REGγ对LPS介导的腹膜炎调节作用
2017-09-23孙锦霞
孙锦霞,黎 丽,王 瑞,黄 钟
1)深圳大学医学部,广东深圳518060;2)聊城大学药学院,山东聊城252059

【生物工程/Bioengineering】
REGγ对LPS介导的腹膜炎调节作用
孙锦霞1,黎 丽1,王 瑞2,黄 钟1
1)深圳大学医学部,广东深圳518060;2)聊城大学药学院,山东聊城252059
为研究蛋白酶体激活因子REGγ对脂多糖(lipopolysaccharides,LPS)介导的腹膜炎的调节作用,采用腹腔注射LPS的方法以建立小鼠腹膜炎模型.首先,通过骨髓细胞过继转移的方法构建骨髓嵌合体小鼠;利用存活率实验检测REGγ敲除对小鼠生存率的影响;然后,通过酶联免疫吸附测定实验检测促炎因子的表达. 结果显示,与野生型(WT)小鼠相比,REGγ敲除(KO)小鼠表现为较低的存活率,且细胞因子TNFα(tumor necrosis factor-α)、IL-1β(interleukin-1β)和MCP-1(monocyte chemoattractant protein-1)的表达也较低.提取WT和KO小鼠来源的骨髓细胞,分别过继转移至X-射线辐照过的WT小鼠,获得WTchimera和KOchimera骨髓嵌合体小鼠. 腹腔注射LPS于WTchimera和KOchimera嵌合体小鼠,结果发现,KOchimera也表现为较低的存活率和TNFα、IL-1β和MCP-1表达.研究结果表明,REGγ在造血系统来源的炎症细胞中敲除,可以加速LPS介导的小鼠存活率下降,且抑制机体促炎因子TNFα、IL-1β和MCP-1的表达.
腹膜炎;蛋白酶体;过继转移;骨髓嵌合体;造血系统;酶联免疫吸附测定法
脓毒症是机体受到严重感染或损伤引发的全身炎症性反应,也是重症监护患者的主要死因.其特点是早期机体会出现过度炎症性反应,又称系统性炎症性反应综合征(systemic inflammatory response syndrome, SIRS),随后过渡为免疫抑制状态,又称补偿性抗炎反应综合征(compensatory anti-inflammatory response syndrome, CARS)[1-3].尽管过去几十年早期定向治疗提高了脓毒症患者的整体存活率,但目前脓毒症仍严重威胁重症患者的生命.大肠杆菌在腹腔感染引发的腹膜炎是发病率和死亡率最高的脓毒症疾病之一,LPS来源于革兰氏阴性细菌的细胞壁,通过结合免疫细胞表面的TLR4(Toll-like receptor-4)受体激活胞内炎症信号通路,上调 TNFα、IL-1β和IL-6等多种炎症因子的表达,介导机体炎症反应[4-5].白酶体激活因子REGγ通过泛素和ATP非依赖的方式降解P53[6]、SIRT1[7]、c-Myc[8]、KLF2[9]、IκBε[10]和SIRT7[11]等底物蛋白,参与调节多种生理病理进程.已知REGγ通过降解KLF2参与调节脓毒症进程[9],但是其与LPS诱导的腹膜炎之间的关系尚不清楚.本研究通过腹腔注射LPS建立腹膜炎模型,并利用REGγ敲除小鼠来研究REGγ在腹膜炎中的作用,为脓毒症治疗提供新的思路.
1 材料与方法
1.1主要试剂与仪器
脂多糖(lipopolysaccharides,LPS)购自美国Sigma公司;HBSS缓冲液((Hank’s平衡盐缓冲液))购自美国Gibico公司;MCP-1、TNFα和IL-1βElisa检测试剂盒购自北京旷博生物有限公司;NH4Cl、KHCO3和Na2EDTA等化学试剂购自上海生工生物工程股份有限公司;生物安全柜购自苏州安泰空气技术有限公司;X-Ray生物辐照仪购自美国Radsource公司.
1.2骨髓嵌合体小鼠构建
选取体重和性别相同的8~12周野生型和REGγ敲除小鼠,分别记做WT和KO小鼠. 转移至生物安全柜内,经体积分数为75%的酒精消毒后,脱颈椎处死.分别取出WT和KO小鼠的股骨和胫骨,用10mLHBSS缓冲液(无Ca2+和Mg2+,含10g/L牛血清白蛋白(bovineserumalbumin,BSA)和5mmol/LHepes)冲出骨髓细胞,并用红细胞裂解液(含0.15mol/LNH4Cl、10mmol/LKHCO3和0.1mmol/LNa2EDTA)裂解红细胞,将所得骨髓细胞用HBSS缓冲液(无Ca2+和Mg2+)稀释至1×107mL-1,置于冰上备用.
利用X-射线生物辐照仪照射实验用WT小鼠,照射剂量为10Gy. 辐照处理WT小鼠4h后,经尾静脉分别注射200μLWT或KO小鼠来源的骨髓细胞悬液(含2×106个细胞), 骨髓细胞过继转移3个月后成功获得骨髓嵌合体小鼠,分别记做WTchimera和KOchimera小鼠,可用于腹膜炎模型的建立.
1.3腹膜炎模型建立和检测
选取体重和性别相同的8~12周WT和KO小鼠,或者已经构建成功的WTchimera和KOchimera骨髓嵌合体小鼠,分别腹腔注射200μLLPS(21mg/kg)以建立LPS-诱导的腹膜炎模型. 每8h统计1次小鼠的存活率,共72h.此外,于LPS注射4h后,分别麻醉WT和KO小鼠或者WTchimera和KOchimera骨髓嵌合体小鼠,经眼眶静脉取血,于4℃1600r/min离心5min,获得上清液即血浆,并冻存于-80℃,用于ELISA检测促炎因子的表达.按Elisa试剂盒说明书对促炎因子TNFα、IL-1β和MCP-1的表达进行检测.
1.4统计学方法
实验所得数据经GraphPadPrism软件处理,数据以平均值(x±s)表示,两组数据差异性比较采用t-test(非参数检验)方法进行分析.小鼠存活率经Kaplan-Meier生存分析,数据差异性比较采用log-ranktest分析方法.P<0.05表示差异具统计学意义,P<0.01表示差异具显著性,P<0.001表示差异具极其显著性.
2 结果与分析
2.1REGγ敲除降低LPS注射小鼠的存活率
为了研究蛋白酶体激活因子REGγ对LPS介导的腹膜炎的调控作用,分别腹腔注射LPS于WT和KO小鼠各11只.每8h记录1次小鼠存活率变化,结果如图1.在注射LPS8h后,KO小鼠死亡率达到20%,且在32h内全部死亡.与WT小鼠相比,注射LPS后KO小鼠存活率显著下降,如图1.
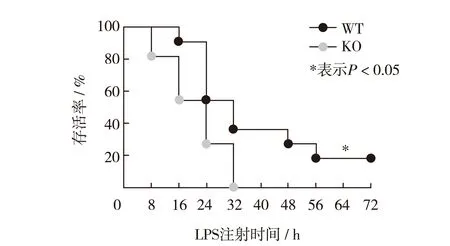
图1 注射LPS后,野生型和REGγ敲除小鼠小鼠存活率的变化Fig.1 The change of the survival rate of WT and KO mice after LPS injection
2.2REGγ敲除下调机体促炎因子表达
为了深入研究REGγ对LPS诱导的腹膜炎的调控作用, 分别腹腔注射LPS于WT和KO小鼠各6只,另设对照组,分别腹腔注射PBS于WT和KO小鼠各4只.4h后经眼眶静脉取血,通过ELISA方法分别检测血浆中促炎因子IL-1β、TNFα和MCP-1的表达,结果如图2.与对照组小鼠相比,注射LPS后促炎因子表达显著上调.但是,注射LPS后KO小鼠血浆中IL-1β、TNFα和MCP-1的表达明显低于WT小鼠,如图2.
2.3造血系统中REGγ缺失降低LPS注射小鼠的存活率
本研究所用REGγ敲除小鼠为完全敲除,已知非免疫细胞如内皮细胞,也能参与机体的免疫调节作用.因此,为深入研究造血系统中REGγ敲除对LPS诱导的腹膜炎的调控作用.分别腹腔注射LPS于WTchimera和KOchimera小鼠各12只,每8 h记录1次小鼠的存活率.结果如图3,与完全敲除小鼠结果相似,KOchimera小鼠的存活率的下降速度显著快于WTchimera小鼠.
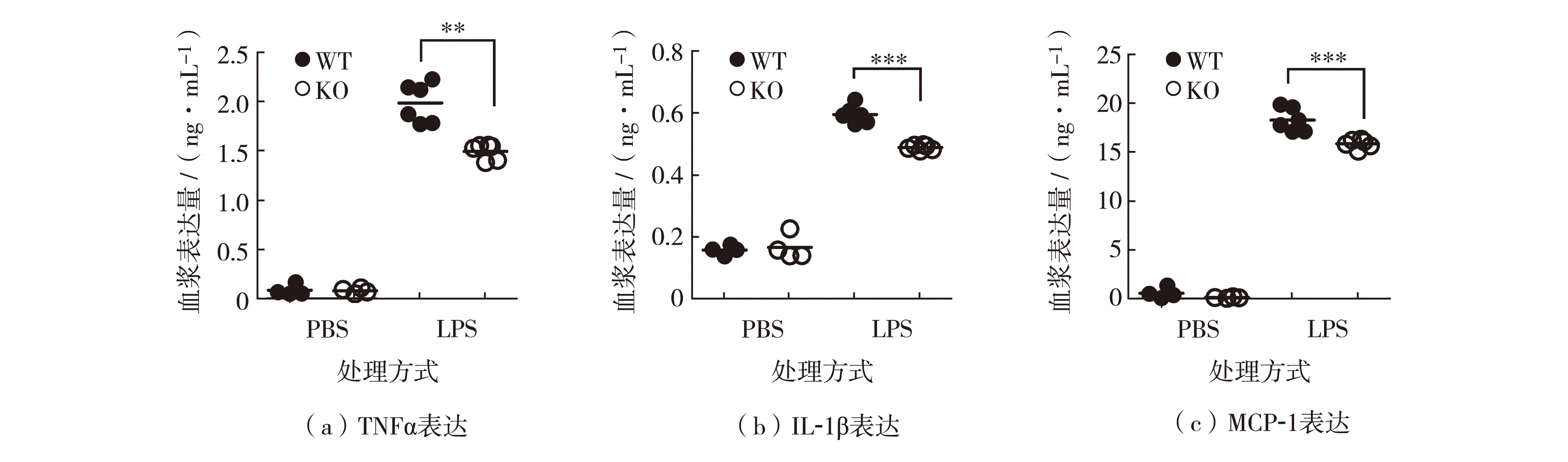
**表示P<0.01, *** 表示P<0.001图2 野生型和REGγ敲除小鼠中促炎因子TNFα、IL-1β 和 MCP-1表达Fig.2 Expression of proinflammatory factors of TNFα, IL-1β and MCP-1 in WT and KO mice
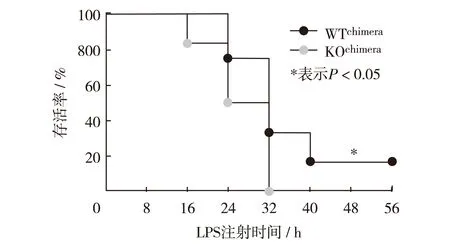
图3 注射LPS后,WTchimera和KOchimera小鼠存活率变化Fig.3 The change of the survival rate of WTchimera and KOchimera mice after LPS injection
2.4造血系统中REGγ缺失下调机体促炎因子表达
为了深入研究造血系统中REGγ缺失对LPS诱导的腹膜炎的调控作用,腹腔分别注射LPS于WTchimera和KOchimera小鼠各6只,另设对照组,分别注射PBS于WTchimera和KOchimera小鼠各4只.4小时后经眼眶静脉取血,通过ELISA的方法分别检测血浆中促炎因子IL-1β、TNFα和MCP-1的表达.结果如图4,与完全敲除小鼠结果相似,LPS注射可以显著上调机体促炎因子表达.但是,LPS注射后KOchimera小鼠血浆中TNFα(图4(a))、IL-1β(图4(b))和MCP-1(图4(c))的表达明显低于WTchimera小鼠.
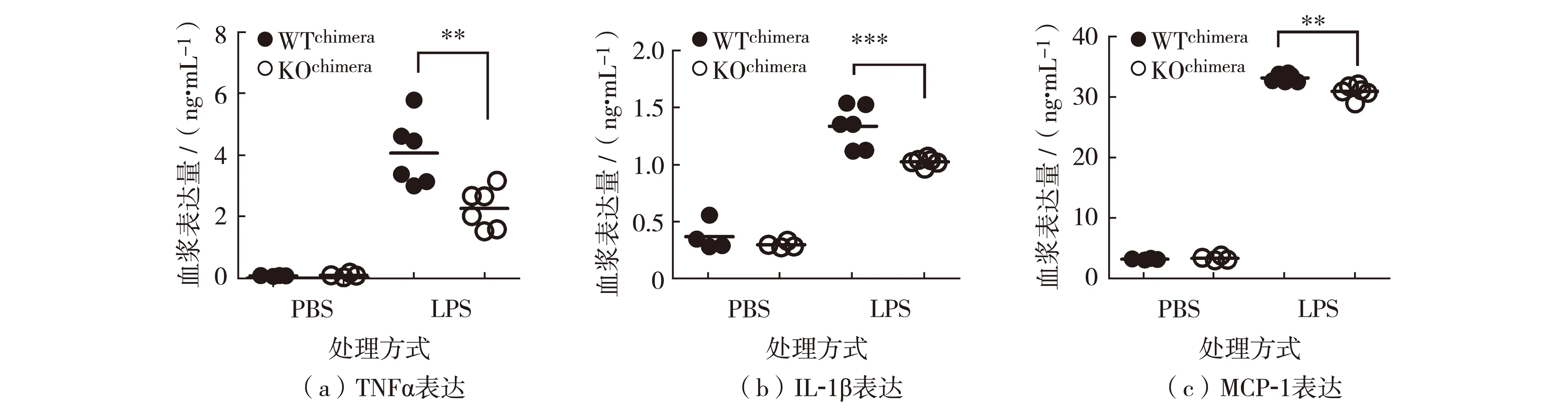
**表示P<0.01, ***表示P<0.001图4 WTchimera and KOchimera骨髓嵌合体小鼠中促炎因子TNFα、IL-1β 和 MCP-1表达Fig.4 Expression of proinflammatory factors of TNFα, IL-1β and MCP-1 in WTchimera and KOchimera mice
3 讨 论
脓毒症是感染后继发的一种系统性炎症反应综合征,由于过度的炎症反应导致全身多器官功能障碍,甚至死亡,其主要致病机制源于感染后大量内毒素的释放[12].但是,IL-1和TNF拮抗剂的使用并未能降低临床上脓毒症患者的死亡率[13-14].脓毒症患者和动物模型的研究数据显示,脓毒症后期机体进入免疫抑制状态,免疫功能的降低利于病原体入侵,可能是导致死亡的主要原因[15-16].机体出现免疫抑制的的相关机制尚不清楚,可能与调节性T细胞(regulatory T cells, Treg)和IL-10、TGFβ(transforming growth factor-β)等抑炎因子相关[17].此外,研究发现,脓毒症患者B、T和树突状细胞(dendritic cells,DC)等细胞的凋亡明显提高,利用凋亡抑制剂抑制免疫细胞凋亡有效改善小鼠的脓毒症病情[12,18-19].因此,免疫细胞的凋亡可能也是机体免疫抑制的重要原因之一.
近期研究表明,另一种细胞程序性坏死方式细胞焦亡,是一种正常的天然免疫反应,也是介导脓毒症导致的机体多器官损伤的主要原因,如NLRP3炎性小体的过度活化可以介导肝细胞焦亡,导致肝损伤[20].此外,Caspase-1/-11或gasderminD基因缺失小鼠体内由于炎性小体活性和细胞焦亡缺失,导致小鼠抵抗内毒素介导的内毒素休克[21-22].因此,细胞焦亡在细菌感染和脓毒症进程中发挥重要的调节作用.
已知REGγ通过降解KLF2可以调节NF-κB活性,参与抑制吞噬细胞杀伤细菌的能力,REGγ敲除小鼠在李斯特菌感染后表现为较低的存活率[9].此外,REGγ敲除也可以明显抑制中性粒细胞和单核吞噬细胞向感染部位的浸润能力[23],从而加重小鼠体内感染,引发败血症,加速小鼠死亡.
本研究通过腹腔注射LPS于野生型和REGγ敲除小鼠,建立腹膜炎模型.结果表明,REGγ完全敲除小鼠和造血细胞中敲除的骨髓嵌合体小鼠,在LPS处理后均表现为加快的存活率下降和较低的促炎因子TNFα、IL-1β和MCP-1的表达.因此,本研究发现REGγ缺失抑制了LPS介导的腹腔炎症反应,从另一方面揭示了REGγ在脓毒症进程的重要调节作用.但REGγ缺失小鼠存活率的变化并非细胞因子风暴所致,可能存在其他调节机制,如免疫抑制、细胞凋亡和细胞焦亡等,尚待深入研究.
结 语
革兰氏阴性菌仍是引发脓毒症的主要病原菌,通过释放大量内毒素,刺激机体内TNFα、IL-1β、IL-6等多种促炎因子的表达显著升高,引发机体内细胞因子风暴,导致死亡. 蛋白酶体激活因子REGγ敲除后虽然没有导致机体内细胞因子风暴的发生,但仍然加速了LPS-介导的小鼠存活率的下降,提示REGγ敲除对LPS-介导的腹膜炎的调控可能通过其他机制实现,仍需后续更多实验验证.
/
:
[1] Bosmann M, Ward P A. The inflammatory response in sepsis[J]. Trends Immunol, 2013, 34: 129-136.
[2] Otto G P, Sossdorf M, Claus R A, et al. The late phase of sepsis is characterized by an increased icrobiological burden and death rate[J]. Critical Care, 2011, 15(4): R183.
[3] Cai Bolin, Deitch E A, Ulloa L. Novel insights for systemic inflammation in sepsis and hemorrhage[J]. Mediators of Inflammation, 2010,2010:642462.
[4] Thomas R C, Bath M F, Stover C M, et al. Exploring LPS-induced sepsis in rats and mice as a model to study potential protective effects of the nociceptin/orphanin FQ system[J]. Peptides, 2014, 61: 56-60.
[5] Rivera C A, Wheeler M D, Enomoto N, et al. A choline-rich diet improves survival in a rat model of endotoxin shock[J]. The American Journal of Physiology, 1998, 275(1): 862-867.
[6] Zhang Zhuo, Zhang Ruiwen. Proteasome activator PA28 gamma regulates p53 by enhancing its MDM2-mediated degradation[J]. The EMBO Journal, 2008, 27(6): 852-864.
[7] Dong Shuxian, Jia Caifeng, Zhang Shengping, et al. The REGγ proteasome regulates hepatic lipid metabolism through inhibition of autophagy[J]. Cell Metabolism, 2013, 18(3): 380-391.
[8] Li Shaungxi, Jiang Cong, Pan Jingjing, et al. Regulation of c-Myc protein stability by proteasome activator REGγ[J]. Cell Death and Differentiation, 2015, 22(6): 1000-1011.
[9] Sun Jinxia, Luan Yi, Xiang Dong, et al. The 11S proteasome subunit PSME3 is a positive feedforward regulator of NF-κB and important for host defense against bacterial pathogens[J]. Cell Reports, 2016, 14(4): 737-749.
[10] Xu Jinjin, Zhou Lei, Ji Lei, et al. The REGγ-proteasome forms a regulatory circuit with IκB? and NFκB in experimental colitis[J]. Nature Communications, 2016, 7: 10761.
[11] Sun Lianhui, Fan Guangjian, Shan Peipei, et al. Regulation of energy homeostasis by the ubiquitin-independent REGγ proteasome[J]. Nature Communications, 2016, 7: 12497.
[12] Boomer J S, Green J M, Hotchkiss R S. The changing immune system in sepsis Is individualized immuno-modulatory therapy the answer?[J]. Virulence, 2014, 5(1): 45-56.
[13] Opal S M, Fisher C J, Dhainaut J F, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The interleukin-1 receptor antagonist sepsis investigator group[J]. Critical Care Medicine, 1997, 25(7): 1115-1124.
[14] Reinhart K, Menges T, Gardlund B, et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES study[J]. Critical Care Medicine, 2001, 29(4): 765-769.
[15] Knaus W A, Harrell F E, Labrecque J F, et al. Use of predicted risk of mortality to evaluate the efficacy of anticytokine therapy in sepsis. The rhIL-1ra phase III sepsis syndrome study group[J]. Critical Care Medicine, 1996, 24(1): 46-56.
[16] Xiao Hongyan, Siddiqui J, Remick D G. Mechanisms of mortality in early and late sepsis[J]. Infection and Immunity, 2006, 74(9): 5227-5235.
[17] Huo Ruichao, Wang Lili, Wang Xiaoya, et al. Removal of regulatory T cells prevents secondary chronic infection but increases the mortality of subsequent sub-acute infection in sepsis mice[J]. Oncotarget, 2016, 7(10): 10962-10975.
[18] Hotchkiss R S, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy[J]. Nature Reviews Immunology, 2013, 13(12): 862-874.
[19] Luan Yingyi, Yao Yongming, Xiao Xianzhong, et al. Insights into the apoptotic death of immune cells in sepsis[J]. Journal of Interferon & Cytokine Research: the Official Journal of the International Society for Interferon and Cytokine Research, 2015, 35(1): 17-22.
[20] Chen Yuanli, Xu Guo, Liang Xiao, et al. Inhibition of hepatic cells pyroptosis attenuates CLP-induced acute liver injury[J]. American Journal of Translational Research, 2016, 8(12): 5685-5695.
[21] Shi Jianjin, Gao Wenqing, Shao Feng. Pyroptosis: Gasdermin-Mediated programmed necrotic cell death[J]. Trends in Biochemical Sciences, 2017, 42(4): 245-254.
[22] Jorgensen I, Miao E A. Pyroptotic cell death defends against intracellular pathogens[J]. Immunological Reviews, 2015, 265(1): 130-142.
[23] 孙锦霞,王 瑞,黄 钟. REGγ敲除对细胞浸润的抑制作用[J]. 深圳大学学报理工版,2017, 34(2):111-116. Sun jinxia, Wang Rui, Huang Zhong. The inhibitory effect of REGγ-knockout on cell infiltration[J]. Journal of Shenzhen University Science and Engineering, 2017, 34(2): 111-116.(in Chinese)
【中文责编:晨兮;英文责编:艾琳】
RegulationofREGγonLPS-mediatedperitonitis
SunJinxia1,LiLi1,WangRui2,andHuangZhong1
1)HealthScienceCenter,ShenzhenUniversity,Shenzhen518060,GuangdongProvince,P.R.China2)CollegeofPharmacy,LiaochengUniversity,Liaocheng252059,ShandongProvince,P.R.China
Mouse peritonitis model is established by LPS peritoneal injection to analyze the roles of proteasome activator REGγ in LPS-mediated peritonitis. Bone marrow chimera mice are generated by adoptive transfer of bone marrow cells. Survival assay is used to research the effect of REGγ deficiency on survival rate. Expression of proinflammatory factors are detected via enzyme-linked immunosorbent assay (ELISA). As compared to the wild-type (WT) mice, the REGγ-knockout (KO) mice shows significantly lower survival rate and lower expression of the proinflammatory factors TNFα, IL-1β and MCP-1. Furthermore, bone marrow from WT and KO mice is isolated and transferred adoptively to X-ray irradiated WT mice to generate WTchimeraand KOchimerabone marrow chimera mice. The results suggest that KOchimeramice also manifest obviously lower survival rate and proinflammatory factors expression of TNFα, IL-1β and MCP-1after peritoneal injection of LPS to WTchimeraand KOchimera. Thus, these data suggest that REGγ deficiency in inflammatory cells of hematopoietic origin accelerates LPS-mediated decline of survival rate and suppresses expression of proinflammatory factors TNFα, IL-1β and MCP-1.
peritonitis; proteasome; adoptive transfer; bone marrow chimera; hematopoietic system; enzyme-linked immunosorbent assay
2017-02-16;Accepted:2017-06-07
Professor Huang Zhong. E-mail: zhuang809@126.com
R 392
:Adoi:10.3724/SP.J.1249.2017.05471
Foundation:National Natural Science Foundation of China (31401217); Postdoctral Science Foundation of China (2014M0560672)
:Sun Jinxia, Li Li, Wang Rui, et al. Regulation of REGγ on LPS-mediated peritonitis[J]. Journal of Shenzhen University Science and Engineering, 2017, 34(5): 471-475.(in Chinese)
国家自然科学基金资助项目(31401217);中国博士后科学基金资助项目(2014M0560672)
孙锦霞(1986—),女,深圳大学博士后研究人员.研究方向:免疫学. E-mail:jinxia8608@126.com
引文:孙锦霞,黎 丽,王 瑞,等. REGγ对LPS介导的腹膜炎调节作用[J]. 深圳大学学报理工版,2017,34(5):471-475.
