恒电位氧化改性石墨毡及其氧还原电极的电化学性能
2017-09-06何梦娇闫康平王贵欣孙羽涵钟宜霏罗春晖
何梦娇 闫康平 王贵欣 孙羽涵 钟宜霏 罗春晖
(四川大学化学工程学院,成都610065)
恒电位氧化改性石墨毡及其氧还原电极的电化学性能
何梦娇 闫康平*王贵欣 孙羽涵 钟宜霏 罗春晖*
(四川大学化学工程学院,成都610065)
分别采用循环伏安改性法和恒电位氧化法对石墨毡进行改性处理,并采用循环伏安法对其电化学性能进行研究,实验结果表明,恒电位氧化改性较循环伏安改性的石墨毡有较好的氧还原活性。通过XRD、FTIR、接触角和CV针对恒电位氧化处理石墨毡进行了进一步的测试。测试结果显示,随恒电位氧化时间的增加,石墨毡表面亲水性含氧官能团增加,润湿性增强。恒电位氧化改性处理25 min的石墨毡氧还原峰电位及电流密度分别为~-0.43 V和~0.003 4 mA·cm-2,显示出很好的电化学催化性能。基于以上结果,恒电位氧化法改性处理能够极大提高石墨毡的氧阴极活性。
氧阴极;石墨毡;改性;恒电位氧化;循环伏安
0 Introduction
Lithium-oxygen(Li-O2)batteries occupy a leading position in the electric vehicle power competition,in which the substance O2involved in the positive reaction comes from the air outside instead of storing an oxidizer internally with the notable features of high energy density and no pollution[1-4].The battery shows a theoretical specific energy of 1.1×104Wh·kg-1, which is 5~10 times[1-6]more than that of the state-of-the-art Li-ion batteries.However,Li-O2batteries are plaguedbymanyproblems,especiallythelittle catalyticeffectsofthecathodematerialused currently,which limit its rapid development to a great extent[7-9].
Graphite felt has the advantages of high corrosion resistance,largespecificsurfacearea,excellent conductivity and good thermal stability,which is suitable for applying as the electrode material of Li-O2batteries.However,the electrode prepared directly with graphite felt exhibits a poor electrochemical performance due to the low surface energy of graphite fiber and the existence of large amount of hydrophobic groups[10].
Itwasreportedthatthesurfaceenergyof graphitefeltcouldbeenhancedbydifferent modifications.Guan et al.[11]investigated the effects of the heat treatment on different carbon materials,and concluded that the electrochemical properties of the treated carbon were improved significantly.Zhong[12], Sun et al.[13-14]foundthattheacidand heat treatment on graphite felt could greatly improve the surface activity of carbonLiu et al.[15]used the modifiedgraphitefeltsastheelectrodeofthe vanadiumbatteries,andfoundthattheelectrochemical performances of the graphite felts modified by electrochemical oxidation were better than that by acid and heat treatments.
Presently,most of the researches are focused on the effects of the current density on the generation of surfacefunctionalgroupsandoftheelectrolyte concentration on the anodic oxidation degree in the method of galvonostatic or potentiostatic oxidation[16-18]. In this work,the graphite felts were respectively modified by cyclic voltammetry and potentiostatic oxidation,and the electrochemical performances of the modified materials in aqueous solution were studied. The influences of the potentiostatic oxidation duration on wettability,functional groups and the electrochemical properties were investigated.It was shown that the electrocatalytic performance of the graphite felt treated by potentiostatic oxidation was improved obviously,andtheoptimaloxidationtimewas determined.
1 Experimental
The Graphite felts obtained by polyacrylonitrile graphitization with the carbon purity of≥99.5%were cut into 5 cm×6 cm wafers with the thickness of 2 mm,further ultrasonic cleaned with anhydrous ethanol and distillated water,and finally dried at 70℃for 24 h in an electric thermostatic drying oven.
Then the graphite felt wafers were respectively modified at room temperature by cyclic voltammetry (CV)in the potential range of 2.0~-0.01 V with the scan rate of 10 mV·s-1and potentiostatic oxidation (PO)at 2.0 V on a PAR273A Potentiostat/Galvanostat and a 5210 lock-in amplifier controlled by Powersuite software(Princeton Applied Research,USA),in which the as-prepared graphite felt wafer,the graphite electrode,the saturated calomel electrode(SCE)and 1 mol·L-1H2SO4solution were used as the working electrode(WE),thecounter electrode(CE),the reference electrode(RE),and the electrolyte,respectively.
The crystalline structures of the graphite felts were examined by X-ray diffraction(XRD on a Philips X′Pertpro MPD)with Cu Kα radiation(λ=0.154 06 nm,U=40 kV,I=40 mA)ranging from 10°to 70°at a scan rate of 0.04°·s-1.The functional groups on the surface of the graphite felts were evaluated by Fourier transform infrared spectroscopy(FTIR)on a thermo Nicolet Magna IR 560 spectrometer in the range of 900~3 800 cm-1.The hydrophilicity of the graphite felts was analyzed by sessile drop contact angle measurementusingacontactanglemeasuring apparatus(JC2000C1,Shanghai Zhongchen digital technic apparatus Co.,Ltd).
The electrochemical performances of the graphite felt materials were examined by cyclic voltammetric (CV)measurements using the same apparatus for the modification introduced above,except that the counter electrode was replaced by a platinum net.The CV measurements of the pristine graphite felts and the CV modified samples were carried out in the potential range of-0.8 and+0.6 V,and the graphite felts modified by PO were carried out in the potentialrange of-0.9 and+0.4 V,which were performed at room temperature with a scan rate of 10 mV·s-1.
The graphite felts involved in CV measurements were partly sealed with wax to remain an effective area of 1 cm×0.5 cm exposed.All electrodes were mounted in an airtight container[11],and dipped in the electrolyte of 0.1 mol·L-1LiOH solution,which was saturated by oxygen flushing for 30 min before CV measurements.
2 Results and discussion
2.1 XRD characterization of the raw graphite felt
The XRD pattern of the pristine graphite felt is shown in Fig.1,which exhibits a sharp diffraction peak at~26°,and two weak peaks at~43°and~53°, corresponding respectively to(002),(100)and(004) faces of the hexagonal structure[19-20],consisting well with the diffraction pattern of graphite(JPCDS:No.65-6212).
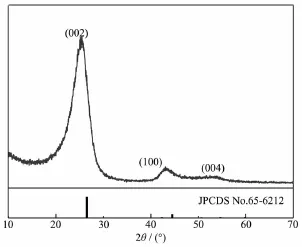
Fig.1X-ray diffraction pattern of the pristine graphite felt
2.2 Modification of the graphite felts
2.2.1 Determination of the modification methods
The cyclic voltammetric curves of the graphite felts with and without modification are given in Fig.2.
The CV curves of both modified samples exhibit obvious reduction peaks compared to that of the untreated graphite felt,implying that the electrochemical reaction activity is improved by modification,which was also confirmed by Georgioua et al.[21]and Shao et al.[17].
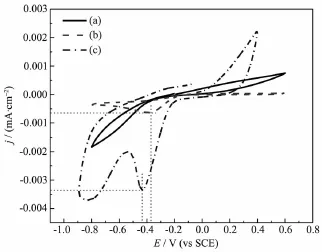
Fig.2Cyclic Voltammetric curves of graphite felts with different treatment(a)without modification; (b)mmodified by CV;(c)modified by PO for 25 min
The reduction peak potentials and the reduction peak current densities of the CV modified sample along with the PO modified sample are~-0.39 V and~0.000 6 mA·cm-2along with~-0.43 V and~0.003 4 mA·cm-2,respectively.Apparently,the reduction peak potentials of the two modified samples are of the same order of the magnitude,whereas the reduction peak current density of the PO modified sample is~4.7 times higher than that of the CV modified.Theoretically,the reduction peak potential refers to the oxygen reduction reaction(ORR)activity,while the reduction peakcurrentdensitytotheamountofreduced oxygen[10-11].Higher current density implies more oxygen consumption,namely the PO modification is more effective.Therefore,potentiostaticoxidationwas determined as the modification method to perform the further investigations in this work.
2.2.2 PO modification of the graphite felts
The change of the current intensity with the potentiostatic oxidation time is shown in Fig.3.
The current intensity descends sharply due to polarization within the beginning 61 s followed by a slight increase,and maintains nearly stable after 200 s.The area under the curve represents the electric consumption during oxidation process,which could be determined by integrating the equation of dQ=dt×dI. Apparently,with the increase of the modification time, the electric consumption increases correspondently,implying that the oxidation degree of graphite felt materials increases simultaneously.The mechanism is discussed in detail below in this article.
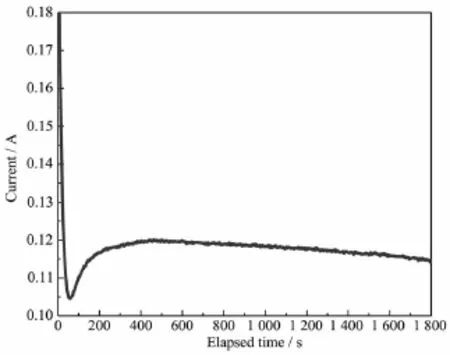
Fig.3Current-time relationship curve during PO modification
2.3 FTIR characterization of the graphite felt
The dispersibility,conductivity and wettability of the graphite-felt electrode have remarkable effects on theelectrochemicalperformances,whichresults eventually from the surface functional groups on graphite felts[22-24].The effects of potentiostatic oxidation modifications on the surface functional groups werecharacterizedbyFouriertransforminfrared spectroscope in this work.The typical FTIR spectra of the graphite felts with and without potentiostatic oxidation(PO)treatment are shown in Fig.4.
The FTIR spectrum of the pristine graphite felt exhibits two broad peaks at around 701 and 1 142 cm-1,which correspond to the out-of-plane C-H bending vibration and the C-O stretching vibration in COOH[25],respectively.All as-oxidized samples present extra five spectral peaks compared with the pristine graphite felt.The peaks at around 2 900 and 1 650 cm-1correspond to the existence of CH&CH2and stretching of C=C,respectively.The peak at around 1 480 cm-1is attributed to the stretching vibrations of asymmetric OH or COOH.The peaks ranging from 1 180 to 1 049 cm-1are the absorbing of C-O[24-28]. Apparently,some hydrophilic functional groups such as OH and COOH[27,29]are generated by modification, which was also confirmed by Yue et al.[30].A schematic description of the formation mechanism of the functional groups is given in Fig.5[31].It is reasonable to suggest that potentiostatic oxidation is an effective method to improve the wettability of the graphite felts.

Fig.4FTIR spectra of the graphite felts modified by PO for(a)0 min;(b)15 min;(c)20 min;(d)25 min; (e)30 min
The FTIR spectra of all treated samples show the similar absorption peaks wavenumbers as mentioned above,indicatingthatthetypesofthesurface functional groups of the modified graphite felts are also similar.However,the absorption peak intensities differ from each other of different oxidation times.The functional groups such as OH,CH and CH2exhibit small intensity variation,while the peak intensities of the C-O stretching and the stretching vibrations of asymmetricCOOHincreasedistinctlywiththe oxidation time increasing,implying that the amount of the corresponding hydrophilic functional groups suchas COOH increases.That means,the hydrophilicity of the graphite felts should also increase with the oxidation time increasing,which is further discussed below in this article.
2.4 Contact angle characterization of the graphite felt surface
The effects of the potentiostatic oxidation on the wettability of graphite felts were investigated by observing the contact angle,which is illustrated in Fig.6.It is shown that the contact angles of the untreatedandthemodifiedsamplesarequite different.The absorption capacity of the untreated graphite felt is relative low,whereas the graphite felt modified for 30 min absorbs the water completely. The effect of the oxidation time on the contact angle is summarized in Fig.7.

Fig.6Contact angle characterization of graphite felts modified by PO for(a)0 min;(b)25 min;(c)30 min

Fig.7Contact angle of graphite felts at different PO time
The contact angle of the pristine sample as shown in Fig.7 is 146°,which exhibits the strong hydrophobic property,that agrees with the results reported by Sun et al.[13].The contact angles of the modifiedsamplesaresmallerthanthatofthe untreated sample.With the extension of oxidation time from 15 to 30 min,the contact angles reduced gradually.The graphite felt modified for 30 min shows the strongest hydrophilic property with the contact angle 0°.The change tendency of the contact angle withtheoxidationtimeindicatesthatthe hydrophilicity is improved by PO modification,and the hydrophilic degree ascends with the modification timeincreasing.ReferringtotheFTIRresults obtained above,it could be concluded thatthe extended modification time intensifies the oxidation degree,which results in the increase of the wettability and the number of the hydrophilic functional groups on the surface of graphite felt.
2.5 Electrochemical behavior
Tofurtherevaluatetheelectrochemical performances of the modified graphite felts,the cyclic voltammetrymeasurementswereperformed.The cyclic voltammetric curves of the samples with and without potentiostatic oxidation are shown in Fig.8. The oxygen reduction potentials and the corresponding current densities of the reduction peaks for the graphite felts modified by potentiostatically oxidizingfor 15,20,25,30 min respectively are presented in Fig.9.

Fig.8Cyclic Voltammetric curves of graphite felts modified by PO for(a)0 min,(b)15 min, (c)20 min,(d)25 min and(e)30 min
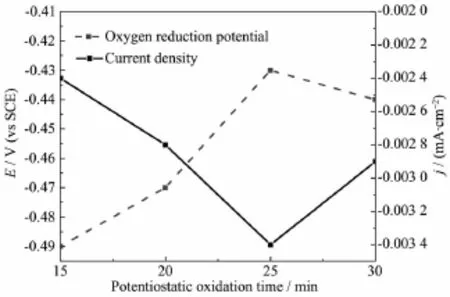
Fig.9Change of the reduction peak potentials and the current densities with PO time
No oxygen reduction peaks appear on the cyclic voltammetriccurveofthepristinegraphitefelt, implying that the ORR activity of the untreated sample is relative poor due to its strong hydrophobicity as discussed above.All cyclic voltammetric curves of the modified graphite felts exhibit the reduction peaks,but the corresponding reduction peak potentials and current densities are relative different. The reduction peak potential and the current density of the graphite felt modified for 15 min are the lowest. With the modification time increasing from 15 to 25 min,the oxygen reduction peak potentials change from~-0.49 to~-0.43 V representing the reducing of polarization,and then the current densities increase from~0.002 4 to~0.003 4 mA·cm-2gradually, indicating that the electrochemical performances of the modified graphite felts are improved with the modification time increasing.However,both oxygen reduction peak potential and current density of the material modified for 30 min exhibit the contrary change tendency,suggesting that the ORR activity of thegraphitefeltisdegradedforthelongtime oxidation.
Not only the amount but also the type of the oxygen-containing functional groups on the surface of thegraphitefeltsincreaseafterpotentiostatic oxidation.Furthermore,with the modification time increasing,the amount of the hydrophilic functional groups increases correspondingly and the hydrophilicity of the graphite felt is accordingly improved[16].The hydrophilic oxygen-containing groups are redox-active, which are able to directly participate in the electrochemical reaction.Therefore,the modified graphite felts exhibit the oxygen reduction reaction activity, which was confirmed by the existence of the reduction peaks on CV curves[32].However,with the oxidation time increasing over 25 min,the ORR activity decreases,indicating that excessive hydrophilic functional groups have negative effects on the electrochemical performances.An interpretation of the phenomenon is schematically described in Fig.10.The surface of the unmodified graphite felts is mostly covered by oxygen due to its low wettability as shown in Fig.10(a),which ORR activity is very poor.The hydrophilic functional groups on the surface obtained by PO modification make the graphite felts to possess the electrochemical reaction activity.At the same time,oxygen as the reactant takes also part in the reaction,where the oxygen channels must be provided to ensure the contact between oxygen and the graphite felts as illustrated in Fig.10(b).Over high wettability resulted from the longtime oxidation causes that most area of the surface is surrounded by water,which blocks the contactofoxygenwiththegraphitefelts,andaccordingly hinders the electrochemical reactions[33]as shown in Fig.10(c).Consequently,the optimal potentiostatic oxidation is supposed to not only increase the amount of the hydrophilic functional groups on the surface but also remain the enough space for oxygen to pass through the water layer and contact with the electrode materials.The graphite felts modified by potentiostatically oxidizing at 2.0 V for 25 min exhibits the preferable electrochemical performance in this work,which is determined as the optimal PO modification conditions.

Fig.10Schematic description of the solid-liquid interfaces(a)hydrophobic surface; (b)partially hydrophilic surface;(c)hydrophilic surface
3 Conclusions
Theoptimalmodificationconditionsforthe graphite felts as the electrode of Li-O2batteries were determined in this work.Both potentiostatic oxidation modification and cyclic voltammetric treatment are able to improve the electrochemical performances of graphite felts.The graphite felts modified by potentiostatic oxidation are more electrochemical active than that treated by cyclic voltammetry.The improvement of the oxygen reduction reaction activity for the PO modified graphite felts is attributed to the increase of the hydrophilicity,owing to the formation of the hydrophilic oxygen-containing functional groups on the surface.Furthermore,the amount of the hydrophilicfunctionalgroupsincreaseswiththePO modification time increasing.However,over high wettability from long time oxidation results in the contact difficulty between oxygen and the graphite felt electrode,and accordingly hinders the electrochemical reactions.As a result,the graphite felts modified by potentiostatically oxidizing at 2.0 V for 25 min exhibits the optimal electrochemical performances.
[1]Abraham K M,Jiang Z.J.Electrochem.Soc.,1996,143(1):1-5
[2]Girishkumar G,Mccloskey B,Luntz A C,et al.J.Phys. Chem.Lett.,2010,1(14):2193-2203
[3]Kraytsberg A,Ein-Eli Y.J.Power Sources,2011,196(3):886-893
[4]Liu T,Leskes M,Yu W,et al.Science,2015,350(6260):530-533
[5]Kuboki T,Okuyama T,Ohsaki T,et al.J.Power Sources, 2005,146(1/2):766-769
[6]Cheng F,Chen J.Chem.Soc.Rev.,2012,41(6):2172-92
[7]Yuasa M,Matsuyoshi T,Kida T,et al.J.Power Sources, 2013,242(35):216-221
[8]Débart A,Bao J,Armstrong G,et al.J.Power Sources,2007, 174(2):1177-1182
[9]Rychcik M,Skyllas-Kazacos M.J.Power Sources,1988,22 (1):59-67
[10]Lu Y,Li W,Sun F,et al.Carbon,2010,48(11):3079-3090
[11]Guan P,Wang G,Luo C,et al.Electrochim.Acta,2014,129 (16):318-326
[12]Zhong S,Padeste C,Kazacos M,et al.J.Power Sources, 1993,45(1):29-41
[13]Sun B,Skyllas-kazacos M.Electrochim.Acta,1992,37(7): 1253-1260
[14]Sun B,Skyllas-kazacos M.Cheminform,1992,23(49):18
[15]LIU Di(刘迪),TAN Ning(谭宁),HUANG Ke-Long(黄可龙), et al.Chinese J.Power Sources(电源技术),2006,30(3):224-223
[16]Ishifune M,Suzuki R,Mima Y,et al.Electrochim.Acta, 2005,51(1):14-22
[17]Shao Y,Yin G,Zhang J,et al.Electrochim.Acta,2006,51 (26):5853-5857
[18]Noel M,Santhanam R.J.Power Sources,1998,72(1):53-65
[19]Lee G W,Kim J,Yoon J,et al.Thin Solid Films,2008,516 (17):5781-5784
[20]CHEN Teng-Yuan(陈腾远),ZHANG Chen-Jun(陈晨军),LI Zai-Jun(李在均),et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30(12):2691-2698
[21]Georgiou P,Walton J,Simitzis J.Electrochim.Acta,2010, 55(3):1207-1216
[22]Seredych M,Hulicova-Jurcakova D,Gao Q L,et al.Carbon, 2008,46(11):1475-1488
[23]Qiao W,Korai Y,Mochida I,et al.Carbon,2002,40(3):351-358
[24]Nian Y R,Teng H.J.Electroanal.Chem.,2003,540(2):119-127
[25]Mawhinney D B,Naumenko V,Kuznetsova A,et al.J.Am. Chem.Soc.,2000,122(10):2383-2384
[26]El-Hendawy A N A.J.Anal.Appl.Pyrolysis,2006,75(2): 159-166
[27]Szabó T,Berkesi O,Forgó P,et al.Chem.Mater.,2006,18 (11):2740-2749
[28]Szabó T,Tombácz E,Illés E,et al.Carbon,2004,44(3):537-545
[29]Li L,Quinlivan P A,Knappe D R U.Carbon,2002,40(12): 2085-2100
[30]Yue Z R,Jiang W,Wang L,et al.Carbon,1999,37(11):1785-1796
[31]HUANG Qiao(黄桥),SUN Hong-Juan(孙红娟),YANG Yong -Hui(杨勇辉).Chinese J.Inorg.Chem.(无机化学学报), 2011,27(9):1721-1726
[32]Frackowiak E,Béguin F.Carbon,2001,39(6):937-950
[33]Moreira J,Ocampo A L,Sebastian P J,et al.Int.J.Hydrogen Energy,2003,28(6):625-627
Electrochemical Performance of Graphite Felts Modified by Potentiostatic Oxidization for Oxygen Reduction Cathode
HE Meng-JiaoYAN Kang-Ping*WANG Gui-XinSUN Yu-HanZHONG Yi-YeiLUO Chun-Hui*
(College of Chemical Engineering,Sichuan University,Chengdu 610065,China)
The graphite felts were respectively modified by cyclic voltammetry(CV)and potentiostatic oxidation (PO),which electrochemical performances were evaluated by cyclic voltammetric experiments.As a result,PO modification is more effective on improving the oxygen reduction reaction(ORR)activity of the graphite felts than CV treatment.The PO modified graphite felts were further investigated by XRD,FTIR,Contact angle and CV.It is found that the wettability of the graphite felts increases with the increase of potentiostatic oxidation time,due to the increase of the hydrophilic oxygen-containing functional groups on surface.The graphite felt modified by PO for 25 min in this work exhibits the preferable electrochemical performances with the reduction potential~-0.43 V and the current density~0.003 4 mA·cm-2of the reduction peak on CV curve.Consequently,potentiostatic oxidation is an effective and feasible treatment for improving the electrochemical properties of the graphite felts as the electrode material of Li-O2batteries.
oxygen cathode;graphite felts;modification;potentiostatic oxidation;cyclic voltammetry
O613.71
A
1001-4861(2017)02-0315-08
10.11862/CJIC.2017.018
2016-05-18。收修改稿日期:2016-10-27。
*通信联系人。E-mail:cyankp@scu.edu.cn,luochunhui@scu.edu.cn
