人工湿地氮去除关键功能微生物生态学研究进展
2017-11-03肖润林吴金水
陈 亮,刘 锋,*,肖润林,吴金水,*
1 中国科学院亚热带农业生态研究所,亚热带农业生态过程重点实验室, 长沙 410125 2 中国科学院亚热带农业生态研究所,长沙农业环境监测研究站, 长沙 410125
人工湿地氮去除关键功能微生物生态学研究进展
陈 亮1,2,刘 锋1,2,*,肖润林1,2,吴金水1,2,*
1 中国科学院亚热带农业生态研究所,亚热带农业生态过程重点实验室, 长沙 410125 2 中国科学院亚热带农业生态研究所,长沙农业环境监测研究站, 长沙 410125
人工湿地是一种能有效处理水体氮素污染的生态技术,其中微生物是驱动人工湿地系统中氮素去除的重要引擎。近20年来,随着分子生物学技术的广泛应用,有关人工湿地氮去除功能微生物生态学方面研究取得了一些重要进展。以硝化-反硝化作用和厌氧氨氧化作用这两种重要的人工湿地微生物脱氮途径为主,针对氨氧化细菌/古菌、厌氧氨氧化菌和反硝化菌等关键脱氮功能微生物的研究,重点归纳总结了目前有关这几类关键功能菌群在人工湿地中的丰度、活性、多样性、分布特征与影响因素,及其对废水中氮去除的作用,并在此基础上对今后的重点研究工作提出了展望。面向未来人工湿地氮去除关键功能微生物的研究应侧重其在污水净化和温室气体减排等方面的生态功能研究,同时加强其代谢过程与机制以及不同功能菌群间的关联研究。
人工湿地;氨氧化细菌/古菌;厌氧氨氧化菌;反硝化菌
随着社会经济和农业生产的发展,大量外源氮素进入自然水体中造成了严重的河流湖泊等水环境富营养化问题。当前,人工湿地已成为有效削减水体中外源氮素的重要技术手段,在处理非点源污染带来的氮负荷更是如此。人工湿地是由基质(原位土壤或人工填料)、生长在其上的水生植物和附着与悬浮在二者上的微生物所组成的生态系统。根据污水在系统中流动方式的差异,人工湿地污水处理系统通常可分为表面流(surface flow constructed wetlands, SFCW)和潜流(subsurface flow constructed wetlands, SSFCW)两种类型,后者又分为水平流(horizontal subsurface flow constructed wetlands, HSSFCW)和垂直流(vertical subsurface flow constructed wetlands, VSSFCW)两种进水方式;与传统污水处理工艺相比,人工湿地具有氮去除效果好、耐冲击负荷能力强、运行管理费用低和生态环境友好等优点[1]。
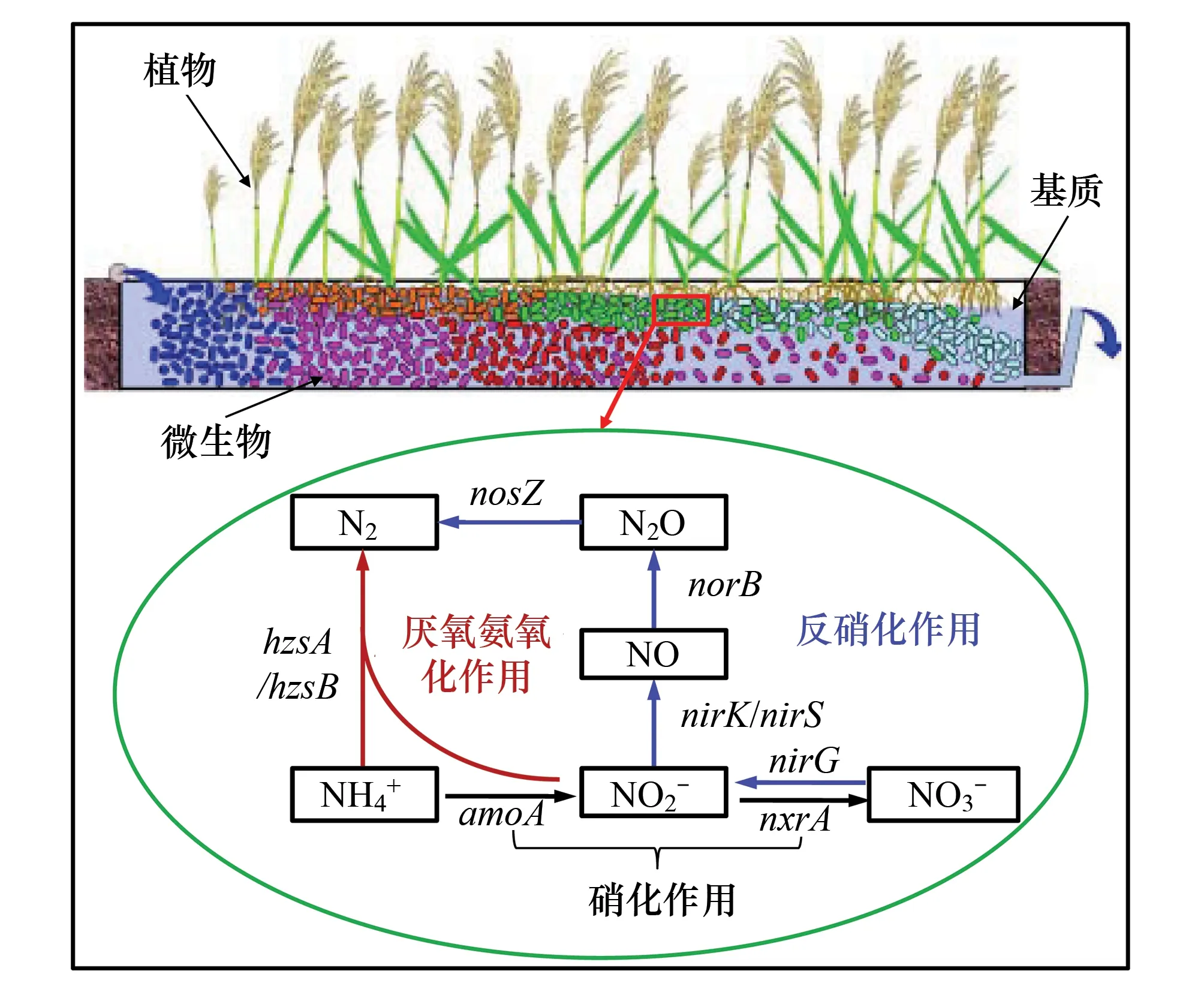
图1 人工湿地氮去除关键微生物过程及相应功能基因 Fig.1 The key microbial N-removal pathways and their related functional genes in constructed wetland

1 氨氧化细菌或古菌(AOB/AOA)
氨氧化是硝化作用的第一个反应步骤,也是限速步骤,是人工湿地氮去除过程的重要环节。典型的氨氧化过程通常认为是一个主要由变形菌门(Proteobacteria)中的一小部分细菌类群所进行的专性好氧的化能自养过程,这类细菌被称为氨氧化细菌(AOB)。然而近年来越来越多的证据显示,在自然界原核生物组成中占重要比例的中温泉古菌(non-thermophilic Crenarchaeota)(后单独划分为奇古菌门(Thaumarchaeota))也具有氨氧化能力,这类古菌被称作氨氧化古菌(AOA)[6]。这两类具有氨氧化作用的微生物普遍存在于海洋沉积物、湖泊底泥和陆地土壤等不同的生境中。在典型的微生物群落中,AOB所占的比例通常<0.1%,且主要集中在亚硝化球菌属(Nitrosococcus)、亚硝化单胞菌属(Nitrosomonas)和亚硝化螺菌属(Nitrosospira)这3个属[7]。相比之下,AOA无论在数量和多样性上普遍都要高于AOB,已知的主要种属有Nitrosopumilus、Nitrosophaera、Nitrosocaldus、Nitrosotalea等,但目前尚未确定是否所有的奇古菌均具有氨氧化能力[8]。

氨氧化微生物作为驱动人工湿地氮转化的一类重要功能微生物得到了研究者的广泛关注(表1)。氨氧化菌群落在人工湿地中的不同区域分布不同,植物根系是微生物最活跃的区域,根区为微生物提供了结构不同的附着表面并形成了根系分泌物和输氧浓度梯度,通常AOB数量和种类在氧含量较高的基质表层和植物根区相对较多[12]。如靖元孝等[13]研究的风车草(Cyperusalternifolius)人工湿地系统中,根区的硝化微生物数量和硝化强度远高于非根区。此外,AOB的群落结构在人工湿地系统中通常比较稳定,Roberts等[14]利用FISH技术对潜流湿地系统生物膜中氨氧化细菌Nitrosomonas的原位分析发现氨氧化菌从生物膜形成的初期就已经存在,在5个月的实验期间其占总细菌种群的比例约1%,但在提高氨氮浓度的一段时间里,Nitrosomonas很快增加到总细菌种群的7%。Ibekwe等[15]采用变性梯度凝胶技术(DGGE)研究处理奶牛场废水的人工湿地中AOB组成变化情况,发现AOB种类在系统中以Nitrosospira为主且在试验期间没有发生优势种群变化。在人工湿地系统中,AOB主要以Nitrosomonas和Nitrosospira这两种属居多,且前者比后者具有更低的基质亲和力和更高的活性,因此Nitrosospira通常发现存在于低氨氮环境中且更能忍受外界物理化学环境条件的变化,而高氨氮浓度环境下更易导致Nitrosomonas成为占主导地位的唯一优势种[16- 18]。许多不同的环境因素包括温度、pH和盐度,以及氨氮和有机碳负荷等都会影响氨氧化微生物的多样性及组成。Dong等[9]对处理养猪废水的“湿地-塘-湿地”组合人工表面流湿地系统中潜在硝化速率和AOB群落进行了研究,研究发现AOB数量和硝化速率与湿地中氨氮浓度均呈显著正相关关系。Sims等[19]对一污水处理厂出水的表面流人工湿地中AOA和AOB进行了为期两年的季节性采样分析,q-PCR结果显示人工湿地底泥和水中的AOA丰度在冬夏季都普遍高于AOB,进一步采用末端限制性片段长度多态性分析(T-RFLP)发现在冬季AOB普遍具有较低的峰强,表明其对温度的敏感度要高于AOA,研究还发现人工湿地的硝化作用强度只与AOB丰度呈显著相关,表明该湿地中硝化作用主要是由AOB驱动的。人工湿地不同种类的植物以及其它微生物也会对氨氧化菌群结构造成影响。黄娟等[20]对低温域(0—15℃)下黄菖蒲、菖蒲和香蒲人工湿地系统中硝化强度和AOB/AOA数量的研究表明不同植物根际氨氧化过程的主要功能微生物具有一定差异,AOA和AOB对于湿地土壤氮转化均具有不可忽视的作用,并与植物本体、土壤硝化过程微环境之间有一定的耦合关系。Schramm等[21]研究证实氨氧化菌和异养微生物间存在竞争,随着生物膜成熟异养微生物的增加会降低氨氧化菌的种群数量。这些研究表明人工湿地系统中普遍存在氨氧化微生物,且其丰度、群落结构和代谢活性受温度、pH、溶解氧、氧化还原电位、氨氮浓度、植物等外界环境因素的影响。
2 反硝化细菌
微生物的反硝化作用是将氮素以N2O或N2形式从人工湿地中最终去除的过程。反硝化菌为兼性厌氧菌,多为化学异养型菌,在对氮转化的过程中只获得能量,并以有机物为电子供体和细胞生长碳源。具有反硝化能力的微生物种类较多,土壤中重要的反硝化细菌包括芽孢杆菌属(Bacillus)、假单胞菌属(Pseudomonas)、微球菌(Micrococcus);水体中主要的反硝化菌有假单胞菌属(Pseudomonas)、产气单胞菌属(Aeromonas)和弧菌属(Vibrio);其它反硝化菌有气杆菌属(Aerobacter)、产碱杆菌属(Alcalogenes)、短杆菌(Brevibacterium)、黄杆菌属(Flavobactrium)等[29]。

表1 人工湿地系统中氨氧化微生物(AOA/AOB)的国内外研究概括
反硝化作用的4个过程分别由硝酸还原酶(Nar)、亚硝酸还原酶(Nir)、氧化氮还原酶(Nor)和氧化亚氮还原酶(Nitrous oxide reductase, Nos)进行催化,相应编码基因分别为nar、nir、nor、nos[30]。目前针对环境样品研究者较多选择nirK/nirS和nosZ作为分子标记基因来研究反硝化菌群落多样性和丰度情况。反硝化菌活性的测定普遍采取的是乙炔抑制法[31-32]。该方法利用乙炔能够抑制N2O还原成N2的原理,向采得的样品柱通入一定量乙炔气,培养一段时间后,从中抽取一定量气体,用气相色谱分析N2O的浓度,据此计算反硝化速率。
对人工湿地系统中反硝化菌群落的研究普遍发现具有较高的丰度和多样性。Ruiz-Rueda等[33]在处理市政废水的表面流湿地中发现存在丰富的反硝化功能基因nirS。Chon等[34]采用定量PCR技术检测处理污水处理厂出水的人工湿地中反硝化功能基因(narG、nirS和nosZ)丰度都在106—109copies/g间,并呈现季节性差异。Kjellin等[35]通过DGGE研究nosZ功能基因多样性发现越靠近人工湿地进水口反硝化菌群落越简单,且延长水力停留时间会导致表面流湿地底泥中反硝化菌的群落结构更复杂,说明底泥中碳氮浓度会对反硝化菌群具有重要影响,浓度越高菌群结构越简单优势种更明显。Song等[32]认为要使湿地中具有最大的反硝化速率必须兼有最佳的环境条件和代谢活性,该研究利用T-RFLP技术监测了一个新建人工湿地中反硝化群落的年际变化情况,结果发现第二年湿地中反硝化菌的群落结构更简单且稳定,活性也比第一年强,相关分析表明反硝化作用主要受湿地环境中温度,pH和有机碳(DOC)浓度的影响很大。一般来说氧的存在会抑制反硝化酶,但有的人工湿地中存在能够在有氧条件下进行反硝化的微生物[11],这种好氧反硝化菌含有不受氧气抑制的周质硝酸盐还原酶(Nap)。温度变化对反硝化菌有强烈影响,在温度低于5℃时反硝化速度很慢,并且在温度低进行的反硝化通常不彻底会产生N2O和NO。通常增加人工湿地中DOC含量会提高反硝化速率,但Burchell等[36]研究表明冬季低温(7.5℃)情况下反硝化速率对增加有机质并没有响应。反硝化的最佳pH值为6—8,低于5时反硝化菌的活性受到很大抑制。植物对人工湿地反硝化菌群的影响主要是通过根际分泌有机碳,许多研究证实有植物比无植物情况下对NO3-N的去除效率更高,且反硝化功能基因、速率大小会因植物种类不同而不同[33,37-39]。
3 厌氧氨氧化细菌



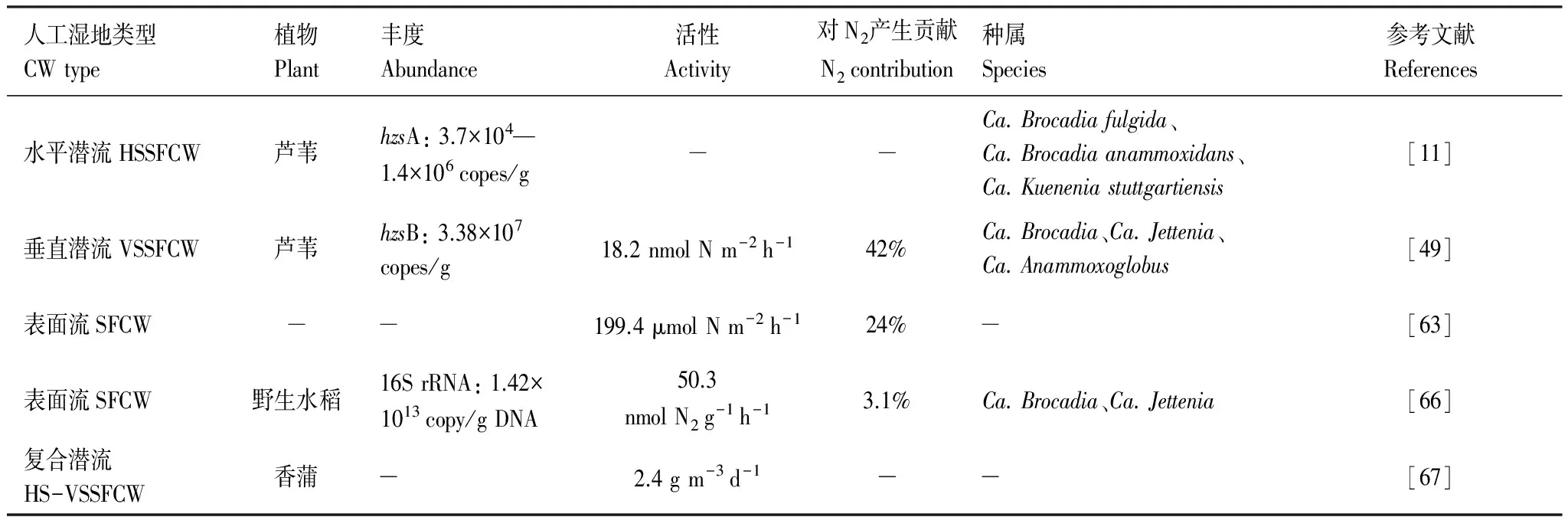
表2 人工湿地系统中厌氧氨氧化菌的国内外研究概括
4 关键功能微生物对人工湿地中氮去除的效应

微生物是人工湿地氮去除能力的重要评价指标,最常见的研究方法是用微生物数量和活性作为评价污染物去除率的参考指标,探讨微生物群落的组成和分布,从而了解人工湿地处理废水时微生物在其中的作用过程。目前明确阐述关键功能微生物类群在人工湿地氮去除作用与机制的研究还较少,如某些氮转化功能微生物对温室气体的释放具有一定的调节作用,而如何应用它们控制温室气体的排放还有待深入研究。
5 展望
综上所述,现代分子生物学技术的发展为人工湿地微生物生态研究开启了新纪元,有关氮转化功能菌群在复杂的人工湿地系统中的存在、多样性组成及活性等得到了广泛研究,这对于我们深刻理解人工湿地氮去除的复杂过程具有重要推动作用。如何提高废水中氮的处理效率依然是未来人工湿地技术需解决的重要问题之一,而氮转化相关功能微生物的研究可谓重点。面向未来的人工湿地功能微生物与氮去除研究,应以新技术新方法为手段,重点开展以下几个方面的研究。
(1)进一步探索与挖掘新型氮转化功能微生物在人工湿地中的群落分布与生态功能。厌氧氨氧化菌、氨氧化古菌和一步硝化菌的发现更新了人们已有的对氮循环的认识,而新型氮转化功能种群在人工湿地系统中的研究报道还非常少,许多问题有待更多研究。例如厌氧氨氧化菌在不同类型和尺度的人工湿地系统以及不同时空变化下的普遍性和活性强度如何还不清楚,如何进一步发挥其在某些特殊废水处理中的应用潜力也需更多研究。尽管氨氧化古菌在一些人工湿地系统中能够检测到很高丰度和多样性,但大多研究表明氨氧化作用主要是由氨氧化细菌主导,对于这类含amoA基因的氨氧化古菌在系统中大量存在和分布具有的生理功能或生态学意义尚不清楚。因此,进一步采用先进分子生物学技术(如高通量测序技术等)并结合各种原位分析手段(如基于DNA分析的稳定同位素探测技术(DNA-SIP)等)明确这两类氨氧化菌群在人工湿地氮转化过程中的作用和其它潜在的生态功能是未来研究的一个重点。
(2)加强人工湿地中氮去除关键微生物的代谢过程与机制的研究。在很多情况下,相关的监测及通量(如进出水中氮浓度变化、氨挥发、N2O释放等)和反应速率(如硝化、反硝化速率、厌氧氨氧化速率等)与氮转化功能微生物的群落多样性信息并没有建立直接关联,而环境因素对其功能作用的表达又具有重要影响,因此加强对这些关键微生物的作用过程及影响因素的认识是发展调控人工湿地氮去除有效方法的重要前提。
(3)不同氮去除关键微生物菌群间的关联研究。当前研究多只针对某一类功能微生物菌群及过程独立开展,同一系统中硝化、反硝化和厌氧氨氧化等菌群间的关系以及它们各自驱动的氮转化过程是如何交替发生的尚不清楚。因此在特定人工湿地系统中研究不同氮转化功能菌群间的耦合关系,以及这种耦合关系下对废水中氮去除的贡献作用,通过关联微生物丰度和功能活性与各形态氮转化通量,构建相关氮去除模型,为最终调控人工湿地微生物过程提高氮去除率提供科学依据。
[1] Vymazal J. Removal of nutrients in various types of constructed wetlands. Science of the Total Environment, 2007, 380(1/3): 48- 65.
[2] 卢少勇, 金相灿, 余刚. 人工湿地的氮去除机理. 生态学报, 2006, 26(8): 2670- 2677.
[3] Truu M, Juhanson J, Truu J. Microbial biomass, activity and community composition in constructed wetlands. Science of the Total Environment, 2009, 407(13): 3958- 3971.
[4] Faulwetter J L, Gagnon V, Sundberg C, Chazarenc F, Burr M D, Brisson J, Camper A K, Stein O R. Microbial processes influencing performance of treatment wetlands: a review. Ecological Engineering, 2009, 35(6): 987- 1004.
[5] 边玉, 阎百兴, 欧洋. 人工湿地微生物研究方法进展. 湿地科学, 2014, 12(2): 235- 242.
[6] Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nature Reviews Microbiology, 2008, 6(3): 245- 252.
[7] Purkhold U, Pommerening-Rōser A, Juretschko S, Schmid M C, Koops H P, Wagner M. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA andamoA sequence analysis: implications for molecular diversity surveys. Applied and Environmental Microbiology, 2000, 66(12): 5368- 5382.
[8] 张丽梅, 贺纪正. 一个新的古菌类群—奇古菌门(Thaumarchaeota). 微生物学报, 2012, 52(4): 411- 421.
[9] Dong X L, Reddy G B. Ammonia-oxidizing bacterial community and nitrification rates in constructed wetlands treating swine wastewater. Ecological Engineering, 2012, 40: 189- 197.
[10] Sims A, Horton J, Gajaraj S, McI ntosh S, Miles R J, Mueller R, Reed R, Hu Z Q. Temporal and spatial distributions of ammonia-oxidizing archaea and bacteria and their ratio as an indicator of oligotrophic conditions in natural wetlands. Water Research, 2012, 46(13): 4121- 4129.
[11] Coban O, Kuschk P, Kappelmeyer U, Spott O, Martienssen M, Jetten M S M, Knoeller K. Nitrogen transforming community in a horizontal subsurface-flow constructed wetland. Water Research, 2015, 74: 203- 212.
[12] Kyambadde J, Kansiime F, Dalhammar G. Distribution and activity of ammonium-oxidizing bacteria in Nakivubo wastewater channel and wastewater treatment wetland, Uganda. Clean Soil Air Water, 2006, 34(1/2): 137- 145.
[13] 靖元孝, 杨丹菁. 风车草(Cyperusalternifolius)人工湿地系统氮去除及氮转化细菌研究. 生态科学, 2004, 23(1): 89- 91.
[14] Silyn-Roberts G, Lewis G.Insituanalysis ofNitrosomonasspp. In wastewater treatment wetland biofilms. Water Research, 2001, 35(11): 2731- 2739.
[15] Ibekwe A M, Grieve C M, Lyon S R. Characterization of microbial communities and composition in constructed dairy wetland wastewater effluent. Applied and Environmental Microbiology, 2003, 69(9): 5060- 5069.
[16] Okabe S, Satoh H, Watanabe Y.Insituanalysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Applied and Environmental Microbiology, 1999, 65(7): 3182- 3191.
[17] Purkhold U, Wagner M, Timmermann G, Pommerening-Röser A, Koops H P. 16S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: extension of the dataset and proposal of a new lineage within the nitrosomonads. International Journal of Systematic and Evolutionary Microbiology, 2003, 53: 1485- 1494.
[18] Bäckman J S K, Hermansson A, Tebbe C C, Lindgren P E. Liming induces growth of a diverse flora of ammonia-oxidising bacteria in acid spruce forest soil as determined by SSCP and DGGE. Soil Biology and Biochemistry, 2003, 35(10): 1337- 1347.
[19] Sims A, Gajaraj S, Hu Z Q. Seasonal population changes of ammonia-oxidizing organisms and their relationship to water quality in a constructed wetland. Ecological Engineering, 2012, 40: 100- 107.
[20] 黄娟, 杨思思, 李润青, 傅大放. 低温域湿地植物根际硝化强度及氨氧化微生物研究. 环境科学研究, 2014, 27(8): 857- 864.
[21] Schramm A, Larsen L H, Revsbech N P, Ramsing N B, Amann R, Schleifer K H. Structure and function of a nitrifying biofilm as determined byinsituhybridization and the use of microelectrodes. Applied and Environmental Microbiology, 1996, 62(12): 4641- 4647.
[22] 黄德锋, 李田. 复合垂直流湿地氨氧化菌种群结构及活性的空间分布. 环境科学, 2008, 29(8): 2160- 2165.
[23] Ruiz-Rueda O, Hallin S, Baeras L. Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. FEMS Microbiology Ecology, 2009, 67(2): 308- 319.
[24] Domingos S S, Dallas S, Skillman L, Felstead S, Ho G. Nitrogen removal and ammonia-oxidising bacteria in a vertical flow constructed wetland treating inorganic wastewater. Water Science and Technology, 2011, 64(3): 587- 594.
[25] Wang F, Liu Y, Ma Y X, Wu X R, Yang H Z. Characterization of nitrification and microbial community in a shallow moss constructed wetland at cold temperatures. Ecological Engineering, 2012, 42: 124- 129.
[26] 仝欣楠, 王欣泽, 何小娟, 孔海南. 人工芦苇湿地氨氮污染物去除及氨氧化菌群落多样性分析. 环境科学研究, 2014, 27(2): 218- 224.
[27] Wang L, Li T. Effects of seasonal temperature variation on nitrification, anammox process, and bacteria involved in a pilot-scale constructed wetland. Environmental Science and Pollution Research, 2015, 22(5): 3774- 3783.
[28] Li X, Zhang M M, Liu F, Li Y, He Y, Zhang S A, Wu J S. The significance ofMyriophyllumelatinoidesfor swine wastewater treatment: Abundance and community structure of ammonia-oxidizing microorganisms in sediments. PLoS One, 2015, 10(10): e0139778.
[29] Grant W P, Long P E. Environmental Microbiology. Glasgow, UK: Thomson Science and Professional, 1981.
[30] 郑平. 环境微生物学(第2版). 杭州: 浙江大学出版社, 2012.
[31] Tiedje J M. Denitrification // Page A L, Ed. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties. Madison: American Society of Agronomy, 1982, 1011- 1026.
[32] Song K, Lee S H, Kang H. Denitrification rates and community structure of denitrifying bacteria in newly constructed wetland. European Journal of Soil Biology, 2011, 47(1): 24- 29.
[33] Ruiz-Rueda O, Trias R, Garcia-Gil L J, Baeras L. Diversity of the nitrite reductase genenirS in the sediment of a free-water surface constructed wetland. International Microbiology, 2007, 10(4): 253- 260.
[34] Chon K, Chang J S, Lee E, Lee J, Ryu J, Cho J. Abundance of denitrifying genes coding for nitrate (narG), nitrite (nirS), and nitrous oxide (nosZ) reductases in estuarine versus wastewater effluent-fed constructed wetlands. Ecological Engineering, 2011, 37(1): 64- 69.
[35] Kjellin J, Hallin S, Wörman A. Spatial variations in denitrification activity in wetland sediments explained by hydrology and denitrifying community structure. Water Research, 2007, 41(20): 4710- 4720.
[36] Burchell M R, Skaggs R W, Lee C R, Broome S, Chescheir G M, Osborne J. Substrate organic matter to improve nitrate removal in surface-flow constructed wetlands. Journal of Environmental Quality, 2007, 36(1): 194- 207.
[37] Bachand P A M, Horne A J. Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecological Engineering, 2000, 14(1/2): 17- 32.
[38] Lin Y F, Jing S R, Wang T W, Lee D Y. Effects of macrophytes and external carbon sources on nitrate removal from groundwater in constructed wetlands. Environmental Pollution, 2002, 119(3): 413- 420.
[39] Hallin S, Hellman M, Choudhury M I, Ecke F. Relative importance of plant uptake and plant associated denitrification for removal of nitrogen from mine drainage in sub-arctic wetlands. Water Research, 2015, 85: 377- 383.
[40] Mulder A, Van De Graaf A A, Robertson L A, Kuenen J G. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiology Ecology, 1995, 16(3): 177- 184.
[41] Thamdrup B, Dalsgaard T. Production of N2through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Applied and Environmental Microbiology, 2002, 68(3): 1312- 1318.
[42] Dalsgaard T, Canfield D E, Petersen J, Thamdrup B, Acua-González J. N2production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica. Nature, 2003, 422(6932): 606- 608.
[43] Kuypers M M M, Sliekers A O, Lavik G, Schmid M, Jørgensen B B, Kuenen J G, Damsté J S S, Strous M, Jetten M S M. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature, 2003, 422(6932): 608- 611.
[44] Zhang Y, Ruan X H, den Camp H J M O, Smits T J M, Jetten M S M, Schmid M C. Diversity and abundance of aerobic and anaerobic ammonium-oxidizing bacteria in freshwater sediments of the Xinyi River (China). Environmental Microbiology, 2007, 9(9): 2375- 2382.
[45] Zhu G B, Wang S Y, Wang W D, Wang Y, Zhou L L, Jiang B, den Camp H J M O, Risgaard-Petersen N, Schwark L, Peng Y Z, Hefting M M, Jetten M S M, Yin C Q. Hotspots of anaerobic ammonium oxidation at land-freshwater interfaces. Nature Geoscience, 2013, 6(2): 103- 107.
[46] Wang S Y, Zhu G B, Peng Y Z, Jetten M S M, Yin C Q. Anammox bacterial abundance, activity, and contribution in Riparian sediments of the Pearl River Estuary. Environment Science & Technology, 2012, 46(16): 8834- 8842.
[47] Hu B L, Shen L D, Zheng P, Hu A H, Chen T T, Cai C, Liu S, Lou L P. Distribution and diversity of anaerobic ammonium-oxidizing bacteria in the sediments of the Qiantang River. Environmental Microbiology Reports, 2012, 4(5): 540- 547.
[48] Hou L J, Zheng Y L, Liu M, Li X F, Lin X B, Yin G Y, Gao J, Deng F Y, Chen F, Jiang X F. Anaerobic ammonium oxidation and its contribution to nitrogen removal in China′s coastal wetlands. Scientific Reports, 2015, 5: 15621.
[49] Zhu G B, Wang S Y, Wang Y, Wang C X, Risgaard-Petersen N, Jetten M S M, Yin C Q. Anaerobic ammonia oxidation in a fertilized paddy soil. The ISME Journal, 2011, 5(12): 1905- 1912.
[50] Yang R X, Li H, Nie S N, Su J Q, Weng B S, Zhou G B, Yao H Y, Gilbert J A, Zhu Y G, Nojiri H. Potential contribution of anammox to nitrogen loss from paddy soils in southern China. Applied and Environmental Microbiology, 2015, 81(3): 938- 947.
[51] Strous M, Fuerst J A, Kramer E H M, Logemann S, Muyzer G, van de Pas-Schoonen K T, Webb R, Kuenen J G, Jetten M S M. Missing lithotroph identified as new Planctomycete. Nature, 1999, 400(6743): 446- 449.
[52] Schmid M, Twachtmann U, Klein M, Strous M, Juretschko S, Jetten M, Metzger J W, Schleifer K H, Wagner M. Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Systematic and Applied Microbiology, 2000, 23(1): 93- 106.
[53] Kartal B, Rattray J, van Niftrik L A, van de Vossenberg J, Schmid M C, Webb R I, Schouten S, Fuerst J A, Damsté J S, Jetten M S M, Strous M.Candidatus“Anammoxoglobus propionicus” a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Systematic and Applied Microbiology, 2007, 30(1): 39- 49.
[54] Quan Z X, Rhee S K, Zuo J E, Yang Y, Bae J W, Park J R, Lee S T, Park Y H. Diversity of ammonium-oxidizing bacteria in a granular sludge anaerobic ammonium-oxidizing (anammox) reactor. Environmental Microbiology, 2008, 10(11): 3130- 3139.
[55] Lam P, Lavik G, Jensen M M, van de Vossenberg, Schmid M, Woebken D, Gutiérrez D, Amann R, Jetten M S M, Kuypers M M M. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(12): 4752- 4757.
[56] Dang H Y, Zhou H X, Zhang Z N, Yu Z S, Hua E, Liu X S, Jiao N Z. Molecular detection ofCandidatusScalinduapacifica and environmental responsees of sediment anammox bacterial community in the Bohai Sea, China. PLos One, 2013, 8(4): e61330.
[57] Sonthiphand P, Hall M W, Neufeld J D. Biogeography of anaerobic ammonia-oxidizing (anammox) bacteria. Frontiers in Microbiology, 2014, 5: 399.
[58] 聂三安, 於辰佳, 李虎, 杨小茹. 水稻土厌氧氨氧化活性测定的同位素示踪法方法探讨. 农业现代化研究, 2015, 36(4): 680- 683.
[59] Francis C A, Beman J M, Kuypers M M. New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. The ISME journal, 2007, 1(1): 19- 27.
[60] Dong Z Q, Sun T H. A potential new process for improving nitrogen removal in constructed wetlands-Promoting coexistence of partial-nitrification and anammox. Ecological Engineering, 2007, 31(2): 69- 78.
[61] Dong X L, Reddy G B. Soil bacterial communities in constructed wetlands treated with swine wastewater using PCR-DGGE technique. Bioresource Technology, 2010, 101(4): 1175- 1182.
[62] Ligi T, Truu M, Oopkaup K, Nõlvak H, Mander U, Mitsch W J, Truu J. The genetic potential of N2emission via denitrification and anammox from the soils and sediments of a created riverine treatment wetland complex. Ecological Engineering, 2015, 80: 181- 190.
[63] Erler D V, Eyre B D, Davison L. The contribution of anammox and denitrification to sediment N2production in a surface flow constructed wetland. Environmental Science & Technology, 2008, 42(24): 9144- 9150.
[64] Nie S A, Li H, Yang X R, Zhang Z J, Weng B S, Huang F Y, Zhu G B, Zhu Y G. Nitrogen loss by anaerobic oxidation of ammonium in rice rhizosphere. The ISME Journal, 2015, 9(9): 2059- 2067.
[65] Jin R C, Yang G F, Yu J J, Zheng P. The inhibition of the anammox process: a review. Chemical Engineering Journal, 2012, 197: 67- 79.
[66] Waki M, Yasuda T, Suzuki K, Komada M, Abe K. Distribution of anammox bacteria in a free-water-surface constructed wetland with wild rice (Zizanialatifolia). Ecological Engineering, 2015, 81: 165- 172.
[67] Tao W D, Wang J. Effects of vegetation, limestone and aeration on nitritation, anammox and denitrification in wetland treatment systems. Ecological Engineering, 2009, 35(5): 836- 842.
[68] Zhi W, Yuan L, Ji G D, He C G. Enhanced long-term nitrogen removal and its quantitative molecular mechanism in tidal flow constructed wetlands. Environmental Science & Technology, 2015, 49(7): 4575- 4583.
[69] Li X, Zhang M M, Liu F, Li Y, He Y, Zhang S N, Wu J S. Abundance and distribution of microorganisms involved in denitrification in sediments of aMyriophyllumelatinoidespurification system for treating swine wastewater. Environmental Science and Pollution Research, 2015, 22(22): 17906- 17916.
[70] Liu F, Zhang S N, Wang Y, Li Y, Xiao R L, Li H F, He Y, Zhang M M, Wang D, Li X, Wu J S. Nitrogen removal and mass balance in newly-formedMyriophyllumaquaticummesocosm during a single 28-day incubation with swine wastewater treatment. Journal of Environmental Management, 2016, 166: 596- 604.
[71] Paredes D, Kuschk P, Mbwette T S A, Stange F, Müller R A, Köser H. New aspects of microbial nitrogen transformations in the context of wastewater treatment-a review. Engineering in Life Sciences, 2007, 7(1): 13- 25.
[72] Zhu G B, Wang S Y, Feng X J, Fan G N, Jetten M S M, Yin C Q. Anammox bacterial abundance, biodiversity and activity in a constructed wetland. Environmental Science & Technology, 2011, 45(23): 9951- 9958.
ResearchadvancesinmicrobialecologyforN-removalinconstructedwetlands
CHEN Liang1,2, LIU Feng1,2,*, XIAO Runlin1,2, WU Jinshui1,2,*
1KeyLaboratoryofAgro-ecologicalProcessesinSubtropicalRegion,InstituteofSubtropicalAgriculture,ChineseAcademyofSciences,Changsha410125,China2ChangshaResearchStationforAgricultural&EnvironmentalMonitoring,InstituteofSubtropicalAgriculture,ChineseAcademyofSciences,Changsha410125,China
A constructed wetland (CW) is an effective technology for the treatment of nitrogen (N) pollution in water bodies, with microorganisms being important engines driving N-removal. Over the past two decades, with the development of culture-independent molecular techniques, break-through progress has occurred in microbial ecology for N-removal in CWs. Nitrification-denitrification and anammox processes have been recognized as the two main microbial pathways for N-removal in CWs. In the present study, we reviewed the available literature regarding research progress in N-removal communities including archaeal and bacterial ammonia oxidizers, anammox bacteria, and denitrifying bacteria in CWs. Case studies on bacterial abundance, activities, diversity, distribution, influence factors, and contribution to N-removal are summarized, and future perspectives for this research field are presented. Future microbial ecology studies of N-removal in CWs should focus on the role of microorganisms in purification of sewage and emission reduction in greenhouse gases, and improve research on their N-metabolic processes, mechanisms, and interactions.
constructed wetland; ammonia oxidizing bacteria/archaea; anammox bacteria; denitrifying bacteria
中国博士后基金项目(2014M560648; 2015T80878);国家自然科学基金项目(41601272)
2016- 06- 15; < class="emphasis_bold">网络出版日期
日期:2017- 04- 25
*通讯作者Corresponding author.E-mail: liufeng@isa.ac.cn; jswu@isa.ac.cn
10.5846/stxb201606151159
陈亮,刘锋,肖润林,吴金水.人工湿地氮去除关键功能微生物生态学研究进展.生态学报,2017,37(18):6265- 6274.
Chen L, Liu F, Xiao R L, Wu J S.Research advances in microbial ecology for N-removal in constructed wetlands.Acta Ecologica Sinica,2017,37(18):6265- 6274.
