The Correlation of Indices in r-TEG with Intra-operative Blood Loss in Neurosurgical Patients
2017-08-07XueZhangXuerongYuandYuguangHuang
Xue Zhang, Xuerong Yu, and Yuguang Huang*
The Correlation of Indices in r-TEG with Intra-operative Blood Loss in Neurosurgical Patients
Xue Zhang, Xuerong Yu, and Yuguang Huang*
Department of Anesthesiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
blood transfusion management; rapid-thrombelastography; intra-operative blood loss; neurosurgerya
Objective Intra-operative coagulopathy has a close relationship with blood loss and the prognosis of patients. Rapid-thrombelastography (r-TEG) is a comprehensive assessment of coagulation abnormalities and also an effective way for constructing blood transfusion. This study attempts to investigate the correlation of r-TEG indices with intra-operative hemorrhage.
Methods Patients who underwent transphenoidal hypophysectomy and craniotomy from January 15 to April 30, 2013 in Peking Union Medical College hospital were recruited. All patients had pre- and post-operative r-TEG and conventional coagulation tests (CCTs). Patients’ information and intra-operative blood loss as a percentage of estimated blood volume were recorded. Spearman’s correlation analyses were used for discovering the relationship between indices in r-TEG or CCTs and the intra-operative blood loss. The significant correlated index of r-TEG was further investigated using linear regression analysis.
Results A total of 181 patients participated in this study. Intra-operative change of α-angle, which reflects the fibrinogen level and function, was the only r-TEG index that correlated with blood loss significantly (=0.013,=-0.184), thus challenging the current empirical cognition of the effects of intra-operative hemorrhage on coagulation. As intra-operative blood loss increased, α-angle decreased, and every 1% loss of estimated blood volume (EBV) led to0.60 degree decrease of α-angle. As for CCT results, changes of fibrinogen and platelet count were also significantly correlated with blood loss (=0.015 and=0.001, respectively).
Conclusions Peri-operative change of α-angle, as an index of r-TEG, exhibited a significant negative correlation with intra-operative blood loss. The impact of hemorrhage on fibrinogen, instead of clotting factors, should be scrutinized.
Chin Med Sci J 2017; 32(2):69-74. DOI:10.24920/J1001-9294.2017.011
NTRA-OPERATIVE coagulopathy is closely related with blood loss volume and the prognosis of patients. The American Society of Anesthesiologists (ASA) Transfusion Guidelines1provides some suggestions on transfusion strategy for blood components, most of which relies on the indices of conventional coagulation tests (CCTs),including prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR), fibrinogen (FBG), and platelet count (PLT). However, the CCTs were time-consuming,so that it is rarely clinically constructed for guiding blood transfusion. At present, transfusion of blood products is mainly based on the visual assessment of anaesthesiologists or surgeons, which is actually subjective and experiential. The information that precisely defines when to perform a transfusion of blood component (except red blood cells) is still not available in the literature. Fresh frozen plasma (FFP) is always the first choice of correcting coagulopathy empirically during operation to supple the consumption of clotting factors. Nevertheless, the rationality of this method has not been proven, and the side effects of plasma transfusion, such as transfusion-associated cardiac overload (TACO), severe infection, as well as complications of the respiratory system,2-4have raised many concerns.
Rapid-thrombelastography (r-TEG) provides a better way to guide blood product transfusion and treatment of the evolving coagulopathy, because it offers an integral reflection of the patient’s coagulation status compared with currently widely used CCTs.5In addition, r-TEG results return more promptly than those of traditional kaolin-activated TEG and CCTs.6The initial r-TEG index activated clotting time (ACT) reflects the overall clotting factor availability and function. K-time and α-angle are indices that are supposed to be corresponding to fibrinogen content and its performance. Maximum amplitude (MA) represents overall effect of platelets, influenced by both number and function.7When ACT increases, there is a need for fresh frozen plasma transfusion; when K-time increases or α-angle decreases, there is a need for fibrinogen transfusion. Platelet transfusion is indicated while MA decreases.
In the previous studies, it has been suggested that r-TEG may effectively guide transfusion therapy, and result in reduced administration of blood components,8less blood loss volume,9and better prognosis of patients.10-12However, the relationship between r-TEG indices and intra-operative blood loss still remains unclear. As a result, the aim of this study was to investigate the correlation of r-TEG indices with intra-operative hemorrhage.
PATIENTS AND METHODS
Participants recruiting
This prospective study was carried out in Peking Union Medical College Hospital between January 15, 2013 and April 30, 2013. It was ethically approved by the institutional ethics research committee. Written informed consent was acquired from participants before the enrollment. The inclusion and exclusion criteria were listed as follows.
Inclusion criteria:
1) Inpatients who were scheduled for transsphenoidal sellar region tumor removal or craniotomy;
2) Age between 15 and 65;
3) ASA grade Ⅰor Ⅱ.
Exclusion criteria:
1) Pre-operative CCT abnormalities: prothrombin time (PT)>15.6s, activated partial thromboplastin time (aPTT)> 41.8s, platelet count (PLT) <100×109/L or > 400×109/L;
2) Liver function abnormality: alanine aminotransferase (ALT) or aspartate aminotransferase (AST) > 50U/L;
3) Renal function abnormality: serum creatinine (Scr)> 120mmol/L;
4) History of pre-operative coagulation-related drugs administration;
5) Homologous transfusion before opening the skull;
6) Unqualified blood sample for staying over 2 hours after venipuncture.
Anesthesia and intra-operative management
Intra-operative anesthesia and fluid management were consistent in all patients in order to control potential sources of bias. General anesthesia was induced with midazolam 0.05 mg/kg, propofol 2-2.5 mg/kg, fentanyl 2-3 µg/kg, rocuronium 0.5-1.0 mg/kg, followed by endotracheal intubation. Anesthesia was maintained with sevoflurane 1.2-1.5 MAC and nitrous oxide 0.5 L/min. Fentanyl was infused to maintain analgesia, and rocuronium was resorted to maintain muscular blockade. Ventilation was controlled with a tidal volume of 10 ml/kg, and respiratory rate was adjusted to maintain a carbon dioxide end-tidal concentration of 35-40 mmHg. The Holliday- Segar Method (4-2-1 rule) was applied to control the intra- operative fluid infusion.13The ratio of crystalloids (Ringer) to colloids (Gelofusine) was 2:1.
At the end of surgery, the residual neuromuscular block was antagonized with neostigmine 40 µg/kg and atropine 20 µg/kg when sevoflurane MAC decreased to 0.2. As patients woke up and spontaneous ventilation resumed, the endotracheal tube was removed.
Compiling parameters and measurements
The following parameters were compiled from the hospital medical records system before surgery: general information (including name, ID, gender, age, ASA grade, date of surgery, surgeon, and surgery category); pre-operative CCTs; complete blood count (CBC) indices including hemoglobin (HGB), hematocrit (Hct); liver and renal function indices including ALT, AST, and Scr. Before anesthesia induction and at the end of the operation, we took 2.7 ml and 7.4 ml of blood sample respectively through venipuncture, and injected into citrate anticoagulation tube (BD Company, citrate concentration 0.109 mol/L) or EDTA anticoagulation tube (BD Company, EDTA 3.6 mg) for pre-operative and post-operative r-TEG, CCT and CBC tests. R-TEG tests were done in the department of anesthesiology (TEG 5000; Haemonetics Company, USA) 15 min after sample collecting. Indices of r-TEG, including activated clotting time (ACT), K-time, α-angle, and maximum amplitude (MA), were recorded. CCT and CBC tests were done in the department of laboratory. Blood sample exams would be cancelled if it stayed over 2 hours before the tests.
The blood loss was presented as percentage of estimated blood volume (EBV). The estimated volume of blood loss was calculated by the following formula: volume of liquid in suction chamber + volume of liquid in blood drainage bag + (weight of gauze with blood and saline – net weight of gauze) – volume of saline. The EBV was calculated by weight×0.07(L). Transfusion of blood product had exerted a great role in coagulation status, which would influence r-TEG results. Consequently, if autologous or homologous blood transfusion was required during the surgery, we calculated the estimated blood loss, then took the blood sample for laboratory test before transfusion, and ended the research at this point.
Statistical analysis
Data were analyzed using standardized statistical software SPSS (version 20.0). Continuous variables were summarized as means and standard deviations if normally distributed, or as medians and 25th and 75th percentiles if not normally distributed. Correlation between peri- operative changes was assessed with Pearson’s correlation coefficients if normally distributed or Spearman’s rank correlation coefficients if not normally distributed.value less than 0.05 was considered to be significant in statistics.
RESULTS
Demographic and baseline data
In 184 eligible patients (75 males and 109 females), two patients were excluded for receiving homologous transfusion before opening the skull, and one patient was excluded because blood sample coagulation and post-operative r-TEG was unavailable. Therefore there were 181 patients enrolled in the study, including 74 males and 107 females, with the average age of 40.7±12.8 years old. We found significant changes in post-operative r-TEG indices and CCT indices compared to those pre-operatives (Table 1).
Relationship between r-TEG indices and blood loss
Correlation between intra-operative change of r-TEG indices and blood loss was analyzed. We delimited d(index) = indexpost-operative–indexpre-operative, which reflected the intra- operative change of each index of r-TEG. As blood loss volume was not normally distributed, the median (1st-3rd quartiles) blood loss was 6.39% (3.62%-10.87%) of EBV. Among 4 indices of r-TEG, only the change of α-angle showed a significant negative correlation with the blood loss (=−0.184,=0.013) (Table 2).

Table 1.Measurements of r-TEG and CCT indices before and after surgical operation (n=181)§
§:Plus-minus values are mean±SD.r-TEG:Rapid thrombelastography; CCT:conventional coagulation tests; ACT: activated clotting time; MA: maximum amplitude; PT: prothrombin time; APTT: activated partial thromboplastin time; INR: international normalized ratio; FBG: fibrinogen; PLT: platelet count.

Table 2. Spearman’s correlation between peri-operative changes of r-TEG indices and blood loss as percentage of EBV (n=181)
*d(index) = indexpost-operative–indexpre-operative.**.
In order to specifically illustrate how changes of α-angle was correlated with peri-operative blood loss, we divided the patients into subgroups according to dα at intervals of 1 degree, and calculated the mean blood loss of EBV in each subgroup (Table 3).
Seven subgroups (group 4 to group 10) had over 5% of the total participants and were selected for further investigation in linear relationship between dα and average blood loss volume (y=-0.60x + 4.42). The results showed that every 1% blood loss of EBV could cause 0.60 degree decrease of α-angle (Fig. 1).
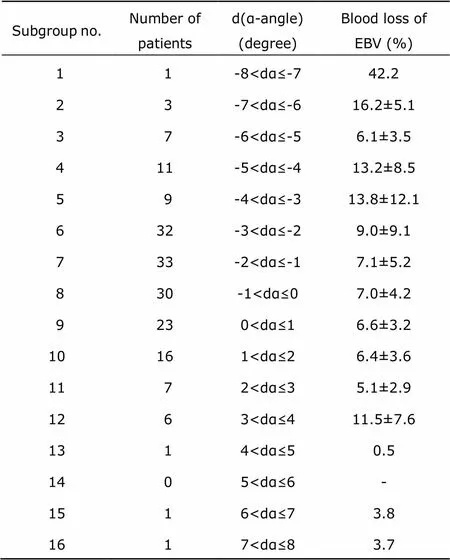
Table 3. Subgrouping according blood loss during operation based on dα-angle (n=181)§
§: Plus-minus values are mean±SD.EBV: Estimated blood volume.
Relationship between CCT indices and blood loss
We also analyzed the correlation between peri-operative change of CCT indices and blood loss. Among 5 CCT indices, changes of FBG and PLT were significantly correlated with blood loss of EBV (=0.015 and=0.001, respectively) (Table 4).
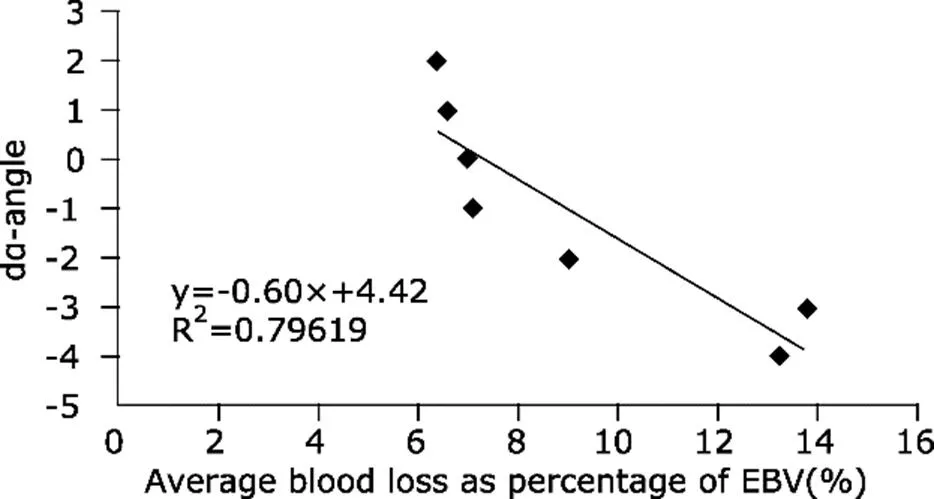
Figure 1.Scatter diagram with regression line showing pre- and post-operative change of α-angle by r-TEG was negatively correlated with average blood loss (percentage of EBV).
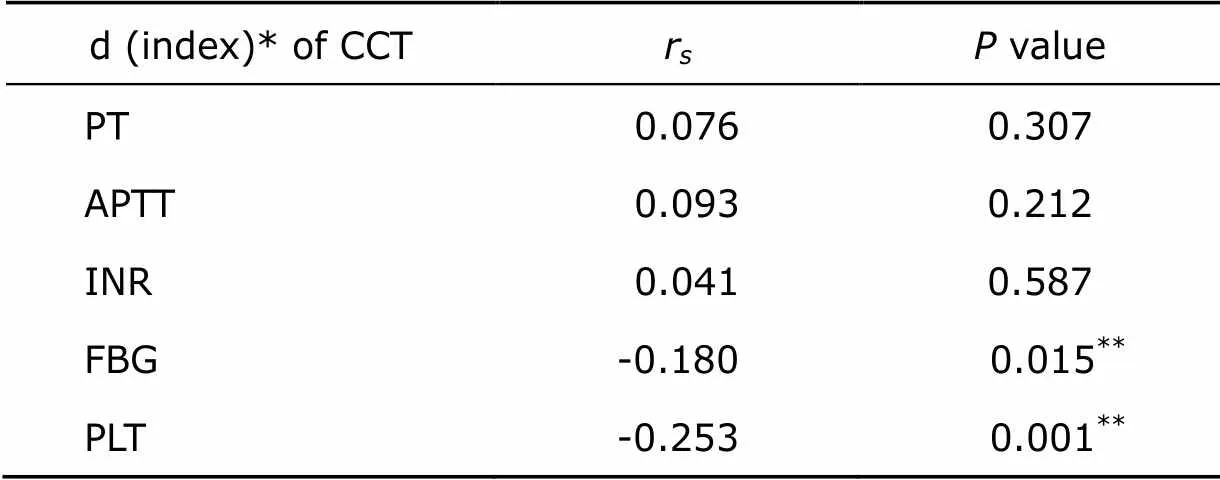
Table 4. Spearman’s correlation between peri-operative changes of CCT indices and blood loss as percentage of EBV (n=181)
*d(index)=indexpost-operative–indexpre-operative.**<0.05.
DISCUSSION
In this study, we collected information from 181 patients and demonstrated that only α-angle in r-TEG, which reflects the amount and function of fibrinogen, was significantly correlated with volume of blood loss during surgery. This was also confirmed by CCT results that peri-operative change of FBG had significant correlation with blood loss. However, we found that ACT, as well as PT, APTT, INR, reflecting the number and function of clotting factors, were not correlated with intra-operative blood loss. As in medical practice, FFP has always been the first choice of index for correcting coagulopathy empirically during surgery; our findings challenged the current empirical transfusion strategy. It was reported that in severe traumatized and massively bleeding patients, fibrinogen could reach critical levels at early stage.14Besides, a few studies also found that the peri-operative blood loss tendency increased when fibrinogen level went below 1.5- 2.0 g/L,15-17which was much higher than the suggested fibrinogen level for blood transfusion in the guideline (0.8g/L).1As a result, current empirical cognition about the effects of peri-operative hemorrhage on coagulation might not be universally correct, and the impact of blood loss on fibrinogen should gain more attention.
Currently, the precise thresholds and dosing strategies for fibrinogen supplementation have not yet been determined.18R-TEG is supposed to be a valuable reference for constructing component blood transfusion. Therefore, we attempted to investigate the relationship between dα-angle and blood loss volume. We found a trend that blood loss volume increased as dα-angle decreased, and that every 1% loss of EBV could cause α-angle to decrease 0.60 degree. This relationship might be an important reference for empirically evaluating whether or not patient needs a fibrinogen transfusion according to the basic α angle and intra-operative blood loss. Meanwhile, based on this relationship, anesthesiologists could also evaluate the appropriate time for patient to take a r-TEG test, which is economically beneficial.
It was expected that the intra-operative blood loss and anesthesia fluid expansion during surgery should cause decrease in fibrinogen amount. However, we were surprised to find that lots of participants had a positive dα-angle during surgery. There are two major reasons that might account for this phenomenon. Firstly, surgical stimulation might activate coagulation system in some patients, thus lead to hypercoagulation state. Secondly, α-angle reflects both the amount and the function of fibrinogen. Intra-operative hemorrhage and anesthesia fluid expansion lead to hemodilution and decrease in fibrinogen count, therefore compensatory mechanisms come into work so that fibrinogen function increases. As a result, the overall effect of hemorrhage on fibrinogen is increased, as shown by peri-operative changes of α-angle.
MA is regarded to be strongly related to PLT according to previous studies.19Nevertheless, converse results were discovered in our study. PLT, acquired through CBC, only reflects the amount of platelets in whole blood. While MA, acquired through r-TEG, reflects both the amount and the function of platelets. Intra-operative blood loss and fluid transfusion lead to hemodilution and the decrease in platelet count; at the same time, compensatory mechanisms come into work, causing platelet function increases. As a result, the overall influence of hemorrhage on platelet reflected by MA is balanced. Since MA is superior to platelet count in predicting platelet transfusion, we speculate that transfusion of platelet is not necessary for minor blood loss.
It must be pointed out that there were some limitations in the present study. Firstly, it was an observational study. The transfusion of blood products was not controlled, which prevented us from observing the effect of blood transfusion after performing intervention guided by r-TEG. Secondly, this was a single institutional study and the recruited participants were only those undergoing neurosurgery. From a perspective of sample size and operation category, multi- center studies with participants undergoing various types of surgery should be carried out. Thirdly, we used citrated whole blood for r-TEG with citrated rapid TEG (CRT) model in the present study. As blood sample tested by CRT model should be obtained 15 minutes before testing, it extends the time to obtain the results. In addition, it has been found that non-citrated whole blood is optimal for evaluation of post-injury coagulopathy with r-TEG, because tissue factors shortens coagulation time by activating factors VII and X, which may influence r-TEG results.20Therefore, we should attempt to assess the utility of non-citrated blood sample in further studies.
To conclude, peri-operative change of α-angle was the only r-TEG index that significantly correlated with intra- operative blood loss volume. The current widely accepted empirical cognition about the effects of intra-operative hemorrhage on coagulation might not be universally correct, and the impact of hemorrhage on fibrinogen should be cautiously scrutinized. Every 1% blood loss of EBV could cause 0.60 degree decrease in α-angle, and this relationship might be used as an important reference to empirically evaluate whether patient would need a fibrinogen transfusion according to the basic α-angle in r-TEG and the volume of intra-operative blood loss.
1. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006; 105:198-208.
2. Dara SI, Rana R, Afessa B, et al. Fresh frozen plasma transfusion in critically ill medical patients with coagulopathy. Crit Care Med 2005; 33: 2667-71. doi: 10.1097/01.CCM.0000186745.53059.F0.
3. Sarani B, Dunkman WJ, Dean L, et al. Transfusion of fresh frozen plasma in critically ill surgical patients is associated with an increased risk of infection. Crit Care Med 2008; 36:1114-8. doi:10.1097/CCM.0b013e318168f89d.
4. Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest 2007; 131:1308-14. doi:10.1378/chest. 06-3048.
5. Cotton BA, Faz G, Hatch QM, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011; 71: 407-17. doi:10.1097/TA.0b013e31821e1bf0.
6. Jeger V, Zimmermann H, Exadaktylos AK. Can Rapid TEG accelerate the search for coagulopathies in the patient with multiple injuries? J Trauma 2009; 66:1253-7. doi:10. 1097/TA.0b013e31819d3caf.
7. Reikvam H, Steien E, Hauge B, et al. Thrombelastography. Transfus Apher Sci 2009; 40:119-23. doi:10.1016/j. transci.2009.01.019.
8. Schöchl H, Nienaber U, Maegele M, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care 2011; 15:R83. doi:10. 1186/cc10078.
9. Wikkelsoe AJ, Afshari A, Wetterslev J, et al. Monitoring patients at risk of massive transfusion with thrombelastography or thromboelastometry: a systematic review. Acta Anaesthesiol Scand 2011; 55:1174-89. doi:10. 1111/j.1399-6576.2011.02534.x.
10. Kunio NR, Differding JA, Watson KM, et al. Thrombelastography-identified coagulopathy is associated with increased morbidity and mortality after traumatic brain injury. Am J Surg 2012; 203: 584-8. doi:10.1016/j.amjsurg. 2011.12.011.
11. Windeløv NA, Welling KL, Ostrowski SR, et al. The prognostic value of thrombelastography in identifying neurosurgical patients with worse prognosis. Blood Coagul Fibrinolysis 2011; 22:416-9. doi:10.1097/MBC. 0b013e3283464f53.
12. Cotton BA, Minei KM, Radwan ZA, et al. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg 2012; 72:1470-5. doi:10.1097/TA.0b013e31824d56ad.
13. Kaye AD, Riopelle JM. Intravascular fluid and electrolyte physiology. In: Miller RD. Miller’s Anesthesia. 7th ed. Philiadephia: Chuichill Livingstone; 2010. p. 1728-30.
14. Fries D. The early use of fibrinogen, prothrombin complex concentrate, and recombinant-activated factor VIIa in massive bleeding. Transfusion 2013; 53:91S-95S. doi:10. 1111/trf.12041.
15. Gerlach R, Tölle F, Raabe A, et al. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 2002; 33:1618-23. doi:10. 1161/01.STR.0000017219.83330.FF.
16. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007; 5: 266-73. doi:10.1111/j.1538-7836.2007.02297.x.
17. Blome M, Isgro F, Kiessling AH, et al. Relationship between factor XIII activity, fibrinogen, haemostasis screening tests and postoperative bleeding in cardiopulmonary bypass surgery. Thromb Haemost 2005; 93:1101-7. doi:10.1160/TH04-12-0799.
18. Levy JH, Welsby I, Goodnough LT. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion 2014; 54: 1389-405. doi:10.1111/trf.12431.
19. Plotkin AJ, Wade CE, Jenkins DH, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma 2008; 64:S64-8. doi:10.1097/TA.0b013e318160772d.
20. Kashuk JL, Moore EE, Le T, et al. Noncitrated whole blood is optimal for evaluation of postinjury coagulopathy with point-of-care rapid thrombelastography. J Surg Res 2009; 156:133-8. doi:10.1016/j.jss.2009. 03.046.
for publication August 07, 2016.
Tel: 010-69152020, E-mail: garybeijing@163.com
杂志排行
Chinese Medical Sciences Journal的其它文章
- Clinical Evaluation of Color Doppler Ultrasound in Selecting the Optimal Treatment Modality for Infantile Hemangioma△
- Neuroprotective Effects of Grape Seed Procyanidin Extract on Ischemia-Reperfusion Brain Injury△
- Effects of Aging on the Proliferation and Differentiation Capacity of Human Periodontal Ligament Stem Cells△
- Abnormal Alterations of Cortical Thickness in 16 Patients with Type 2 Diabetes Mellitus: A Pilot MRI Study△
- Prevalence of Carbapenem-Resistant Klebsiella Pneumoniae (CRKP) and the Distribution of Class 1 Integron in Their Strains Isolated from a Hospital in Central China
- Can Fundus Fluorescein Angiography be Performed for Diabetic Patients on Oral Metformin?△
