磁共振弥散张量和波谱成像在缺血性脑小血管疾病中的应用研究
2017-09-29张慧丽李仕红张颖冬周俊山殷信道
张慧丽,李仕红,张颖冬,周俊山,殷信道
磁共振弥散张量和波谱成像在缺血性脑小血管疾病中的应用研究
张慧丽1,李仕红2,张颖冬3,周俊山3,殷信道4*

目的探讨磁共振弥散张量成像(diffusion tensor imaging,DTI)联合磁共振波谱成像(magnetic resonance spectroscopy,MRS)对于缺血性脑小血管疾病(small vessel disease,SVD)的影像评估价值。材料与方法对42例缺血性SVD患者进行常规MRI、DTI和MRS扫描,测量病灶及健侧对称正常脑白质区域的平均弥散系数(average diffusion coefficient,DCavg)值、各向异性分数(fractional anisotropy,FA)值,测量病灶及其周围正常脑白质区域的N-乙酰天门冬氨酸(N-acetyl aspartic acid,NAA)、胆碱(choline,Cho)、肌酸(creatine,Cr)、肌醇(myo-inositol,MI)等生化代谢物的浓度值,并计算NAA/Cho、NAA/Cr、Cho/Cr、MI/Cr的比值。将42例SVD患者的缺血性病灶按照影像学显示分组,并对各组上述测量指标进行统计学分析。结果选取42例缺血性SVD患者的42个病灶并分成慢性缺血组(30例)和慢性期梗死灶组(12例)。SVD病灶的DCavg值和FA值分别较健侧镜像区正常脑白质的增高和降低(P<0.01),而两组间SVD病灶的DCavg值和FA值差异无统计学意义(P>0.05)。SVD病灶的NAA、Cho和Cr的均数都小于周围正常白质(P<0.01),而两组间的各项MRS代谢值差异均无统计学意义(P>0.05)。Pearson相关性分析,正常脑白质的DCavg值与FA值(r=-0.383,P=0.012)呈负相关,FA值与NAA/Cho (r=0.420,P=0.006)、NAA/Cr (r=0.382,P=0.012)之间均呈正相关,而在SVD病灶组无上述相关性。Spearman相关分析,SVD病灶组的DCavg与高血压呈正相关(r=0.338,P=0.029)。结论对于缺血性SVD,DCavg、FA、NAA、Cho和Cr等检测指标能够共同反映神经髓鞘结构的微观变化及其功能的破坏,联合应用DTI和MRS成像技术对缺血性SVD疾病进行研究,有助于其临床诊断及病理机制的研究。
弥散张量成像;磁共振波谱;脑小血管疾病;磁共振成像
在2013年的中国脑卒中大会上,有报道指出脑血管病已居于中国居民死亡原因第一位,而由脑小血管疾病(small vessel disease,SVD)引起的脑血管病变约占到1/3[1-2]。SVD是指由直径小于400μm的脑小血管病变导致的疾病,包括多发腔隙性脑梗(multiple lacunar infarction,MLI)、皮质下动脉硬化性脑病(subcortical arteriosclerotic encephalopathy,SAE)、脑白质疏松症(leucoaraiosis,LA)等[3-4]。近年来国内外多项研究表明[5-9],SVD与血管性认知障碍、血管性痴呆、脑卒中、下肢功能障碍、抑郁症等疾病有着密切关系。近年来,对于SVD的危险因素、病理机制等研究逐渐增多,其中功能磁共振扫描技术在SVD的研究中得到广泛应用。本研究主要针对脑SVD中的缺血性病变,运用磁共振弥散张量成像(diffusion tensor imaging,DTI)联合磁共振波谱成像(magnetic resonance spectroscopy,MRS)对其临床诊断及病理变化进行研究和探讨。
1 材料与方法
1.1 研究对象
选取至盐城市第三人民医院就诊的SVD患者共42例,对其颅脑进行常规磁共振(magnetic resonance imaging,MRI)、DTI和MRS扫描成像。42例患者中,男20例,女22例,年龄41~78岁,平均64.1岁,高血压患者26例,糖尿病患者13例。所选研究对象的准入标准如下:(1)患者颅脑MRI显示皮层下白质内单个病灶直径小于15 mm;(2)SVD病灶为非对称性的患者,即病灶为单侧性或病灶镜像区无病灶;(3)曾有SVD病史,同时无大血管病变的病灶,本次就诊行MRI常规检查时有脑白质疏松者。排除标准:(1)脑外伤、脑占位性病变、脑积水、一氧化碳中毒、低血糖等导致颅脑MRI检查脑白质异常表现者;(2)脑灰质腔梗患者;(3)腔梗合并大面积脑梗死患者。
1.2 MRI数据采集
MRI检查仪采用GE 3.0 T Signa HDx,线圈选用8通道头颈联合线圈。所有患者均行颅脑MRI常规扫DTI及MRS扫描。
1.2.1 常规MRI扫描
矢状位行T1WI (TR/TE=2956/24 ms),轴位行螺旋桨(Propeller) T2WI (TR/TE=5000/93 ms)、液体衰减反转恢复(fluid affenuated inversion recovery,FLAIR)序列的T1WI (TR/TE=1875/24 ms)和T2WI (TR/TE=8600/165 ms)、弥散加权成像(diffusion weighted imaging,DWI;TR/TE=5000/75.9 ms)。
1.2.2 DTI扫描
采用平面回波序列(echo planar imaging,EPI),在15个方向上行弥散权重采集,b=1000 s/mm2,取轴位扫描,28层,层厚5 mm,层距0 mm,FOV 24 cm×24 cm,NEX=1,TR/TE=6600/87.6 ms,扫描时间为2 min 10 s。
1.2.3 MRS扫描
采用磁共振氢质子波谱(1H-MRS)检查,以轴位T2WI作为MRS扫描定位像,采用PROBE-SI144序列,与扫描控制FWHM≤15,水抑制≥98%,扫描感兴趣区(region of interest, ROI)避开颅骨和脑脊液。扫描参数:TR/TE=1000/144 ms,层厚10 mm,FOV 24 cm×24 cm,NEX=1。
1.3 图像处理
图像处理采用GE公司SUN Workstation 4.3 工作站的Functool 5.4.07软件。
1.3.1 DTI图像处理
对DTI原始图像进行校正、调阈值、降噪、计算后生成平均弥散系数(average diffusion coefficient,DCavg)图和各向异性分数(fractional anisotropy,FA)图。以T2 FLAIR像为参照,在基底节区、侧脑室周围、半卵圆区和放射冠区选择直径小于15 mm的病灶,ROI大小范围在20~55 mm2。同时获得同等ROI大小的病灶及对侧脑白质镜像区正常脑组织的DCavg和FA值。
1.3.2 MRS图像处理
Functool软件自动完成MRS原始图像数据的基线校正、曲线校正、代谢物识别和代谢物波峰下面积的计算。依照所选病灶的DCavg图选择ROI内病灶中心和病灶周围正常脑组织的体素,体素选择时尽量避免脑脊液周边可能匀场不均的体素。1H-MRS采集的数据包括:(1)以下代谢值:N-乙酰天门冬氨酸(N-acetyl aspartic acid,NAA)、胆碱(choline,Cho)、肌酸(creatine,Cr)、肌醇(myoinositol,MI);(2)计算NAA/Cho、NAA/Cr、Cho/Cr、MI/Cr的比值。
1.4 数据统计分析
数据统计分析采用SPSS 16.0统计软件,计量资料用均数±标准差表示,两组间的均数比较采用独立样本t检验,配对设计的计量资料采用配对t检验,计量资料的相关性分析采用Pearson相关分析,对分类变量的相关分析采用Spearman分析。以P<0.05表示差异有统计学意义。
2 结果
2.1 病灶的MRI影像表现及分组情况
根据病灶在T1WI Flair、T2WI Flair及DWI成像序列上的不同信号表现,将42个病灶分成两组(见表1、图1)。
2.2 两组DTI数据分析
取42个病灶及其对侧脑镜像区同等大小ROI区域正常脑白质的DCavg值和FA值进行配对t检验,结果显示SVD病灶区域的DCavg值较正常脑白质区域升高(P<0.01),而病灶的FA值较正常脑白质区域降低(P<0.01),见表2。对慢性缺血灶组和慢性期梗死组病灶的DCavg值和FA值进行独立样本t检验,结果显示两组间的DCavg值和FA值差异均无统计学意义(P>0.05),见表3。
2.3 两组MRS数据分析
将42个SVD病灶中心与病灶周围正常白质区域内MRS各代谢值进行比较,病灶的NAA、Cho和Cr的均数都小于周围正常白质(P<0.01),而病灶的MI/Cr比值较正常白质高(P<0.05)。两组间MI值及其他比值差异无统计学意义(P>0.05),见表4。对慢性缺血灶组和慢性期梗死组病灶的各项MRS代谢值进行独立样本t检验,结果显示两组间的各项MRS代谢值差异均无统计学意义(P>0.05),见表5。

表1 42个病灶的MRI表现及分组情况Tab.1 The group of the 42 lesions by MRI displays
表2 SVD病灶与其对侧脑镜像区正常脑白质DCavg值和FA值的比较Tab. 2 The comparsion of DCavg value and FA value in the SVD lesion and the normal white matter on the contra-side

表2 SVD病灶与其对侧脑镜像区正常脑白质DCavg值和FA值的比较Tab. 2 The comparsion of DCavg value and FA value in the SVD lesion and the normal white matter on the contra-side
注:采用配对t检验,**P<0.01。
组别DCavg (e-10mm2/s)FA病灶组10.186±1.7500.256±0.086对侧正常脑白质组7.963±0.7350.340±0.108t值8.653-5.393P值0.000**0.000**
表3 慢性缺血灶组与慢性期梗死灶组的DCavg值和FA值的比较Tab. 3 The comparison of DCavg value and FA value between chronic ischemic focus group and chronic lacunar infarctions group
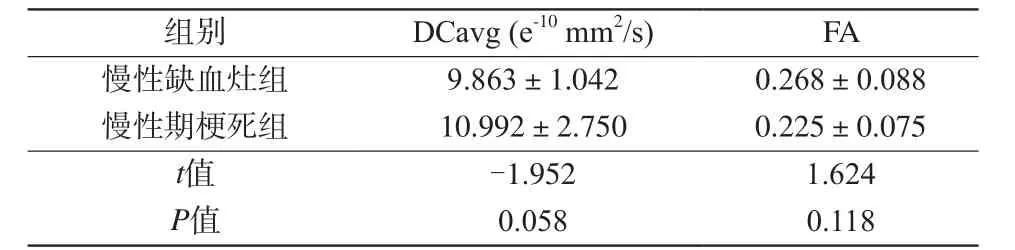
表3 慢性缺血灶组与慢性期梗死灶组的DCavg值和FA值的比较Tab. 3 The comparison of DCavg value and FA value between chronic ischemic focus group and chronic lacunar infarctions group
注:采用独立样本t检验,P<0.05为差异有统计学意义。
组别DCavg (e-10mm2/s)FA慢性缺血灶组9.863±1.0420.268±0.088慢性期梗死组10.992±2.7500.225±0.075t值-1.9521.624P值0.0580.118

图1 A:T2 Flair 显示右侧侧脑室前脚前方的低信号慢性期梗死灶;B:DWI显示病灶为低信号;C:DCavg图示病灶为高信号,小圆圈为在病灶及对侧镜像区设置的同等大小的ROI,测量DCavg值;D:FA显示病灶区域低信号,小圆圈为在病灶及对侧镜像区设置的同等大小的ROI,测量FA值Fig. 1 A: T2 Flair shows a chronic lacunar infarction with low signal in the front of right lateral ventricle forefoot; B: DWI shows the infarction with low signal; C: DCavg shows the infarction with high signal, the circles both in the infarction area and the symmetrical side white matter area are the same size ROI that used to measure DCavg value; D: FA shows the infarction with low signal, the circles both in the infarction area and the symmetrical side white matter area are the same size ROI that used to measure FA value.
表4 SVD病灶与病灶周围正常脑白质MRS各代谢值的比较Tab.4 The comparison of MRS metabolites in SVD lesions and the normal white around the lesions

表4 SVD病灶与病灶周围正常脑白质MRS各代谢值的比较Tab.4 The comparison of MRS metabolites in SVD lesions and the normal white around the lesions
注:采用配对t检验,**P<0.01表示差异有统计学意义。
组别NAAChoCrMINAA/ChoNAA/CrCho/CrMI/Cr SVD病灶9227.262±3047.5395520.143±2023.8874907.881±1517.6471078.619±374.7491.741±0.5181.909±0.5081.152±0.2320.235±0.071病灶周围正常白质11448.452±3811.0846725.190±2557.4545799.167±1802.0491138.690±385.2231.755±0.5091.983±0.390 1.152±0.2310.204±0.083t值-6.095-4.885-4.990-1.161-0.259-1.4910.0272.342P值0.000**0.000**0.000**0.2520.7970.1440.9790.024*
表5 慢性缺血灶组与慢性期梗死灶组的MRS各代谢值的比较Tab.5 The comparison of MRS metabolites between chronic ischemic focus group and chronic lacunar infarctions group

表5 慢性缺血灶组与慢性期梗死灶组的MRS各代谢值的比较Tab.5 The comparison of MRS metabolites between chronic ischemic focus group and chronic lacunar infarctions group
注:采用独立样本t检验,P<0.05为差异有统计学意义。
组别NAAChoCrMINAA/ChoNAA/CrCho/CrMI/Cr慢性缺血组9220.567±2941.3175598.233±2074.5394942.000±1626.8841047.333±348.2891.729±0.5141.903±0.4261.143±0.2260.221±0.066慢性期梗死组9244.000±3436.3515324.917±1965.4494822.583±263.3031156.833±440.7951.770±0.5501.923±0.6931.177±0.2560.270±0.073t值-0.022-0.401-0.254-0.770-0.224-0.110-0.407-2.028P值0.9820.6930.8020.4520.8520.9130.6890.057
2.4 两组DTI分析指标、MRS分析指标及高血压、糖尿病间的相关性分析
将SVD病灶的DCavg值和FA值,及其MRS的NAA、Cho、Cr、MI的代谢值与患者是否患有高血压或糖尿病进行Spearman相关分析,其中DCavg与高血压呈正相关(r=0.338,P=0.029),而SVD病灶对侧脑镜像区域的脑白质的DCavg值和FA值以及SVD病灶周围正常脑白质的NAA、Cho、Cr、MI的代谢值与高血压和糖尿病无相关性(P>0.05)。将SVD病灶的DCavg值、FA值与MRS的各代谢值之间进行Pearson相关性分析,各检测指标之间无任何相关性(P>0.05)。然而,在正常脑白质测得的DCavg值与FA值(r=-0.383,P=0.012)呈负相关,测得的FA值与NAA/Ch (r=0.420,P=0.006),以及FA值与NAA/Cr (r=0.382,P=0.012)之间均呈正相关。
3 讨论
SVD是指由脑的小血管及微血管(直径<400μm)病变而引起的一组疾病[10],它们临床表现相似、影像表现相近[11]。近10多年来,众多研究发现SVD与脑卒中、认知障碍、血管性痴呆、慢性肾脏疾病以及抑郁症等有着密切关系[7,12],从而引起了广泛关注及学者们的研究兴趣。随着功能磁共振技术的发展,MRI检查特别是DTI和MRS技术被越来越多地运用到对SVD的临床诊断以及病理机制的研究中[13-15]。
DTI成像技术是利用组织中水分子在人体组织中扩散运动存在的各向异性来探究组织超微结构的成像方法。常用的测量参数是平均弥散系数和部分FA,分别反映的是水分子扩散的能力和神经髓鞘的完整性、纤维致密性及平行性,即白质纤维束的完整程度。FA值越大,神经传导功能便越强。Van Norden等[16-17]认为MD值可作为衡量SVD进展的重要指标,且DCavg值和FA值与认知能力有着较强相关性,特别是FA值可被看作较T2WI更能反映认知能力的指标。
MRS是一种分子水平的成像,可以无创性地通过MRS扫描来了解人体一定体积的组织中化学物质的含量和浓度。本次研究所采集的检测指标是脑组织1H-MRS中较为常用的检测指标。NAA的含量可以反映成熟神经元的数量和功能状态,NAA较少意味着神经元发生不可逆转损伤。Cho是髓鞘形成、细胞代谢和胶质增生的指标,也是细胞膜的重要组成成分,反映了细胞膜磷脂代谢的情况,Cho增高说明细胞膜更新加快,细胞密度增大。Cr在脑组织中分布均匀且在各病理状态下数值相对稳定,因此常被用作比较其他代谢物浓度变化的参考值。NAA/Cr、Cho/Cr比值常作为反映神经元功能的定量指标,可判断神经元及髓鞘的完整性和损伤程度。MI则被认为是神经胶质的标志物[18]。
本研究中两组缺血性SVD病灶的DCavg值较健侧正常脑白质升高,而FA值则降低,MRS也检测到病灶区代谢物NAA、Cho和Cr较正常脑白质减低,这均提示两组SVD病灶都存在神经髓鞘的破坏,白质纤维束结构不完整,因此髓鞘对水分子垂直于神经纤维束方向上的运动限制减小,水分子弥散速度加快,这与相关文献报道一致[13,15]。而慢性缺血组和慢性期梗死灶组病灶的DCavg值和FA值之间差异没有统计学意义,同样,两组的MRS各代谢物数值及代谢物比值之间差异无统计学意义,原因可能有以下几个方面:(1)慢性缺血灶和慢性期梗死灶在病理变化上无明显差别或存在交叉病理变化;(2) DCavg值、FA值、NAA值及其他代谢物值只能反映神经髓鞘结构遭到破坏,结构不完整,而与结构破坏的程度无关[19];(3)可能与本研究中所选样本个体差异及抽样误差有关。
本研究中还发现缺血性SVD病灶的DCavg值与高血压因素呈正相关,而正常脑白质的DCavg与高血压无相关性,可能提示高血压对缺血性SVD病灶病理变化有影响。其次,正常脑白质区的DCavg值与FA值呈负相关,且FA值与NAA/Ch和NAA/Cr呈正相关,然而SVD病灶的上述指标并无明显相关性,提示上述DTI及MRS检测指标之间的相关性可以为SVD的诊断提供影像学依据,但其准确性还有待进一步研究。
综上所述,磁共振DTI和MRS两种功能成像技术分别从水分子弥散变化及组织代谢两个方面反映了脑白质神经纤维髓鞘结构的病理变化,对于缺血性SVD在临床诊断及病理机制研究等方面较传统MRI检查有着明显的优越性。
[References]
[1] Blanco-Rojas L, Arboix A, Canovas D, et al. Cognitive profile in patients with a first-ever lacunar infarct with and without silent lacunes: a comparative study. BMC Neurol, 2013, 13(1): 203.
[2] Fang M, Feng C, Xu Y, et al. Microbleeds and silent brain infarctions are differently associated with cognitive dysfunction in patients with advanced periventricular leukoaraiosis. Int J Med Sci, 2013, 10(10):1307-1313.
[3] Potter GM, Marlborough FJ, Wardlaw JM. Wide variation in definition, detection, and description of lacunar lesions on imaging.Stroke, 2011, 42(2): 359-366.
[4] Zhang HL, Zhang YD, Yin XD, et al. A preliminary study of diffusion tensor iimaging in cerebral small vessel disease. Journal of Nanjing Medical University (Natural Sciences), 2013, 33(11): 1604-1607,1612.张慧丽, 张颖冬, 殷信道, 等.磁共振弥散张量成像在脑小血管病变的应用初步研究. 南京医科大学学报(自然科学版), 2013, 33(11):1604-1607,1612.
[5] Lam A, Hamilton-Bruce MA, Jannes J, et al. Cerebral small vessel disease: genetic risk assessment for prevention and treatment. Mol Diagn Ther, 2008, 12(3): 145-156.
[6] Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain, 2005, 128(Pt 9): 2034-2041.
[7] Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol,2010, 9(7): 689-701.
[8] Verdelho A, Madureira S, Moleiro C, et al. White matter changes and diabetes predict cognitive decline in the elderly: the LADIS study.Neurology, 2010, 75(2): 160-167.
[9] Lin Q, Huang WQ, Teng CM. Genetic associations of leukoaraiosis indicate pathophysiological mechanisms in white matter lesions etiology. Rev Neurosci, 2015, 26(3): 343-358.
[10] Schmidtke K, Hull M. Cerebral small vessel disease: how does it progress?. J Neurol Sci, 2005, 229-230(3): 13-20.
[11] Liu N, Gao PY. Research situation on magnetic resonance imaging of cerebral small vessel disease. Chin J Stroke, 2014, 9(5): 450-454.刘妮, 高培毅. 脑小血管病磁共振影像研究概况. 中国卒中杂志,2014, 9(5): 450-454.
[12] Zhang AJ, Yu XJ, Wang M. The clinical manifestations and pathophysiology of cerebral small vessel disease. Neurosci Bull,2010, 26(3): 257-264.
[13] Liu J, Zhang LL, Zhang XP, et al. MRS and DTI study of cerebral white matter in small vessel ischemic disease. J Community Med,2014, 12(21): 1-4.刘晋, 张鲁临, 张小鹏, 等. 脑白质小血管缺血性病变的MRS、DTI研究. 社区医学杂志, 2014, 12(21): 1-4.
[14] Wen CY, Wang SY, Wang MH, et al. DTI study of relativity between cerebral small vessel disease and cognitive impairment. J Med Res,2013, 42(2): 174-177.闻彩云, 王溯源, 王美豪, 等. 脑小血管病变与认知功能损害相关性的DTI研究. 医学研究杂志, 2013, 42(2): 174-177.
[15] Yuan Y, Gu JP, Yin XD. Study of cerebral white matter in small vessel ischemic disease with MRS and DTI. Pract Geriatr, 2014,28(11): 941-944.袁勇, 顾建平, 殷信道. 脑白质小血管缺血性病变的MRS、DTI研究. 实用老年医学, 2014, 28(11): 941-944.
[16] van Norden AG, de Laat KF, van Dijk EJ, et al. Diffusion tensor imaging and cognition in cerebral small vessel disease: the RUN DMC study. Biochim Biophys Acta, 2012, 1822(3): 401-407.
[17] Charlton RA, Schiavone F, Barrick TR, et al. Diffusion tensor imaging detects age related white matter change over a 2 year followup which is associated with working memory decline. J Neurol Neurosurg Psychiatry, 2010, 81(1): 13-19.
[18] Rango M, Cogiamanian F, Marceglia S, et al. Myoinositol content in the human brain is modified by transcranial direct current stimulation in a matter of minutes: a1H-MRS study. Magn Reson Med, 2008,60(4): 782-789.
[19] Nitkunan A, McIntyre DJ, Barrick TR, et al. Correlations between MRS and DTI in cerebral small vessel disease. NMR Biomed, 2006,19(5): 610-616.
The application research of diffusion tensor imaging and magnetic resonance spectroscopic imaging in ischemic cerebral small vessel disease
ZHANG Hui-li1, LI Shi-hong2, ZHANG Ying-dong3, ZHOU Jun-shan3, YIN Xin-dao4*1School of Radiology, Jiangsu Vocational College of Medicine, Yancheng 224000,China
2Department of Radiology, Yancheng Fumin hospital, Yancheng 224000, China
3Department of Internal Neurology, Nanjing Hospital Affiliated to Nanjing Medical University, Nanjing 210006, China
4Department of Radiology, Nanjing Hospital Affiliated to Nanjing Medical University,Nanjing 210006, China
Co-first Author: LI Shi-hong
Objective:To investigate the medical imaging evaluative value of diffusion tensor imaging combined with magnetic resonance spectroscopy in ischemic cerebral small vessel disease.Materials and Methods:Forty-two cases of ischemic SVD were imaged with conventional MRI, DTI and MRS. Average diffusion coefficient (DCavg) and fractional anisotropy (FA) were measured symmetrically,which in the lesion regions and the contralateral normal white matter area. Then the absolute metabolite concentrations of N-acetylaspartate (NAA), total cholines (Cho),total creatines (Cr) and myo-inositol (MI) in SVD lesion regions and the normal white matter regions around the lesions were detected, and the ratios of NAA/Cho, NAA/Cr, Cho/Cr, MI/Cr were calculated. To divide the 42 lesions of ischemic SVD into groups according to the imaging display, all the indexes above mentioned of each group were statistically analyzed.Results:The 42 lesions of ischemic SVD were divided into chronic ischemic focus group (30 cases) and chronic lacunar infarction group (12 cases). The DCavg values of SVD lesions significantly raised compared with those of the contralateral normal white matter area(P<0.01), while the FA values of SVD lesions reduced (P<0.01). Neither DCavg nor FA values between chronic ischemic focus group and chronic lacunar infractions group were significantly different (P>0.05). The mean values of NAA, Cho and Cr of the SVD lesions were all less than those of the normal white matter regions around the lesions (P<0.01), but there was no significant difference of MRS metabolic values between chronic ischemic focus group and chronic lacunar infarctions group (P>0.05).Pearsoncorrelation analysis showed that there was a negative correlation between DCavg value and FA (r=-0.383,P=0.012) in normal white matter, the FA value was positively correlated with NAA/Cho (r=0.420,P=0.006), NAA/Cr (r=0.382,P=0.012),but there was no correlation in the SVD lesions. The DCavg value was actively associated with hypertension in SVD lesions bySpearmancorrelation analysis (r=0.338,P=0.029).Conclusion:For the ischemic SVD, the values of DCavg, FA, NAA, Cho and Cr can reflect the micro variations and the functional damages of nerve neurolemma. Combined application of DTI and MRS could have a great contribution to clinical diagnose and pathological mechanism in ischemic SVD.
Diffusion tensor imaging; Magnetic resonance spectroscopy; Small vessel disease; Magnetic resonance imaging
29 Nov 2016, Accepted 25 Jan 2017
作者单位:
1.江苏医药职业学院医学影像学院,盐城 224000
2.盐城阜民医院医学影像科,盐城224000
3.南京医科大学附属南京医院神经内科,南京 210006
4.南京医科大学附属南京医院医学影像科,南京 210006
南京市卫生青年人才培养工程项目(第一层次)(编号:QRX11035);南京市2015年度科技发展计划项目(编号:201503021);盐城市2014年度科技计划项目(编号:YK2014056)
并列第一作者:李仕红
殷信道,E-mail:y.163yy@163.com
2016-11-29
接受日期:2017-01-25
R445.2;R743.9
A
10.12015/issn.1674-8034.2017.06.004
张慧丽, 李仕红, 张颖冬, 等. 磁共振弥散张量和波谱成像在缺血性脑小血管疾病中的应用研究. 磁共振成像, 2017,8(6): 418-423.
*Correspondence to: Yin XD, E-mail: y.163yy@163.com
