1,2-环己二胺缩邻香兰素单核与四核镱稀土配合物的近红外性质
2017-08-07范仲天董艳萍李光明
范仲天 高 博 董艳萍*,,2 李光明*,
1,2-环己二胺缩邻香兰素单核与四核镱稀土配合物的近红外性质
范仲天1高 博1董艳萍*,1,2李光明*,1
(1黑龙江大学化学化工与材料学院,功能无机材料化学省部共建教育部重点实验室,哈尔滨 150080)
(2《黑龙江大学工程学报》编辑部,哈尔滨 150080)
通过配体1,2-环己二胺缩邻香兰素(H2L)和不同的镱盐反应,合成了4个镱稀土配合物[Yb(H2L)2](ClO4)3·2CH3OH·H2O(1),[Yb4(L)4(NO3)2(H2O)2](PF6)2·4CH3CN(2),[Yb4(L)4(H2O)2Cl2](PF6)2·2CH2Cl2·2H2O(3)和[Yb4(L)4(NO3)2(H2O)2][Yb(NO3)3(H2O)2(CH3OH)](NO3)2·4CH2Cl2·6CH3OH(4)。X射线单晶衍射分析表明配合物1为零维的单核结构,配合物2~4均为四核结构。研究了4个配合物的近红外发光性能。
近红外发光;镱配合物;结构
0 Introduction
Much recent interest has been focused on the design and preparation of the polynuclear lanthanide complexes,because they could be potentially used in fluorescence[1-5],magnetism[6-11].However,direct excitation of Ln3+ions is difficult because of the weak (Laporte-forbidden)nature of their f-f transitions.In order to hurdle it,well-designed organic chromophores have been used to act as antennas[12-14].It is wellknown that salen type ligands are able to stabilize differentmetalsin variouscoordination environment[15-16]. And a series ofsalen type ligands have been employed in the synthesis ofnumerous metalcomplexes,such as a number of salen type homo-polynuclear complexes, some of which exhibit intriguing magnetic[17-20]and luminescent properties[21-23].Previously,Jones and coworkers have reported some salen type ligands to stabilize Ln3+centers and provide the antenna for lanthanide NIR luminescence,e.g.,Jones et al.have reported the first salen type sandwich Yb3complex which shows NIR luminescence of the Yb3+ion in 2005[24].In 2012,they have presented the homoleptic cyclic tetranuclear complexes which exhibit NIR luminescence of the Nd3+ions and Yb3+ions[25-26].However, the synthesis and structures are often influenced by a variety of factors,such as the structure of the ligand, the ionic radius of the lanthanide ions and the nature of the counter ions.To the best of our knowledge,few mono-nuclear lanthanide complexes constructed from flexible hexa-dentate salen type ligands with the outer O2O2moiety has been documented.In view of recent emerging significance of homo-polynuclear lanthanide complexes in NIR luminescence and magnetism,based on the hexa-dentate ligand H2L(N,N′-bis(3-methoxy -salicylidene)cyclohexane-1,2-diamine)with the richness of coordination codes(L2-and H2L modes,Scheme 1).The semi-ridged salen type ligand ofH2L and various ytterbium salts were employed in the experiment to explore the structures and NIR luminescence of the salen type lanthanide complexes.As a result,a series of four mono-and tetra-nuclear ytterbium complexes have been isolated.Their structures have been determined and described as well as their NIR luminescence have been investigated and discussed.
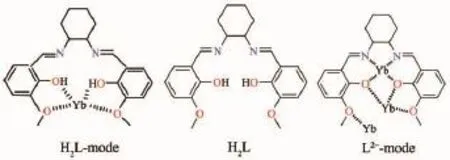
Scheme 1 Molecular structure and bonding modes of the salen type ligand H2L for mono-and tetranuclear ytterbium complexes 1~4
1 Experimental
1.1 Materials and general methods
All chemicals except YbCl3·6H2O,Yb(NO3)3· 6H2O,Yb(ClO4)3·9H2O and H2L were obtained from commercial sources and used without further purification.YbCl3·6H2O,Yb(NO3)3·6H2O,Yb(ClO4)3·9H2O were prepared by the reactions of Ln2O3and hydrochloric acid,nitric acid and perchloric acid in aqueous solution,respectively.H2L was prepared according to the reported method[25].Elemental(C,H and N)analyses were performed on a Perkin-Elmer 2400 analyzer.FTIR spectra were obtained on a Perkin-Elmer Spectrum 100 spectrophotometer by using KBr disks in the range of 4 000~500 cm-1.UV-Vis absorption spectra were recorded on a Perkin-Elmer Lambda 35 spectrometer.Thermal analyses were conducted on a Perkin-Elmer STA 6000 with a heating rate of 10℃·min-1in a temperature range from 30 to 800℃ under atmosphere.Excitation and emission spectra were measured with an Edinburgh FLS 920 fluorescence spectrophotometer.Luminescence lifetimes were recorded on a single photon counting spectrometer from Edinburgh Instruments(FLS 920)with a microsecond pulse lamp as the excitation.
1.2 Syntheses of complexes 1~4
1.2.1 Synthesis of[Yb(H2L)2](ClO4)3·2CH3OH·H2O(1)
To a stirred solution of H2L (0.2 mmol,0.077 g) in absolute CH2Cl2(10 mL),a solution of Yb(ClO4)3· 9H2O(0.1 mmol,0.063 g)in absolute CH3OH(10 mL) were added.The resultant mixed solution was allowed to stir for 10 h at room temperature.The solution was filtered and petroleum ether was allowed to diffuse slowly into the filtrate at room temperature and yellow crystals were obtained in a week.For 1:Yield:0.083 g,63%.Anal.Calcd.for C46H60YbCl3N4O23(%):C,41.97; H,4.59;N,4.26;Found(%):C,41.92;H,4.72;N,4.24. IR(KBr,cm-1):3 392(w),2 952(w),1 643(s),1 503(m), 1 451(m),1 228(s),1 122(s),1 093(s),745(w),627(w). UV-Vis(CH3OH,λmax/nm):226,274,361.
1.2.2 Synthesis of[Yb4(L)4(NO3)2(H2O)2](PF6)2· 4CH3CN(2)
To a stirred solution of H2L (0.2 mmol,0.077 g)in absolute CH3CN (10 mL),a solution of Yb(NO3)3· 6H2O(0.2 mmol,0.093 g)in absolute CH3OH(10 mL) were added.The mixed solution was allowed to stir for 2 h at room temperature,and then NH4PF6(0.3 mmol, 0.049 g)wasadded to the solution.The resultantmixture was allowed to stir for 10 h at room temperature.The yellow solution was then filtered and diethyl ether was allowed to diffuse slowly into the filtrate at room temperature and yellow crystals were obtained in a week.For 2:Yield:0.072 g,53%.Anal.Calcd.for C96H112Yb4F12N14O24P2(%):C,40.77;H,3.99;N,6.93; found:C,39.98;H,3.91;N,6.91%.IR (KBr,cm-1): 3 423(w),2 937(m),2 857(m),1 650(s),1 619(m), 1 472(s),1 384(m),1 290(m),1 227(m),844(s),742 (m),559(m).UV-Vis(CH3OH,λmax/nm):221,264,338.
1.2.3 Synthesis of[Yb4(L)4(H2O)2Cl2](PF6)2·2CH2Cl2· 2H2O(3)
The synthesis of 3 is similar to 2 except that YbCl3·6H2O(0.2 mmol,0.078 g)was used instead of Yb(NO3)3·6H2O.For 3:Yield:0.067g,48%.Anal. Calcd.forC90H104Yb4Cl6F12N8O20P2(%):C,38.43;H,3.73; N,3.98;Found(%):C,38.36;H,3.90;N,3.96.IR(KBr, cm-1):2 936(m),2 859(m),1 648(s),1 618(m),1 386 (m),1 292(m),1 226(m),844(s).UV-Vis(CH3OH,λmax/ nm):224,271,342.
1.2.4 Synthesis of[Yb4(L)4(NO3)2(H2O)2][Yb(NO3)3 (H2O)2(CH3OH)](NO3)2·4CH2Cl2·6CH3OH(4)
To a stirred solution of H2L(0.2 mmol,0.077 g) in absolute CH2Cl2(25 mL),a solution of Yb(NO3)3· 6H2O(0.2 mmol,0.093 g)in absolute CH3OH(5 mL) were added.The yellow solution was then filtered and petroleum ether was allowed to diffuse slowly into the filtrate at room temperature and yellow crystals were obtained in a week.For 4:Yield:0.078 g,49%.Anal. Calcd.for C99H135Yb5Cl8N13O42(%):C,35.73;H,4.09; N,5.47;Found(%):C,35.15;H,3.81;N,5.59.IR (KBr,cm-1):3 425(w),2 936(m),2 860(m),1 651(s), 1 508(m),1 470(m),1 385(m),1 313(m),1 227(m), 742(m).UV-Vis(CH3OH,λmax/nm):223,274,353.
1.3 X-ray crystallography
Suitable single crystals of 1~4 were selected for X-ray diffraction analysis.Single-crystal X-ray data of 1~4 were collected using an Oxford Xcalibur Gemini Ultra diffractometer with graphite-monochromated Mo Kαradiation(λ=0.071 073 nm)at 293 K.The structures were solved by the direct methods and refined by full-matrix least-squares on F2using the SHELXTL-97 program[27].The Yb3+,NO3-and Cl-were easily located,and then non-hydrogen atoms (C,N and O)were placed from the subsequent Fourierdifference maps.All non-hydrogen atoms were refined anistropically.Crystallographic data and structure refinement parameters for the complexes are presented in Table 1.The SQUEEZE results were consistent with TG-DSC and elemental analysis.The CH3CN, CH2Cl2,CH3OH and H2O molecules have been included in the formula for the calculation of intensive properties. The SQUEEZE results have been appended to the CIF files.
CCDC:916031,1;916032,2;916034,3;916033,4.
2 Results and discussion
2.1 Synthesis and spectral analysis
As shown in Scheme 2,complex 1 was synthesized by the reaction of H2L with Yb(ClO4)3·9H2O with the ligand-to-metal molar ratio of 2∶1,while complexes 2~4 were synthesized by the reaction ofH2L with Yb(NO3)3·6H2O or YbCl3·6H2O and/or NH4PF6with the ligandto-metal molar ratio of 1∶1.In the FT-IR spectra(Fig.S1 in Supporting information),the characteristic strong absorptions of theν(C=N)vibration at 1 643~1 651 cm-1for complexes 1~4,are slightly blue-shifted by 17~25 cm-1compared to those for the free ligands (1 626 cm-1)upon coordination of the Ln3+ion.The presence of ClO4-in complex 1 as a counter ion was indicated by its IR spectrum,which shows a strong characteristic band at 627 cm-1for ClO4-.Four bands around 1 470,1 385,1 225 and 742 cm-1forcomplexes 2 and 4 were observed,which can be assigned to vibrations of coordinated nitrate groups,respectively (ν1,ν2,ν3andν4).In addition,the absorption bands at 844 cm-1for complexes 2 and 3 are attributed to the stretching vibrations of the PF6-anions.TG-DSC analyses of complexes 1~4(Fig.S2~S5)reveal that complex 1 loses a gradual weight loss of 6.16%in the range of 33~85℃,which corresponds to the loss of two methanol molecules and one water molecules (Calcd.6.53%).The loss of four acetonitrile molecules (Obsd.5.78%,Calcd.5.81%)is observed for 2 in the range of 33~250℃.Complex 3 loses two dichloromethane molecules and two water molecules in the temperature range of 33~330℃(Obsd.8.90%,Calcd. 7.32%).For 4,the weight loss between 33 and 180℃ can be attributed to the loss of four dichloromethane molecules and six methanol molecules(Obsd.14.90%, Calcd.15.98%).
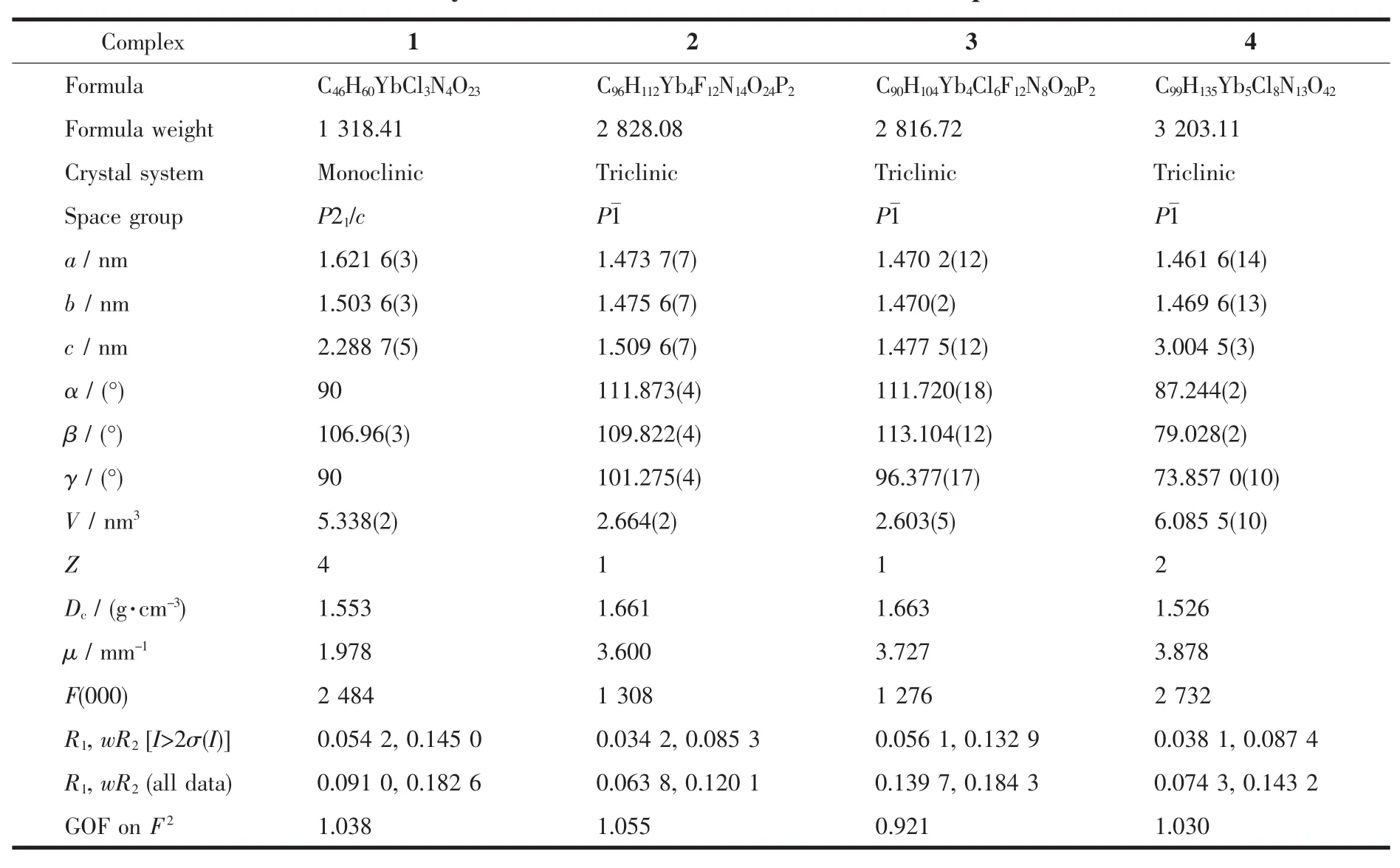
Table 1 Crystaldata and structure refinement for complexes 1~4
2.2 Structuraldescription of complex 1

Fig.1 View of the cationic structure in complex 1
X-ray crystallographic analysis reveals that complex 1 crystallizes in a monoclinic space group P21/c with an ionic mononuclear structure(Fig.1). Complex 1 is composed of one[Yb(H2L)2]3+cation, three free ClO4-anions,two crystalline CH3OH and one H2O molecules.The Yb3+ion is eight-coordinated to eight oxygen atoms from two ligands forming a distorted square antiprism geometry (Fig.1),and shielded in the outer O2O2 cavity (two phenol oxygen atoms and two methoxy oxygen atoms)of the ligand, while the nitrogen atoms ofimine remain uncoordinated. Notably,the two ligands coordinate to the Yb3+ion in a crossover configuration.The dihedral angle between the coordination planes of two O2O2 cavities is 79.793(1)°.The dihedral angles between the two neighboring aromatic rings in different ligands are 28.330(3)°and 36.974(3)°.The Yb-O bond distances from phenol and methoxy groups are in the range of 0.221 4(6)to 0.247 2(6)nm and the average distance is 0.233 6 nm.The three free ClO4-ions do not participate in the coordination structure but balance the positive charge of the complex.Noticeably,such an ionic crossover mononuclear Yb3+complex is unique with a crossover structure involving pure oxygen atoms coordination.It is similar to the reported mononuclear complex[Yb(H2L1)2CH3OH](ClO4)3(H2L1=N,N′-bis(2-hydroxy-3-methoxybenzylidene)-1,3-propanediamine): the Yb3+ion is nine-coordinated to nine-oxygen atoms from two ligands and one methanol[28].Previously,two kinds of salen type mononuclear lanthanide complexes have been reported,e.g.complexes [(H2L2)Nd(NO3)3] (H2L2=N,N′-bis(3-methoxysalicylidene)propane-1,2-diamine)[29],[Nd(H2L3)(NO3)3](H2L3=N,N′-ethylene-bis (3-methoxysalicylideneimine)[30],which are ofthe neutral crab-like structure with a ratio of ligand to lanthanide of1∶1;[Ce(salophen)2](salophen=N,N′-bis(salicylidene) -1,2-(phenylenediamine))[31]are of crossover structure and distorted sandwich structure with a ratio of ligand to lanthanide of 2∶1,in which the Ce ions are+4 oxidation state and the N atoms are coordinated to the Ceions.
2.3 Structural description of complexes 2~4
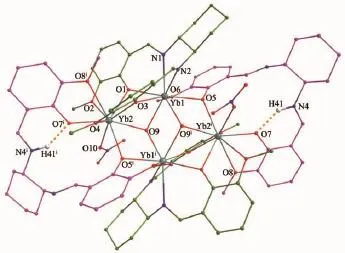
Fig.2 View of the cationic structure of complex 2
Single-crystal X-ray diffraction analyses indicate that complexes 2,3 and 4 are isomorphous,so only structure ofcomplex 2 is descripted in detail.Complex 2 crystallizes in a triclinic space group of P1 featuring a discrete ionic structure with a defect-dicubane Yb4core.The structural unit is composed of one [Yb4(L)4 (NO3)2(H2O)2]2+cation,two free PF6-anions,and four solvate acetonitrile molecules (Fig.2).The [Yb4(L)4 (NO3)2(H2O)2]2+cation lying about an inversion centre, two equivalent Yb2(L)2moieties are bridged by twoμ-O phenoxide atoms (O5 and O5i)of two salen type ligands with O4tetradentate mode(Scheme 1)and two O atoms(O9 and O9i)of two coordinatedμ3-OH-groups,resulting in the formation of a homoleptic cyclic tetranuclear Yb4(L)2(HL)2host structure.In each of two equivalent Yb2(L)(HL)moieties,two Yb3+(Yb1 and Yb2)ions with differentcoordination environments are also linked by twoμ-O phenoxide atoms(O1 and O3)of one L2-ligand with N2O4hexa-dentate mode and one O atom (O9)ofthe coordinated H2O molecule.The unique inner Yb3+ion (Yb1)is eight-coordinate and bound by the N2O2core of the salen type L2-ligand in addition to two O atom (O5 of MeO group and O6 ofμ-O phenoxide atom)from the salen type ligand and two O atoms of two coordinated H2O molecule.Meanwhile the outer Yb3+ion(Yb2)is ninecoordinated including seven oxygen atoms from two outer O2O2moieties of two salen type ligands,where four O atoms(two ofMeO groups and two ofphenoxide atoms)are from one salen type ligand and three O atoms(one of MeO groups and two ofphenoxide atoms) from another salen type ligand.It completes its coordination environment with one O atom from the monodentate NO3-anion and one O atom from the coordinated H2O molecule.The unique Yb…Yb distances are different,with the distances of 0.343 00(4), 0.370 28(6)and 0.379 12(5)nm for Yb1…Yb2,Yb1…Yb1iand Yb2…Yb1i,respectively,in which each of the Yb1…Yb2 separations in the equivalent Yb2L2moieties is slightly shorter than that(Yb1…Yb1ior Yb2…Yb1iseparation)between two equivalent Yb2L2moieties.For the inner Yb3+ion(Yb1 or Yb1i),the Yb -O bond length(0.227 2(4)~0.228 4(5)nm;O atoms from coordinated H2O molecule)or the Yb-N bond lengths(0.238 6(7)~0.243 9(6)nm)are in the range of the Yb-O bond lengths(0.227 2(5)~0.231 2(5)nm) with O atoms from the phenoxo groups.For the outer Yb3+ion (Yb2 or Yb2i),the Yb-O bond lengths also depend on the nature of the oxygen atoms,varying from 0.222 2(5)to 0.269 5(5)nm,in which the bond lengths(0.254 8(5)~0.269 5(5)nm)from the oxygen atoms ofMeO groups are distinctively longerthan those (0.222 2(5)~0.239 6(4)nm,0.232 7(6)and 0.231 7(4) nm)from the phenoxo oxygen atoms,monodentate NO3-anion or coordinated H2O molecule.It is interesting to note the presence of an ‘apical’triply bridged H2O molecule for each of the two central O atoms(O9 and O9i),which could be shown from the reasonable directionality of the interactions with three Yb3+ions. Furthermore,as to the cation[Yb4(L)4(NO3)2(H2O)2]2+, the charge is balanced by the protonation of one(N4 or N4i)of the imino nitrogen atoms for two of the deprotonated salen type L2-ligands,which endows the formation of two strong intramolecular H-bond interactionswith the shortN4…O7 distance(0.269 8(10) nm)(Fig.2).The PF6-is not involved in the coordination to lanthanide ions,and it plays a chargebalancing role.
Notably,although complex 2 is isomorphic to previous reported analog of the salen type homoleptic tetranuclear complexes,e.g.[Yb4(L)2(HL)2(NO3)2(OH)2] (NO3)2[25]and[Yb4(L)2(HL)2(μ3-OH)2Cl2]Cl2[26],the PF6-ions instead of NO3-or Cl-ions act as the counter ions balancing the positive charge in 2.Furthermore, there are obvious differences between the composition of complexes 2,3 and 4,e.g.the two coordination NO3-anions in 2 are replaced by two Cl-anions in 3, while a neutral molecule of[Yb(NO3)3(H2O)2(CH3OH)] is crystalline in 4.
2.4 NIR luminescence
The UV-Vis absorption spectra of the ligand H2L and complexes 1~4 were recorded in CH3OH solution (Fig.3).For free ligand,the typical absorptions at 220 and 261 nm are attributed to theπ-π*transition of the aromatic ring and azomethine chromophores.The peak at 334 nm is attributed to n-π*transition of R band which belongs to azomethine.For complexes 1~4,the similar ligand-centered solution absorption bands(221~226,264~274,338~361 nm)are observed and red-shifted as compared to those (220,261 and 334 nm)for ligand due to the changes in the energy levels of the ligand orbitals upon the coordination.Complexes 2~4 show the higher molar absorption coefficients than thatfor complex 1,which is attributed to more ligands in complex 1.

Fig.3 UV-Vis spectra of the ligand H2L and complexes 1~4 in CH3OH solution
The NIR photoluminescence spectra ofcomplexes 1~4 were recorded with the excited wavelength at 370 nm in the isoabsorptive solution at room temperature. NIR photoluminescence spectra of complexes 1~4 exhibit that the typical NIR emission bands of Yb3+ion could be observed at 975 nm which is assigned to the2F5/2→2F7/2transition(Fig.4).Notably,the emission of Yb3+ion in complexes 1 and 4 is not a sharp transition that well-split NIR emission peaks are observed,where it appears as a series of bands with two otherbroad bands centered around 1 024 and 1 056 nm.Similar results have been proposed in previously report which is attributed to the crystal-field or stark splitting[32].The absence of the typical Yb3+ion excitation bands in the excitation spectra and the ligandcentered luminescence in the emission spectra of 1~4 reveal the occurrence of the ligand-to-metal energy transfer.Obviously,the NIR signal of Yb3+ion for complex 1 is the strongest among the four complexes which can be ascribed to two reasons.Firstly,it might be owing to the existence of the coordinated counter ions,such as NO3-anions and Cl-anions in 2~4, which are believed to quench the NIR fluorescence. Secondly,the Yb3+ion of complex 1 is well enclosed in the crossover structure,which contributes to preventing the NIR luminescence quenching from solvent molecule effectively.In addition to the steadystate emission,we also perform time-resolved measurements for complexes 1 and 4 in the NIR region by using the time-correlated single photon counting (TCSPC)technique.The decay of the emission band at 975 nm gives a satisfactory fitto a monoexponential decay with lifetimes of 2.90μs for 1 and 2.59μs for 4.The lifetimes of 2 and 3 cannot be obtained due to the weakness of the signal.

Fig.4 NIR spectra of complexes 1~4 in CH3OH with the excited wavelength at 370 nm in the isoabsorptive solution
3 Conclusions
We have isolated a series of four salen type ytterbium complexes featuring an ionic crossover mononuclear structure for complex 1 and defectdicubane core structures for complexes 2~4 through the self-assembly of the semi-ridged salen type H2L with various ytterbium salts.It demonstrates that the flexible ligand and the different ytterbium counter ions dominate the structures of salen type ytterbium complexes.All complexes 1~4 exhibit the similar typical NIR luminescence of Yb3+ions proposing that the energy transfer from H2L to Yb3+ions in 1~4 takes place effectively and the crossover structure and the defect-dicubane structure can encapsulate the lanthanide ions efficiently prevent the NIR luminescence quenching from solvent molecules.
Acknowledgments:This work is financially supported by the National Natural Science Foundation of China (Grants No. 51402092,21601132).
Supporting information is available athttp://www.wjhxxb.cn
[1]Rocha J,Carlos L D,Paz F A A,et al.Chem.Soc.Rev., 2011,40:926-940
[2]Carlos L D,Ferreira R A S,De Zea B,et al.Chem.Soc. Rev.,2011,40:536-549
[3]Kieran G,Julia M,Michael A S,et al.Cryst.Growth Des., 2017,17:1524-1538
[4]Wang G T,Zhang J C,Tang Z Y,et al.CrystEngComm, 2016,18:2437-2445
[5]Elena A M,Anastasiya V Y,Matthias Z,et al.Dalton Trans.,2017,46:3457-3469
[6]Sabdeep K G,Stuart K L,Kamma S,et al.Inorg.Chem., 2017,56:3946-3960
[7]Dong Y P,Yan P F,Zou X Y,et al.Dalton Trans.,2016, 9148-9157
[8]Mattgew G.Nitholas F C,Ana-Maria A,et al.Chem.Sci., 2016,7:155-165
[9]Sunri L,Takuji O.Chem.Lett.,2017,46:10-18
[10]Chen Y C,Liu J L,Ungur L,et al.J.Am.Chem.Soc.,2016, 138:2829-2837
[11]Liu J,Chen Y C,Liu J L,et al.J.Am.Chem.Soc.,2016, 138:5441-5450
[12]Akine S,Utsuno F,Taniguchi T,et al.Eur.J.Inorg.Chem., 2010:3143-3150
[13]Albrecht M,Osetska O,Bünzli J C G,et al.Chem.Eur.J., 2009,15:8791-8799
[14]Chauvin A S,Comby S,Song B,et al.Chem.Eur.J.,2008, 14:1726-1735
[15]Dalla Cort A,De Bernardin P,Forte G,et al.Chem.Soc. Rev.,2010,39:3863-3874
[16]ZOU Xiao-Yan(邹晓艳),MA Hui-Yuan(马慧媛),PANG Hai-Jun(庞海军),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(9):1647-1652
[17]Zhu J,Song H F,Yan P F,et al.CrystEngComm,2013,15: 1747-1752
[18]Yan P F,Lin P H,Habib F,et al.Inorg.Chem.,2011,50: 7059-7065
[19]Zou X Y,Yan P F,Dong Y P,et al.RSC Adv.,2015,5: 96573-96573
[20]Dong Y P,Yan P F,Zou X Y,et al.J.Mater.Chem.C, 2015,3:4407-4415
[21]Yang X,Jones R A,Rivers J H,et al.Dalton Trans.,2009: 10505-10510
[22]Yang X,Lam D,Chan C,et al.Dalton Trans.,2011,40: 9795-9801
[23]Liu T Q,Yan P F,Luan F,et al.Inorg.Chem.,2015,54: 221-228
[24]Yang X,Jones R A.J.Am.Chem.Soc.,2005,127:7686-7688
[25]Feng W,Zhang Y,LüX,et al.CrystEngComm,2012,14: 3456-3463
[26]Feng W,Zhang Y,Zhang Z,et al.Inorg.Chem.,2012,51: 11377-11385
[27]Sheldrick G M.Acta Crystallogr.Sect.A,2008,A64:112-122
[28]Zou X Y,Yan P F,Zhang J W,et al.Dalton Trans.,2013, 42:9482-9489
[29]Sun W B,Yan P F,Li G M,et al.Inorg.Chim.Acta,2009, 362:1761-1766
[30]Gao T,Yan P F,Li G M,et al.Inorg.Chim.Acta,2008, 361:2051-2058
[31]Terzis A,Mentzafos D,Tajmir-Riahi H.Inorg.Chim.Acta, 1984,84:187-193
[32]Kang T S,Harrison B S,Bouguettaya M,et al.Adv.Funct. Mater.,2003,13:205-210
关键词:合成;单桥连双环戊二烯;Friedel-Crafts酰基化反应;铼羰基配合物;催化
DOI:10.11862/CJIC.2017.153
Abstract:Thermal treatment of two p-phenylene-and p-biphenylene-bridged biscyclopentadienes(C5Me4H)E (C5Me4H)(E=C6H4,(C6H4)2)with Re2(CO)10in refluxing mesitylene gave the corresponding complexes(E)[(η5-C5Me4) Re(CO)3]2(E=C6H4(1),(C6H4)2(2)),which were separated by chromatography,and characterized by elemental analysis,IR,1H NMR and13C NMR spectroscopy.The molecular structures ofcomplexes 1 and 2 were characterized by X-ray crystal diffraction analysis,showing both them are monobridged biscyclopentadienyl dinuclear rhenium carbonyl complexes.In addition,the catalytic performance of complexes 1 and 2 was also tested,and it was found thatboth complexes were obviously active for Friedel-Crafts acylation reactions.CCDC:1506755,1;1506754,2.
Keywords:synthesis;mono-bridged biscyclopentadiene;Friedel-Crafts acylation reaction;rhenium carbonylcomplex;catalysis
0 Introduction
Much attention has been focused on the synthesis of a series of biscyclopentadienyl carbonyl ruthenium complexes in recent decades,which mainly include non-bridged [1-2], singly bridged [3-8] and doubly bridged[9-10]biscyclopentadienyl complexes.Bridged bis (cyclopentadienyl)ligands have been extensively studied as frameworks for dinuclear metal complexes that are resistant to fragmentation and maintain twometal centers in close proximity even after metalmetal bond cleavage[11-13].Especially,rhenium carbonyl complexes have been studied as catalysts for many reactions due to their catalytic activity.When compared to mononuclear rhenium complexes,bridged dicyclopentadienyl dirhenium analogues,in which the bridging ligand binds two reactive metal centers,may promote the distinctive chemical reactivity and catalytic properties.However,only a few examples of Friedel-Crafts reactions catalyzed by rhenium carbonyl complexes have been reported up to now[14-16]. Recently,Our group have reported the synthesis and catalytic activity of three monobridged bis (cyclopentadienyl)rhenium carbonyl complexes[17], showing that these rhenium carbonyl complexes have catalytic activity for Friedel-Crafts alkylation reactions.To develop a deeper understanding of the structures and reactivity of bridged bis (cyclopentadienyl)rhenium carbonyl complexes,here in this paper we selected two bridged ligand precursors(C5Me4H)E(C5Me4H)(E=C6H4,(C6H4)2),in which both two bridging groups have planer structure, and expected to see the catalytic reactivity of both pphenylene- and p-biphenylene-bridged bis (cyclopentadienyl)rhenium carbonylcomplexes.
1 Experimental
1.1 General considerations
All procedures were performed under an argon atmosphere by using standard Schlenk techniques. Solvents were distilled from appropriate drying agents under nitrogen atmosphere.Elemental analyses of C and H were performed with a Vario ELⅢ elemental analyzer.The IR spectra were recorded as KBr disks on a Thermo Fisher is50 spectrometer. Gas chromatograms were recorded with an Agilent 6820 gas chromatograph.1H and13C NMR spectra were recorded on a Bruker AVⅢ-500 (or 600)instrument in CDCl3.The ligand precursors (C5Me4H)E(C5Me4H) (E=C6H4, (C6H4)2)were synthesized according to the literature[18-20].
1.2 Synthesis of complex 1
A solution of free ligand (C5Me4H)C6H4(C5Me4H) (0.19 g,0.6 mmol)and Re2(CO)10(0.2 g,0.3 mmol)in mesitylene (15 mL)was refluxed for 48 h.After removal of solvent,the residue was placed on an alumina column.Elution with petroleum ether/CH2Cl2(2∶1,V/V)developed a colorless band,which was collected,and after concentration,afforded (C6H4)[(η5-C5Me4)Re(CO)3]2(1)as a white solid.Yield:52.1% (0.134 g).m.p.316℃;Anal.Calcd.for C30H28O6Re2(%): C,42.05;H,3.29.Found(%):C,42.32;H,3.13.1H NMR(CDCl3,500 MHz):δ2.16(s,12H,C5Me4),2.25(s, 12H,C5Me4),7.28(s,4H,C6H4).13C NMR(CDCl3,125 MHz):δ10.9,11.4,97.7,102.2,104.0,131.8,132.4, 197.37.IR(KBr,cm-1):1 900(s),2 002(s).
1.3 Synthesis of complex 2
Using a procedure similar to that described above,ligand precursor(C5Me4H)(C6H4)2(C5Me4H)was reacted with Re2(CO)10in refluxing mesitylene for 48 h.After chromatography with petroleum ether/CH2Cl2, (C6H4)2)[(η5-C5Me4)Re(CO)3]2(2)was obtained as white crystals.Yield:77.8% (0.217 g).m.p.313℃;Anal. Calcd.for C36H32O6Re2(%):C,46.34;H,3.46.Found (%):C,45.98;H,3.59.1H NMR(CDCl3,500 MHz):δ 2.17 (s,12H,C5Me4),2.26 (s,12H,C5Me4),7.38(d, 4H,J=8.0 Hz,C6H4),7.61(d,4H,J=8.5 Hz,C6H4).13C NMR (CDCl3,125 MHz):δ10.9,11.3,97.7,102.1, 104.4,127.0,131.4,133.0,139.8,197.4.IR(KBr, cm-1):1 903(s),2 012(s).
1.4 Crystallographic analysis
Crystallographic data for complexes 1 and 2 were collected at 298 K on a Bruker AXS SMART 1000 CCD diffractometer with Mo Kαradiation (λ=0.071 073 nm)using theφ-ωscan technique.The crystal structures were solved by direct method and refined on F2by full-matrix least-squares technique using the SHELXL-97 program package[21].All non-hydrogen atoms were found from the Fourier difference maps refined anisotropically,hydrogen atoms were included in calculated positions riding on the parent atoms and refined with fixed thermal parameters. Crystallographic data and experimental details for structural analysis of the complexes are summarized in Table 1.
CCDC:1506755,1;1506754,2.

Table 1 Crystal data and structure refinement parameters for complexes 1 and 2
1.5 General procedure for catalytic tests
The catalytic reactions were carried out under an argon atmosphere with magnetic stirring.The required complexes (0.02 mmol)was mixed with 1,2-dichloroethane (3.5 mL)in a 25 mL round-bottom flask at room temperature.Aromatic compounds and acylation reagents were added by syringe.The reaction mixture was heated at 80℃ for 24 h.After cooling to room temperature,the solid catalyst was separated from the reaction mixture by filtration.The solvent was removed by rotary evaporation,and the residue was purified by Al2O3column chromatography, eluting with petroleum ether and ethyl acetate to give a white solid.
2 Results and discussion
2.1 Preparation of complexes 1 and 2
Reactions of ligand precursors (C5Me4H)E (C5Me4H)(E=C6H4,(C6H4)2)with Re2(CO)10in refluxing mesitylene for 48 h afforded the corresponding complexes(E)[(η5-C5Me4)Re(CO)3]2(E=C6H4(1),(C6H4)2(2))in yield of 52%and 78%,respectively(Scheme 1).The IR spectra of complexes 1 and 2 all exhibited only terminalcarbonyl bands(1:1 900,2 002 cm-1;2: 1 903,2 012 cm-1).The1H NMR spectra of 1 and 2 all displayed two groups of singlets for the four methyl protons,indicating the symmetrical structure in solution,in addition,complex 1 showed a singlet atδ7.28 for the phenylene protons and complex 2 showed two doublets atδ7.38 and 7.61 for the biphenylene protons.

Scheme 1 Synthesis of complexes 1 and 2
2.2 Crystal structures of complexes 1 and 2
Slow evaporation of the solvent from the complexes in hexane-CH2Cl2 solution gave single crystals 1 and 2 suitable for X-ray diffraction. Selected bond parameters are presented in Table 2 and their structures are depicted in Fig.1 and 2, respectively.
Single-crystal X-ray diffraction analysis reveals that 1 and 2 crystallize in the triclinic space group P1 Complexes 1 and 2 are monobridged bis (cyclopentadienyl)rhenium carbonyl complexes and similar to each other.Both structures show that the molecule consists oftwo[(η5-C5Me4)Re(CO)3]moieties linked by a single bridge,with each rhenium atom isη5-coordinated to the cyclopentadienyl ring and three terminal CO ligands.Two Re(CO)3units are located on the opposite site of the monobridged ligand and anti to each other,the Re atoms exhibit a three-legged pianostool geometry.Re-Re bonds are not observed in both complexes.Complex 1 has a symmetric structure,the molecule consists oftwo [(η5-C5Me4)Re(CO)3]moieties linked by a p-phenylene-bridge.Complex 2 also has similar structure,consisting oftwo[(η5-C5Me4)Re(CO)3] moietieslinked by a p-biphenylene-bridge.The dihedral angle between the two Cp ring planes is 0°and the torsion angle Re1-Cp(centroid)…Cp(centroid)-Re1iis -180°,indicating two Cp rings are parallel to each other.For complexes 1 and 2,the distances of Re1-CEN and Re1i-CEN(CEN means the centroid ofthe cyclopentadienylring)are equal(Table 2).The Re-CEN distance in 1 is 0.195 6 nm and the Re-CEN distancein 2 is 0.194 6 nm,which compare very well with those values in the analogues [(η5-C5H4)2C(CH2)5][Re (CO)3]2(0.195 7 and 0.196 0 nm)and [(η5-C5H4)2 Si (CH3)2][Re(CO)3]2(0.194 6 and 0.195 2 nm)[17].From above data,we can conclude that the different ligand bridges in these complexes have no obvious effect on their structures.
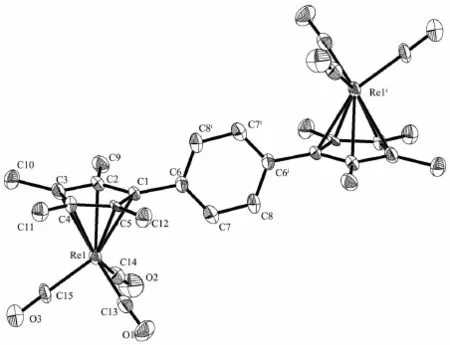
Fig.1 Molecular structure of complex 1

Fig.2 Molecular structure ofcomplex 2

Table 2 Selected bond distances(nm)and angles(°)for complexes 1 and 2
2.3 Catalysis of Friedel-Crafts acylation reactions In order to test the catalytic capability in Friedel -Crafts acylation reactions(Scheme 2~6)catalyzed by two complexes,considering factors such as the reaction time,yield,economic considerations,the following experimental conditions were chosen for next work:reaction time 24 h;1,2-dichloroethane as solvent;80℃;the molar ratio of aromatic substrates and acylation reagents was 1∶3;the amount of catalyst was 1.0%(n/n)(substrate as reference).
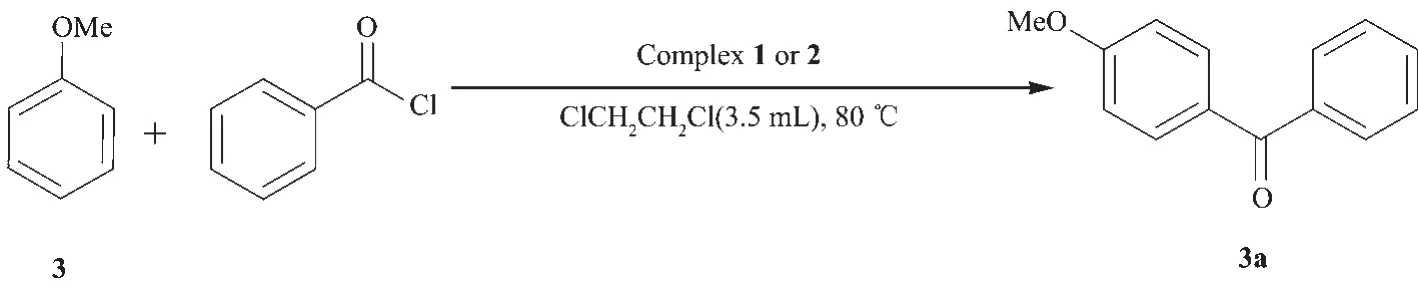
Scheme 2 Complex 1 or 2 catalyzed Friedel-Crafts acylation reaction of anisole with benzoyl chloride

Scheme 3 Complex 1 or 2 catalyzed Friedel-Crafts acylation reactions of anisole and methylbenzene with acyl chlorides

Scheme 4 Complex 1 or 2 catalyzed Friedel-Crafts acylation reactions of anisole and methylbenzene with acetic anhydride

Scheme 5 Complex 1 or 2 catalyzed Friedel-Crafts acylation reactions of acyl chlorides
With the above studies,the yields(%)were found to vary with aromatic substrates with different acylation reagents,the catalytic results of complex 1 and 2 are shown in Table 3.Friedel-Crafts acylation reactions are electrophilic substitution reactions, however halogen element and carbonyl formed p-π conjugation in acylating agent,thus the acylating agents were difficult to lose halogen element to form carbocation.Benzoyl chloride,phenylacetyl chloride, cinnamoyl chloride and cyclohexanecarboxylic acid chloride could be used as acylation reagents in these reactions and the corresponding products were obtained with high selectivity for the para-products without detection of di-substituted in all cases, suggesting that the catalytic reaction has highregioselectivity.The order of increasing reactivity was found to be:4-bromoanisole<4-methyl anisole<methylbenzene <2-bromoanisole <anisole <2-methylanisole, which was consistent with the characteristics ofthe aromatic electrophilic substitution mechanism.Overall,both complexes gave similar results,showing that the different ligand bridges have only a smallinfluence on the catalytic behavior.

Scheme 6 Complex 1 or 2 catalyzed Friedel-Crafts acylation reactions of acetic anhydride

Table 3 Catalyzed Friedel-Crafts acylation reaction of aromatic substrates with different acylation reagents

Continued Table 3
3 Conclusions
Two new bridge d bis(cyclopentadienyl)rhenium carbonyl complexes(E)[(η5-C5Me4)Re(CO)3]2(E=C6H4(1),(C6H4)2(2))have been synthesized and structurally characterized.Friedel-Crafts reactions of aromatic substrates with acylation reagents showed that two complexes have catalytic activity.Compared with traditional catalysts,these complexes have significant practical advantages,namely lower amounts of catalyst,mild reaction conditions,and high selectivity. Further studies to elucidate the reaction mechanism and expand the synthetic utility of these catalysts are in progress.
Supporting information is available athttp://www.wjhxxb.cn
References:
[1]Bailey N A,Radford S L,Sanderson J A,et al.J.Organomet. Chem.,1978,154:343-351
[2]Nataro C,Angelici R J.Inog.Chem.,1998,37:2975-2983
[3]Knox S A R,Macpherson K A,Orper A G,et al.J.Chem. Soc.,Dalton Trans.,1989:1807-1813
[4]Burger P.Angew.Chem.Int.Ed.,2001,40:1917-1919
[5]Zhou X,Zhang Y,Xu S,et al.Inorg.Chim.Acta,1997,262: 109-112
[6]Fox T,Burger P.Eur.J.Inorg.Chem.,2001:795-803
[7]Zhang Y,Wang B,Xu S,et al.Transition Met.Chem., 1999,24:610-614
[8]Zhang Y,Xu S,Zhou X.Organometallics,1997,16:6017-6020
[9]Ovchinnikov M V,Guzei I A,Angelici R J.Organometallics, 2001,20:691-696
[10]Ovchinnikov MV,Wang X,Schultz AJ,etal.Organometallics, 2002,21:3292-3296
[11]Bonifaci C,Ceccon A,Gambaro A,et al.J.Organomet. Chem.,1998,557:97-109
[12]Cuenca T,Royo P.Coord.Chem.Rev.,1999,193-195:447-498
[13]Ceccon A,Santi S,Orian L,et al.Coord.Chem.Rev.,2004, 248:683-724
[14]Nishiyama Y,Kakushou F,Sonoda N.Bull.Chem.Soc.Jpn., 2000,73:2779-2782
[15]Kusama H,Narasaka K.Bull.Chem.Soc.Jpn.,1995,68: 2379-2383
[16]Kuninobu Y,Matsuki T,Takai K.J.Am.Chem.Soc.,2009, 131:9914-9915
[17]Li Z,Ma Z H,Wang H,et al.Transition Met.Chem., 2016,41:647-653
[18]Meng X,Sabat M,Grimes R.N.J.Am.Chem.Soc.,1993, 115:6143-6151
[19]Bunel E E,Campos P,Ruz J,et al.Organometallics,1998, 7:474-476
[20]Yuen H F,Marks T J.Organometallics,2008,27:155-158
[21]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of Göttingen,Göttingen,Germany, 1997.
NIR Luminescent N,N′-Bis(3-methoxy-salicylidene)cyclohexane-1,2-diamine Mono-and Tetra-nuclear Ytterbium Complexes
FAN Zhong-Tian1GAO Bo1DONG Yan-Ping1ZOU Xiao-Yan*,1,2LI Guang-Ming*,1
(1Key Laboratory of Functional Inorganic Material Chemistry(MOE),School of Chemistry and Materials Science,Heilongjiang University,Harbin 150080,China)
(2Editorial Board of Journal of Engineering of Heilongjiang University,Harbin 150080,China)
A series of four N,N′-bis(3-methoxy-salicylidene)cyclohexane-1,2-diamine(H2L)ytterbium complexes, namely,[Yb(H2L)2](ClO4)3·2CH3OH·H2O(1),[Yb4(L)4(NO3)2(H2O)2](PF6)2·4CH3CN(2),[Yb4(L)4(H2O)2Cl2](PF6)2· 2CH2Cl2·2H2O(3)and[Yb4(L)4(NO3)2(H2O)2][Yb(NO3)3(H2O)2(CH3OH)](NO3)2·4CH2Cl2·6CH3OH(4)have been isolated by reactions of H2L with various ytterbium salts.X-ray crystallographic analysis reveals that complex 1 is of a discrete mononuclear structure,and complexes 2~4 are of homoleptic tetra-nuclear structure.The NIR luminescence of all complexes were investigated and discussed.CCDC:916031,1;916032,2;916034,3; 916033,4.
NIR luminescent;ytterbium complexes;structure
Syntheses,Structures and Catalytic Activity of p-Phenylene-or p-Biphenylene-Bridged Biscyclopentadienyl Dinuclear Rhenium Carbonyl Complexes
ZHANG Ning1MA Zhi-Hong*,2LI Su-Zhen3HAN Zhan-Gang1ZHENG Xue-Zhong1LIN Jin*,1
(1College of Chemistry&Material Science,Hebei Normal University,Shijiazhuang 050024,China)
(2College of Basic Medicine,Hebei Medical University,Shijiazhuang 050017,China)
(3Hebei College of Industry and Technology,Shijiazhuang 050091,China)
O614.346
A 文章编号:1001-4861(2017)08-1489-08
10.11862/CJIC.2017.182
614.71+3 文献标识码:A
1001-4861(2017)08-1497-08
2017-05-18。收修改稿日期:2017-06-23。
国家自然科学基金(No.51402092,21601132)资助项目。
*通信联系人。E-mail:zxy_18889@126.com,gmli_2000@163.com
收稿日期:2017-04-07。收修改稿日期:2016-05-09。
国家自然科学基金(No.21372061)、河北省自然科学基金(No.B2017205006)和河北师范大学重点基金(No.L2017Z02)资助项目。
*通信联系人。E-mail:mazhihong-1973@163.com,linjin64@126.com;会员登记号:S06N0210M1305。
