸四环素(美国药典标准级);过硫酸钠、FeSO4·7H2O、浓硫酸、甲醇、氢氧化钠均为分析纯。
UltiMate 3000型高效液相色谱仪(美国赛默飞);pHS-3C型酸度计;FA2004B型电子天平;ZWY型恒温水浴振荡器。
1.2 实验方法
根据实验要求,取50 mL含一定初始浓度的盐酸四环素水溶液加入到100 mL锥形瓶中,加入一定量FeSO4·7H2O,调节溶液pH后快速加入过硫酸钠,于恒温振荡器中振荡并开始计时,控制振荡速度为150 r/min,90 min后取样过0.22 μm滤膜,采用高效液相色谱仪测定上清液中盐酸四环素的剩余浓度,计算去除率,反应温度由恒温水浴振荡器控制。
1.3 色谱条件
色谱内置真空泵及压力传感器,色谱柱为C18柱(250 mm×4.6 mm,5 μm);检测器采用紫外检测器,检测波长为359 nm;流动相为31%(体积分数,下同)乙腈和69%的0.1 mol/L草酸溶液,流动相流速为0.8 mL/min;进样量为20 μL。
2 结果与讨论
2.1 盐酸四环素初始浓度的影响
设定Fe2+摩尔浓度为1.00 mmol/L,过硫酸钠摩尔浓度为2.0 mmol/L,反应温度为30 ℃,pH=3.0,调节盐酸四环素的初始质量浓度分别为30、50、70、100 mg/L,反应时间设为90 min,考查盐酸四环素初始浓度对盐酸四环素去除率的影响,结果如图1所示。

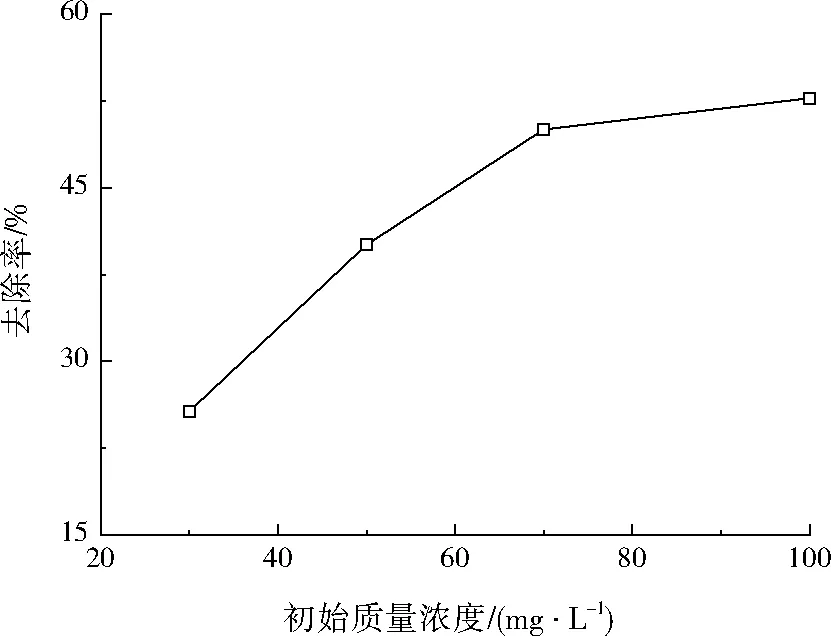
图1 盐酸四环素初始质量浓度对其去除率的影响Fig.1 Effect of initial concentration of tetracycline hydrochloride on its removal rate
2.2 Fe2+浓度的影响
设定盐酸四环素初始质量浓度为50 mg/L,过硫酸钠摩尔浓度为2.0 mmol/L,反应温度为30 ℃,pH=3.0,调节Fe2+摩尔浓度分别为0、0.04、0.08、0.10、0.15、0.20、0.50、1.00、1.50、2.00 mmol/L,反应时间设为90 min,考查Fe2+浓度对盐酸四环素去除率的影响,结果如图2所示。
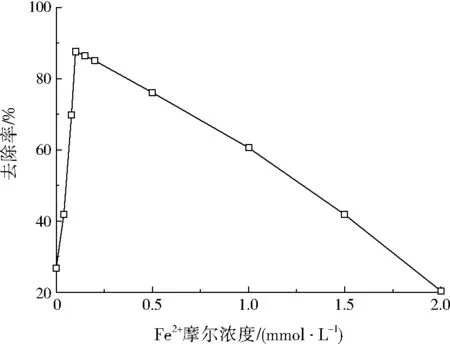
图2 Fe2+摩尔浓度对盐酸四环素去除率的影响Fig.2 Effect of Fe2+ molar concentration on the removal rate of tetracycline hydrochloride

(1)
(2)
2.3 过硫酸钠浓度的影响
设定盐酸四环素初始质量浓度为50 mg/L,Fe2+摩尔浓度为1.00 mmol/L,反应温度为30 ℃,pH=3.0,调节过硫酸钠摩尔浓度分别为0、0.5、1.0、2.0、3.0、5.0 mmol/L,反应时间设为90 min,考查过硫酸钠浓度对盐酸四环素去除率的影响,结果如图3所示。
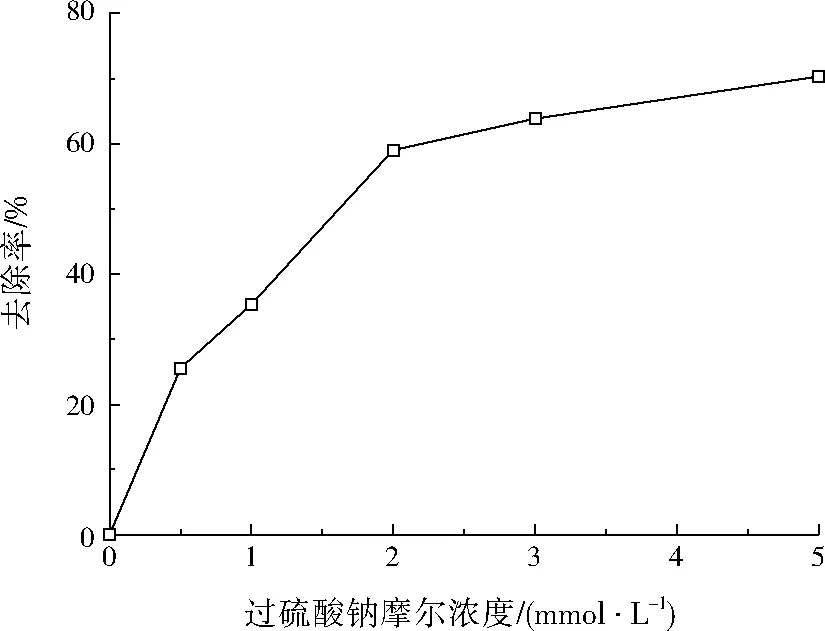
图3 过硫酸钠摩尔浓度对盐酸四环素去除率的影响Fig.3 Effect of molar concentration of sodium persulfate on tetracycline hydrochloride removal rate

2.4 温度的影响
设定盐酸四环素初始质量浓度为50 mg/L,Fe2+摩尔浓度为1.00 mmol/L,过硫酸钠摩尔浓度为2.0 mmol/L,pH=3.0,调节反应温度分别为30、50、70、90 ℃,反应时间设为90 min,考查温度对盐酸四环素去除率的影响,结果如图4所示。

图4 温度对盐酸四环素去除率的影响Fig.4 Effect of temperature on tetracycline hydrochloride removal rate

dct/dt=-kct
(3)
式中:ct为t时刻溶液中盐酸四环素的质量浓度,mg/L;t为反应时间,min;k为一级反应速率常数,min-1。
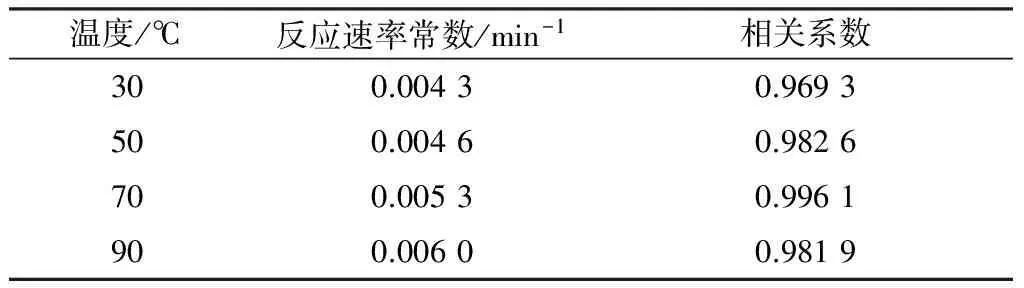
表1 不同温度下盐酸四环素降解的反应速率常数
k值随温度变化可用阿伦尼乌斯方程描述:
k=A×exp (-Ea/RT)
(4)
式中:A为指前因子,min-1;Ea为表观活化能,J/mol;R为摩尔气体常数,取8.314 J/(mol·K);T为热力学温度,K。
根据实验中不同温度下的反应速率常数k,对lnk与1/T作图(见图5),得出在上述反应体系下盐酸四环素降解的活化能为5.173 kJ/mol。
2.5 pH的影响
设定盐酸四环素初始质量浓度为50 mg/L,Fe2+摩尔浓度为1.00 mmol/L,过硫酸钠摩尔浓度为2.0 mmol/L,温度为30 ℃,调节溶液pH分别为3.0、7.0、9.0、12.0,反应时间设为90 min,考查pH对盐酸四环素去除率的影响,结果如图6所示。
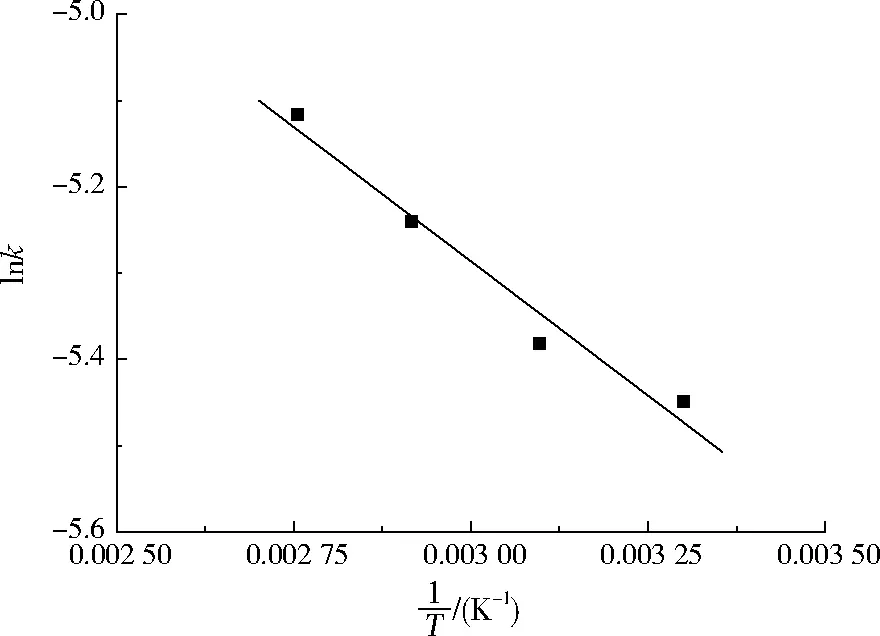
图5 反应速率常数拟合曲线Fig.5 The fitting curve of reaction rate constant
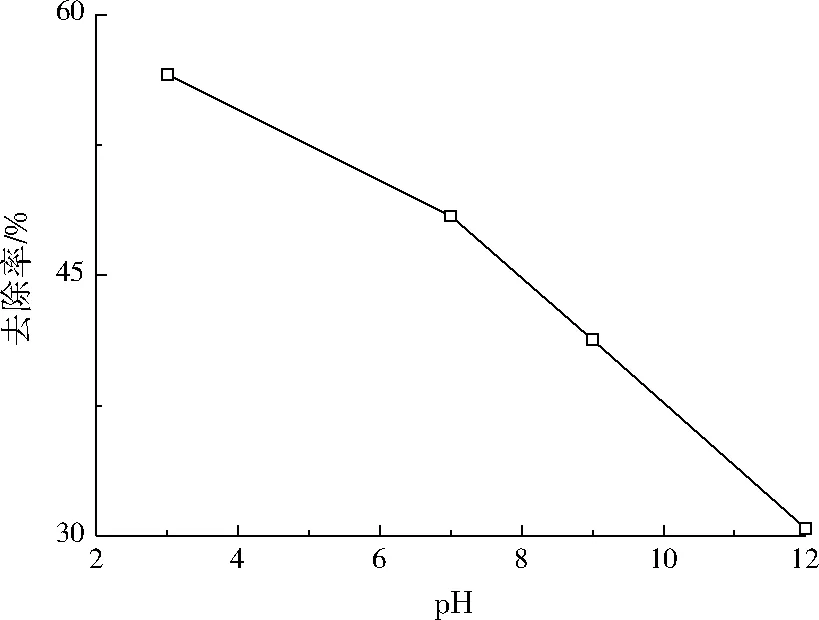
图6 pH对盐酸四环素去除率的影响Fig.6 Effect of pH on tetracycline hydrochloride removal rate
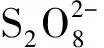
(5)
(6)
(7)
3 结 论
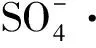
(2) 当盐酸四环素初始质量浓度为50 mg/L,Fe2+摩尔浓度为0.10 mmol/L,过硫酸钠摩尔浓度为2.0 mmol/L,反应温度为30 ℃,pH=3.0时,反应90 min后盐酸四环素去除率可达87.6%。
(3) Fe+/过硫酸钠体系对盐酸四环素的降解遵循一级反应动力学方程。该反应体系下盐酸四环素降解的活化能为5.173 kJ/mol。
[1] GU Cheng,KARTHIKEYAN K G.Interaction of tetracycline with aluminum and iron hydrous oxides[J].Environment Science & Technology,2005,39(8):2660-2667.
[2] 马艳,高乃云,姚娟娟,等.水中盐酸四环素的超声辐照降解[J].华南理工大学学报(自然科学版),2010,38(8):147-152.
[3] REYES C,FERNANDEZ J,FREER J,et al.Degradation and inactivation of tetracycline by TiO2photocatalysis[J].Journal of Photochemistry and Photobiology A:Chemistry,2006,184(1/2):141-146.
[4] KHAN M H,BAE H,JUNG J Y.Tetracycline degradation by ozonation in the aqueous phase:proposed degradation intermediates and pathway[J].Journal of Hazardous Materials,2010,181(1/2/3):659-665.
[5] JIAO Shaojun,ZHENG Shourong,YIN Daqiang,et al.Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria[J].Chemosphere,2008,73(3):377-382.
[6] DRZEWICZ P,PEREZ ESTRADA L,ALPATOVA A,et al.Impact of peroxydisulfate in the presence of zero valent iron on the oxidation of cyclohexanoic acid and naphthenic acids from oil sands process affected water[J].Environment Science & Technology,2012,46(16):8984-8991.
[7] ZHAO Li,JI Yuefei,KONG Deyang,et al.Simultaneous removal of bisphenol A and phosphate in zero-valent iron activated persulfate oxidation process[J].Chemicial Engineering Journal,2016,303:458-466.
[8] GAO Haiping,CHEN Jiabin,ZHANG Yaling,et al.Sulfate radicals induced degradation of triclosan in thermally activated persulfate system[J].Chemicial Engineering Journal,2016,306:522-530.
[9] GAYATHRI P,DORATHI R P J,PALANIVELU K.Sonochemical degradation of textile dyes in aqueous solution using sulphate radicals activated by immobilized cobalt ions[J].Ultrason Sonochem,2010,17(3):566-571.
[10] 廖云燕,刘国强,赵力,等.利用热活化过硫酸盐技术去除阿特拉津[J].环境科学学报,2014,34(4):931-937.
[11] KOLTHOFF I M,MILLER I K.The chemistry of persulfate.Ⅰ. The kinetics and mechanism of the decomposition of the persulfate ion in aqueous medium[J].Journal American Chemical Society,1951,73(7):3055-3059.
[12] CRIQUET J,LEITNER N K V.Degradation of acetic acid with sulfate radical generated by persulfate ions photolysis[J].Chemosphere,2009,77(2):194-200.
[13] ZOU Xiaoli,ZHOU Tao,MAO Juan,et al.Synergistic degradation of antibiotic sulfadiazine in a heterogeneous ultrasound-enhanced Fe0/persulfate Fenton-like system[J].Chemicial Engineering Journal,2014,257:36-44.
StudyondegradationoftetracyclinehydrochloridebyFe2+/sodiumpersulfatesystem
ZHANGLiangbo,XUChunhong,ZHUHuina,LIYing,ZHANGBaozhong.
(SchoolofChemistry,ChemicalEngineeringandEnvironment,HenanUniversityofTechnology,ZhengzhouHenan450001)
10.15985/j.cnki.1001-3865.2017.07.016
2016-12-15)
张良波,男,1979年生,博士,讲师,研究方向为高级氧化技术及新型环境功能材料。
*河南省科技攻关项目(No.172102210031);河南工业大学高层次人才基金资助项目(No.2012BS057);河南工业大学科技创新人才培育计划项目(No.2014CXRC05);河南省高等学校重点科研项目(No.16B610004)。
