二级出水中环丙沙星耐药菌光辐照灭活研究
2017-08-07孙迎雪胡春芳北京工商大学环境科学与工程系北京100048
石 娜,孙迎雪,齐 菲,胡春芳 (北京工商大学环境科学与工程系,北京 100048)
二级出水中环丙沙星耐药菌光辐照灭活研究
石 娜,孙迎雪*,齐 菲,胡春芳 (北京工商大学环境科学与工程系,北京 100048)
针对二级出水中的一株环丙沙星耐药菌,研究了菌株生长特性和光辐照对其灭活、复活及耐药性的影响.结果表明,该菌株对青霉素、氨苄西林、磺胺甲恶唑、四环素和利福平均具有耐受性,在环丙沙星存在的条件下,其最大比生长速率由0.63h-1降低到0.51h-1.光辐照对环丙沙星耐药菌的灭活率基本随光照强度和辐照时间的增加而升高,且基本符合零级或一级化学反应动力学.可见光(100/300/500W汞灯和1000W氙灯(>400nm))辐照60min,环丙沙星耐药菌灭活率达到0.25~0.39log.100/300W汞灯和1000W氙灯(>400nm)辐照下环丙沙星耐药菌的变化符合零级反应动力学,灭活速率为10196.43~11345.24CFU/(mL·min),500W汞灯(>400nm)辐照下环丙沙星耐药菌的变化符合一级反应动力学,灭活速率为 0.01min-1.可见光+UVA(100/300/500W 汞灯和 1000W 氙灯(>300nm))辐照 60min,环丙沙星耐药菌灭活率达到 0.30~5.63log.100W 汞灯(>300nm)辐照下环丙沙星耐药菌的变化符合一级反应动力学,灭活速率为0.01min-1,300W汞灯(>300nm)辐照下环丙沙星耐药菌的变化符合零级反应动力学,灭活速率为2572.02CFU/(mL·min).未完全灭活的耐药菌存在复活,其48h光、暗复活率达到-3.9%~123.4%.在光辐照过程中,只有1000W可见光+UVA辐照影响环丙沙星耐药菌的耐药性,辐照60min,其抑菌圈直径由11.0mm下降到8.0mm.
环丙沙星耐药菌;可见光;UVA;灭活率;环丙沙星耐药性
抗生素的滥用导致抗生素耐药菌的产生和传播日益严重[1-2].据调查,2013年我国抗生素使用量高达 162000t,其中,喹诺酮类抗生素环丙沙星使用量达到5340t[3].抗生素在生物体内不能完全代谢,会以原有的形式或代谢产物的形式排出体外,最终进入城市污水处理厂,而污水处理厂的生物处理和氯消毒、紫外消毒等传统消毒工艺对抗生素和抗生素耐药菌的去除效果有限[1,4],进而导致抗生素和抗生素耐药菌进入水环境[5-6],威胁水生态环境和人类健康.
太阳光具有灭活地表水中微生物的能力,这也是地表水中抗生素耐药菌降解的重要机制之一[1,7-9].有研究发现,太阳光辐照水体可灭活几个数量级的大肠杆菌[10].细菌细胞中的光敏化合物卟啉经400~430nm可见光辐照后会返回基态与氧结合,并转移能量,产生单线态氧、超氧自由基、羟基自由基和过氧化氢等活性氧(ROS)破坏细胞[11].由于太阳光中的短波紫外线(UVC)在经过地球表面同温层时被臭氧层吸收,不能到达地球表面,中波紫外线(UVB)在自然水体中很快衰减,因此,较长波段的光的潜在价值更加重要[12].
本研究选取环丙沙星耐药菌作为研究对象,重点考察可见光和可见光+UVA光辐照对该菌株的灭活、复活的控制效果以及对耐药性的影响,解析光辐照的机理,以期为城市水环境中抗生素耐药菌的控制提供基础数据.
1 材料与方法
1.1 耐药菌的分离与纯化
采用膜过滤法和平板划线分离法从二级出水中(水样取自甘肃省白银市某城市污水处理厂A2/O工艺二级出水)筛选对环丙沙星抗生素有耐药性的大肠杆菌菌株[6].添加抗生素的浓度参考CLSI规定的最低抑菌浓度(MIC)[13].以大肠埃希菌ATCC 25922[13]作为质控菌.
1.2 耐药菌生长特性
将单菌落接种于含 4mg/L环丙沙星的胰蛋白胨大豆肉汤培养基(TSB)中,培养10~12h作为种子培养液(OD600>1),再将种子液接种于含有不同浓度(0、4、8mg/L)环丙沙星的 TSB中培养,同时制作空白培养基,每 2h测定 OD600,测定至12h,20h后,每1h测定OD600,测定至24h.
1.3 耐药菌生长动力学参数确定
用S-Gompertz模型[16]拟合环丙沙星耐药菌的生长曲线,获得环丙沙星耐药菌的最大生长量、最大比生长速率和迟滞时间.具体数学表达式如下:

式中:N为某生长时间t下细菌悬浊液在600nm波长下的吸光度值,cm-1;N0为细菌悬浊液在600nm波长下的初始吸光度值(低于检测限,则初始值定为 0.001),cm-1;Nm为细菌的最大生长量,即细菌悬浊液在 600nm波长下的最大吸光度值,cm-1;μm为细菌的最大比生长速率,h-1;λ为迟滞时间,h;t为生长时间,h.
1.4 耐药菌对典型抗生素的耐受性分析
采用纸片扩散法考察环丙沙星耐药菌对青霉素、氨苄西林、氯霉素、四环素、磺胺甲恶唑和利福平 6种典型抗生素的耐受性[13].(35±2)℃孵育 16~18h,根据抑菌圈直径的大小,判断细菌对6种抗生素的耐受性.
1.5 光辐照控制
1.5.1 样品制备 从平板培养基上挑选单菌落接种至含 4mg/L环丙沙星的营养肉汤培养基中,37℃过夜培养,然后取 20mL的过夜培养菌液,4500g离心5min,弃清液,再用无菌水充分悬浮菌体沉淀,洗去培养基和抗生素,4500g离心5min,反复2次,最后将菌体悬浮于无菌水中,用麦氏比浊法获得1.5×108CFU/mL(0.5麦氏),随后加入到无菌水中,获得预期浓度的反应样品.
1.5.2 光辐照反应 使用光化学反应仪(XPA-7,南京胥江机电厂)进行光辐照实验,反应仪中心部位配有 100/300/500W 汞灯(265.2~579nm)或1000W 氙灯(200~1200nm),另外,将汞灯和氙灯分别配合300或400nm滤波片使用产生不同的辐照条件.利用可见光光辐照计(FZ-A,北京师范大学机电厂)和紫外光辐照计(UVA,北京师范大学机电厂)测定光辐照强度.将准备好的菌液加入到已灭菌的反应试管,然后放入光化学反应仪外围的固定装置中,并进行磁力搅拌,开始光辐照实验.
1.5.3 光复活和暗修复 将1.5.2中光辐照后的最终样品转移至灭菌的烧杯中,用于光复活和暗修复试验.试验条件分别为实验室日光灯下和黑暗条件下磁力搅拌,24和48h测定细菌浓度[14].
1.5.4 耐药菌的检测和灭活率计算 采用平板计数法测定样品中的环丙沙星耐药菌.37℃培养24h,计菌落数,用单位体积水样的菌落形成单位(CFU/mL)表示.

式中:N0为光辐照前样品中环丙沙星耐药菌的菌落数;Nt为光辐照后样品中环丙沙星耐药菌的菌落数.
1.5.5 耐药性分析 采用药敏纸片扩散法考察环丙沙星耐药菌耐药性的变化[13].同1.4.
2 结果与讨论
2.1 环丙沙星耐药菌的生长特性
环丙沙星耐药菌的生长特性能够反映其在不同环境中的增殖特性[15].从表1可以看出,环丙沙星耐药菌的最大生长量(以 OD600计)在 2.58~2.89之间,且随抗生素浓度的增加而升高,在8mg/L环丙沙星条件下最大生长量最高为 2.89,这说明高浓度抗生素条件下菌株利用碳源和合成菌体的能力更强、效率更高[15].环丙沙星耐药菌的最大比生长速率在 0.51~0.63h-1之间,抗生素浓度增加,最大比生长速率从 0.63h-1下降到0.51h-1.在有抗生素的条件下该菌株的迟滞时间增加,0、4、8mg/L环丙沙星条件下菌株的迟滞时间分别为2.06、2.52、2.12h.

表1 不同环丙沙星浓度下耐药菌的最大生长量(Nm)、最大比生长速率(μm)和迟滞时间(λ)Table 1 Maximum increment (Nm), maximum specific growth rate (μm) and lag time (λ) of ciprofloxacinresistant bacteria versus different concentration of ciprofloxacin
2.2 环丙沙星耐药菌对不同抗生素的耐受性
从表2可以看出,环丙沙星耐药菌对青霉素、氨苄西林、磺胺甲恶唑未形成明显抑菌圈,耐受能力很强;对四环素和利福平的抑菌圈直径均为10.5mm,具有一定的耐受性;对氯霉素的抑菌圈直径为 15mm,药敏性为中介.这说明污水中环丙沙星耐药菌对 β-内酰胺类抗生素的耐受能力普遍较高,同时,该菌株对磺胺甲恶唑耐受性也较强,对四环素和利福平的耐受性次之,对氯霉素的耐受能力最弱,这可能与污水处理厂附近居民的抗生素使用类型和使用频率等有关.文献中报道的从污水中分离的环丙沙星多重耐药菌多对 β-内酰胺类抗生素、四环素类抗生素和磺胺类抗生素有耐药性,这与本文的研究结果类似[6,17].

表2 环丙沙星耐药菌对6种抗生素的耐受性Table 2 Resistance of ciprofloxacin-resistant bacteria to six antibiotics
2.3 可见光对环丙沙星耐药菌的影响
100、300、500W汞灯和1000W氙灯配合400nm截止滤波片产生的可见光的光强分别是18.9、27.3、40.2和115.8mW/cm2.从图1(a)可以看出,环丙沙星耐药菌随可见光辐照时间的增加而减少.可见光辐照60min时,环丙沙星耐药菌由1.30×106~1.36×106CFU/mL 降低到 5.35×105~7.6×105CFU/mL,说明可见光对环丙沙星耐药菌具有一定的灭活作用.早在1930年,Gates发现只要增加光通量,波长在400nm以上的可见光与紫外光一样可以灭活细菌[18],当 DNA损伤率超过修复率,细胞即发生死亡[18-19].目前,可见光杀菌的研究光源多为单波长可见光,相关研究发现,可见光辐照是否具有杀菌效果与波长有很大关系,如405、435、470、525、610、630、810和880nm波长的可见光具有杀菌功能[20-25],405nm 可见光可以破坏耐药基因[26],但 625nm 可见光不能杀菌[22].本文中环丙沙星耐药菌的灭活应该是具有杀菌功能的不同单波段可见光共同作用的结果.

图1 可见光辐照对环丙沙星耐药菌的影响Fig.1 Effect of visible light irradiation onciprofloxacin-resistant bacteria
从图1(b)可以看出,环丙沙星耐药菌的灭活率随光照强度增加而升高,光照强度越高,灭活效果越好.可见光辐照60min时,100、300、500和 1000W 可见光辐照下环丙沙星耐药菌的灭活率分别为 0.25、0.31、0.34和 0.39log.1000与500W对环丙沙星耐药菌的灭活效果相当,其灭活率分别是 0.34和 0.39log,而前者光照强度远高于后者光照强度,这可能是因为不同光源发射的可见光各波段光强有所差别.另外,同一光照强度下,环丙沙星耐药菌的灭活率随辐照时间的增加而升高.光照强度在时间上的累积即为辐照剂量,可见光对细菌的影响与辐照剂量密切相关[21-22].例如,470nm 可见光辐照剂量达到10J/cm2以上才能灭活金黄色葡萄球菌[21]; 405nm蓝光和880nm红外光同时辐照可灭活金黄色葡萄球菌和铜绿假单胞菌,最佳辐照剂量20J/cm2分别可灭活金黄色葡萄球菌、铜绿假单胞菌72%和93.8%[25].

表3 可见光辐照0~60min环丙沙星耐药菌灭活动力学拟合结果Table 3 Kinetic of visible light irradiation on ciprofloxacin-resistant bacteria
将可见光辐照 0~60min的环丙沙星耐药菌浓度变化进行动力学方程拟合,如表3所示,得出100、300和1000W辐照下环丙沙星耐药菌的变化符合零级反应动力学,反应速率随光照强度的增加而升高,在1000W辐照下反应速率常数最大为 11345.24CFU/(mL·min),500W 辐照下环丙沙星耐药菌的变化符合一级反应动力学,光辐照反应速率常数为0.01min-1.
2.4 可见光+UVA对环丙沙星耐药菌的影响
100、300、500W汞灯和1000W氙灯配合300nm截止滤波片产生的可见光+UVA的光强分别是25.4、37.3、61.2和118.63mW/cm2,其中UVA光强分别是6.5、10.0、20.0和2.83mW/cm2.从图2可以看出,光辐照60min时,环丙沙星耐药菌由2.90×105~1.30×106CFU/mL降低到0~1.46× 105CFU/mL,灭活率达到0.30~5.63log.500W可见光+UVA对环丙沙星耐药菌的灭活效果最好,反应20min,即辐照剂量为414J/cm2时,环丙沙星耐药菌的灭活率达到5.63log,远远高于500W可见光辐照60min时的灭活率,这说明UVA在耐药菌灭活过程中起主要作用.100、300W辐照下,可见光+UVA消毒效果略好于可见光消毒效果,这可能是因为低功率汞灯发射的 UVA较弱.而1000W辐照下,10min时可见光+UVA与可见光对环丙沙星耐药菌的灭活率相同,随后前者的灭活率低于后者,50min后,前者对环丙沙星耐药菌的灭活率才迅速增加,60min时其灭活率达到1.60log,高于可见光辐照的灭活率 0.39log,这说明UVA在反应前期未对环丙沙星耐药菌产生明显的灭活作用,而在后期开始发挥显著的消毒效果,这可能是UVA氧化损伤积累的结果.UVA对DNA的破坏分为直接损伤和氧化损伤,直接损伤是通过形成环丁烷嘧啶二聚体直接破坏DNA,氧化损伤包括I型和II型光氧化反应[27].

图2 可见光+UVA(>300nm)辐照对环丙沙星耐药菌的影响Fig.2 Effect of visible light with UVA irradiation on ciprofloxacin-resistant bacteria
将可见光+UVA辐照 0~60min环丙沙星耐药菌浓度的变化进行动力学方程拟合,如表4所示,得出 100W辐照下环丙沙星耐药菌的变化符合一级反应动力学,光辐照反应速率常数为0.01min-1,300W 辐照下环丙沙星耐药菌的变化符合零级反应动力学,光辐照反应速率常数为2572.02CFU/(mL·min),其他2种辐照条件下环丙沙星耐药菌的变化既不符合零级反应动力学,也不符合一级反应动力学.

表4 可见光+UVA辐照0~60min环丙沙星耐药菌灭活动力学拟合结果Table 4 Kinetic of visible light with UVA irradiation on ciprofloxacin-resistant bacteria
2.5 光复活和暗修复
从图3可以看出,除500W可见光+UVA辐照的环丙沙星耐药菌完全灭活后无复活外,其他条件辐照后的环丙沙星耐药菌在光照和黑暗条件下复活率随时间的增加而升高,复活率从 24h的-74.5~89.0%增加到48h的-3.9~123.4%.从图3(a)光复活情况可以看出,可见光辐照后的环丙沙星耐药菌的光复活率随光照强度的增加而降低,48h光复活后, 100W可见光辐照后的耐药菌复活率最高为96.0%,1000W可见光辐照后的耐药菌复活率最低为-3.9%;可见光+UVA辐照后,除 500W 外,24h时环丙沙星耐药菌仍在持续消减,48h时光复活率随光照强度的增加而降低,其中100W可见光+UVA光辐照后的耐药菌复活率最高为123.4%, 1000W可见光+UVA光辐照后的耐药菌复活率最低为 27.4%.从图 3(b)暗修复情况可以看出,在可见光中,300W 辐照后的环丙沙星耐药菌复活率最低,24和48h的复活率分别为-29.9%和67.9%,1000W辐照后的环丙沙星耐药菌复活率最高,24和48h的复活率分别为73%和90.8%;可见光+UVA辐照后,除 500W 外,在24h,100W辐照后的环丙沙星耐药菌复活率最低为-74.5%,在48h,1000W辐照后的环丙沙星耐药菌复活率最低为37.7%.
光辐照后环丙沙星耐药菌持续消减可能与残余消毒效果有关.Xiong和 Hu研究了 UVA/ TiO2体系中大肠埃希菌ATCC 700891在30、60、90min间歇照射后0~240min的暗修复情况,发现UVA/TiO2体系在黑暗条件下具有一定的残余消毒效果,且光照时间越长,残余消毒效果越显著[14].残余消毒效果可能与光辐照过程中产生的稳定氧化剂如 H2O2有关[28],H2O2可在水中持续存在数小时,起到抑制细菌再生的作用[29].

图3 光辐照后环丙沙星耐药菌的光复活(a)和暗修复(b)效果(“+”表示环丙沙星耐药菌无存活)Fig.3 The photo reactivation (a) and dark repair (b) of the ciprofloxacin-resistant bacteria after light irradiation (“+”represented neither photo reactivation nor dark repair )
2.6 环丙沙星耐药菌耐药性的去除

图4 可见光+UVA对环丙沙星耐药菌耐药性的影响Fig.4 Effect of visible light with UVA on the ciprofloxacin-resistant bacteria
抗生素耐药性是指细菌在抗生素存在条件下的生存和生长能力[30].根据美国临床和实验室标准协会制定的抗菌药物敏感性试验执行标准,肠杆科细菌对环丙沙星耐受性的判定标准是:抑菌圈直径 D≥21mm为敏感,抑菌圈直径 D=16~20mm为中介,抑菌圈直径 D≤15mm为耐药[13].抑菌圈直径越大,抗生素耐药性越低.结果表明,1000W可见光+UVA辐照的环丙沙星耐药菌抑菌圈直径总体呈下降趋势,辐照 60min,抑菌圈直径由11.0mm下降到8.0mm,即环丙沙星耐药菌耐药性增强,如图4所示,其他光辐照条件均不影响环丙沙星耐药菌的耐药性,其抑菌圈直径大小一直维持在 11.0mm,这与有关太阳光辐照对多重耐药菌耐药性影响的研究结果有所不同, Rizzo等[1]研究发现太阳光辐照 180min,多重耐药性大肠杆菌对环丙沙星的MIC降低33%.
从图5可以看出,100、300W可见光和可见光+UVA辐照后的环丙沙星耐药菌在光复活和暗修复后,抑菌圈直径不变.除 48h光复活,500W可见光辐照后的环丙沙星耐药菌在复活后抑菌圈直径由11.0mm增加到21.0~22.0mm,即环丙沙星耐药菌的耐药性有所降低.1000W可见光辐照后的环丙沙星耐药菌只在暗修复 48h时,抑菌圈直径由11mm减小到10.5mm.而在可见光+UVA中,只有1000W可见光+UVA辐照影响环丙沙星耐药菌在光复活和暗修复后抑菌圈直径,分别从11.0mm减小到10.0mm和9.3mm,即环丙沙星耐药菌耐药性增强,这说明环丙沙星耐药菌对UVA具有一定的耐受性.Huang等发现紫外(UV254)消毒可使四环素耐药菌的半抑制浓度(IC50)降低40%,即四环素耐药菌对 UV254无耐受性[31].可见抗生素耐药菌对不同波长光的耐受性不同.
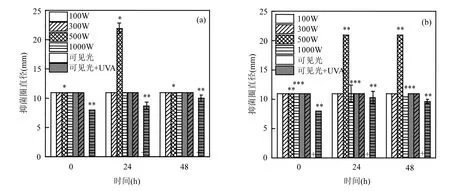
图5 光复活(a)和暗修复(b)后环丙沙星耐药菌抑菌圈直径的变化Fig.5 The Inhibitory circle diameter of ciprofloxacin-resistant bacteria after photo reactivation (a) and dark repair (b)*, P<0.1; **, P<0.05; ***, P<0.01
3 结论
3.1 抗生素对抗生素耐药菌的生长具有一定的影响.该环丙沙星耐药菌对青霉素、氨苄西林、磺胺甲恶唑、四环素和利福平均具有耐受性.
3.2 可见光和可见光+UVA对环丙沙星耐药菌的灭活率基本随光照强度和辐照时间的增加而升高.可见光中,1000W 辐照下环丙沙星耐药菌的灭活效果最好,辐照 60min(辐照剂量为73.44J/cm2),灭活率达到0.39log;可见光+UVA中, 500W 辐照下环丙沙星耐药菌的灭活效果最好,辐照 20min(辐照剂量为 414J/cm2),灭活率达到5.63log.
3.3 不同光辐照条件对环丙沙星耐药菌的光复活和暗修复影响不同.500W 可见光+UVA 辐照的环丙沙星耐药菌完全灭活后无复活,未完全灭活的环丙沙星耐药菌复活率达到-3.9%~123.4%. 3.4 在光辐照过程中,只有1000W可见光+UVA辐照影响环丙沙星耐药菌的耐药性;光复活和暗修复后,100、300W可见光和可见光+UVA辐照后的环丙沙星耐药菌的抑菌圈直径不变,其他条件辐照后的耐药菌抑菌圈直径发生改变.
[1] Rizzo L, Fiorentino A, Anselmo A. Effect of solar radiation on multidrug resistant E. coli strains and antibiotic mixture photodegradation in wastewater polluted stream [J]. Science of the Total Environment, 2012,427-428:263-268.
[2] Dunlop P S, Civola M, Rizzo L, et al. Effect of photocatalysis on the transfer of antibiotic resistance genesin urban wastewater [J]. Catalysis Today, 2015,240:55-60.
[3] Zhang Q Q, Ying G G, Pan C G, et al. Comprehensive evaluation of antibiotics emission and fat in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterialresistance [J]. Environmental Science & Technology, 2015,49(11):6772-6782.
[4] Munir M, Wong K, Irene X. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan [J]. Water Research, 2011,45(2):681-693.
[5] Karaolia P, Michael I, Fernandez I G, et al. Reduction of clarithromycin and sulfamethoxazole-resistant Enterococcus by pilot-scale solar-driven Fenton oxidation [J]. Science of the Total Environment, 2014,468-469:19-27.
[6] 卢 诚,张 俊,王 钊,等.河北潘家口水库氯霉素类抗生素检测及风险评估 [J]. 中国环境科学, 2016,36(6):1843-1849.
[7] Boehm A B, Yamahara K M, Love D C, et al. Covariation and photoinactivation of traditional and novel indicator organisms and human viruses at a sewage-impacted marine beach [J]. Environmental Science & Technology, 2009,43(21):8046-8052.
[8] Davies C M, Evison L M. Sunlight and the survival of enteric bacteria in natural-waters [J]. Journal Applied Microbiology, 1991,70(3):265-274.
[9] Sinton L W, Hall C H, Lynch P A, et al. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters [J]. Applied and Environmental Microbiology, 2002,68(3):1122-1131.
[10] Maraccini P A, Wenk J, Boehm A B. Photoinactivation of eight health-relevant bacterial species: Determining the importance of the exogenous indirect mechanism [J]. Environmental Science & Technology, 2016,50:5050-5059.
[11] Ghate V, Leong A L, Kumar A, et al. Enhancing the antibacterial effect of 461 and 521nm light emitting diodes on selected foodborne pathogens in trypticase soy broth by acidic and alkaline pH conditions [J]. Food Microbiology, 2015,48:49-57.
[12] Kadir K, Nelson K L. Sunlight mediated inactivation mechanisms of Enterococcus faecalis and Escherichia coli in clear water versus waste stabilization pond water [J]. Water Research, 2014, 50:307-317.
[13] CLSI. Clinical and Laboratory Standards Institute. http://www. clsi.org/.
[14] Xiong P, Hu J Y. Inactivation/reactivation of antibiotic-resistant bacteria by a novel UVA/LED/TiO2system [J]. Water Research, 2013,47:4547-4555.
[15] 席劲瑛,黄晶晶,胡洪营,等.污水处理厂二级出水中四环素抗性菌的生长特性与耐药性 [J]. 环境科学, 2014,121(7):1723-1729.
[16] Zwietering M H, Jongenburger I, Rombouts F M, et al. Modeling of the bacteria growth curve [J]. Applied and Evironmental Microbiology, 1990,56(6):1875-1881.
[17] Ferro G, Fiorentino A, Alferez M C, et al. Urban wastewater disinfection for agricultural reuse: effect of solar driven AOPs in thei nactivation of a multidrug resistant E. coli strain [J]. Applied Catalysis B: Environmental, 2015,178:65-73.
[18] Gates F L. A study of the bactericidal action of ultraviolet light. III: The absorption of ultraviolet light by bacteria [J]. The Journal Genral Physiology, 1930,14(1):31-42.
[19] Gad F, Zahra T, Hasan T, et al. Effects of growth phase and extracellular slime on photodynamic inactivation of grampositive pathogenic bacteria [J]. Antimirobial Agents and Chemotherapy, 2004,48(6):2173-2178.
[20] Wilson M. Lethal photosensitization of oral bacteria and its potential in the photodynamic therapy of oral infections [J]. Photochemistry and Photobiology, 2004,3(5):412-418.
[21] Guffey J S, Wilborn J. In vitro bactericidal effect of 405nm and 470nm Blue Light [J]. Phtomedicine and Laser Surgery, 2006, 24(6):684-688.
[22] Kim S, Kim J, Lim W, et al. In vitro bactericidal effect of 625, 525 and 425nm wavelength (red, green, and blue) light-emitting diode irradiation [J]. Photochemistry and Photobiology, 2013, 31(11):554-562.
[23] Nussbaum E L, Lilge L, Mazzulli T. Effects of low level laser therapy (LLLT) of 810nm upon in vitro growth of bacteria: relevance of irradiance and radiant exposure [J]. Journal Clinical Laser Medicine and Surgery, 2003,21(5):283-290.
[24] Nussbaum E L, Lilge L, Mazzulli T. Effects of 630-, 660-, 810-, and 905-nm laser irradiation delivering radiant exposure of 1-50J/cm2on three species of bacteria in vitro [J]. Journal Clinical Laser Medicine and Surgery, 2002,20(6):325-333.
[25] Guffey J S, Wilborn J. Effects of combined 405nm and 880nm light on Staphylococcus aureus and Pseudomonas aeruginosa in vitro [J]. Photomedicine and Laser Surgery, 2006,24(6):680-683.
[26] Enwemeka C S, Williams D, Enwemeka S K, et al. Blue 470nm light kills Methicillin-resistant Staphylococcus aureus (MRSA) in vitro [J]. Photomedicine and Laser Surgery, 2009,27(2):221-226.
[27] Giannakis. Solar disinfection is an augmentable, in situ-generated photo-Fenton reaction-Part 1: A review of the mechanisms and the fundamental aspects of the process [J]. Applied Catalysis B: Environmental, 2016,199(15):199-223.
[28] Rincón A G, Pulgarin C. Bactericidal action of illuminated TiO2on pure Escherichia coli and natural bacterial consortia: post-irradiation events in the dark and assessment of the effective disinfection time [J]. Applied Catalysis B: Environmental, 2004, 49(2):99-112.
[29] Shang C, Cheung L M, Ho C M, et al. Repression of photoreactivation and dark repair of coliform bacteria by TiO2-modified UV-C disinfection [J]. Applied Catalysis B: Environmental, 2009,89(3/4):536-542.
[30] Pruden. Balancing water sustainability and public health goals in the face of growing concerns about antibiotic resistance [J]. Environmental Science & Technology, 2014,48:5-14.
[31] Huang J J, Hu H Y, Tang F, et al. Inactivation and reactivation of antibiotic-resistant bacteria by chlorination in secondary effluents of a municipal wastewater treatment plant [J]. Water Research, 2011,45(5):2775-2781.
Inactivate ciprofloxacin-resistant bacteria in secondary treated effluent by light irradiation.
SHI Na, SUN Ying-xue*, Qi Fei, Hu Chun-fang (Deparment of Environmental Science and Engineering, Beijing Technology and Business University, Beijing 100048, China). China Environmental Science, 2017,37(7):2599~2606
A ciprofloxacin-resistant bacterium strain was isolated from secondary treated effluent, and effects of light irradiation for disinfection of ciprofloxacin-resistant bacterium strain were investigated. The ciprofloxacin-resistant bacterium strain presented resistance to penicillin, ampilicillin, sulfamethoxazole, tetracyline and rifampicin. In the presence of ciprofloxacin, the maximum specific growth rate of the strain decreased from 0.63h-1to 0.51h-1. The inactivation ratio of ciprofloxacin-resistant bacterium strain raised with increasing of light intensity and irradiation time, and the inactivated reaction followed either the zero order or first order kinetics. By irradiation of visible light (100/300/500W mercury lamp and 1000W xenon lamp (>400nm)) for 60min, the inactivation ratio of ciprofloxacin-resistant bacterium strain reached 0.25~0.39log. The inactivated reaction by 100/300W mercury lamp and 1000W xenon lamp(>400nm) irradiation fited in with zero order kinetics, and the reaction rate constant was 10196.43~11345.24CFU/(mL·min). The inactivated reaction by 500W mercury lamp(>400nm) followed first order kinetics, and the reaction rate constant was 0.01min-1. By irradiation of visible light with UVA (100/300/500W mercury lamp and 1000W xenon lamp (>300nm)) for 60min, the inactivation ratio of ciprofloxacin-resistant bacteria reached 0.30~5.63log. The inactivated reaction by 100W mercury lamp(>300nm) followed first order kinetics, and the reaction rate constant was 0.01min-1. The inactivated reaction by 300W mercury lamp(>300nm) irradiation fited in with zero order kinetics, and the reaction rate constant was 2572.02CFU/(mL·min). Both of photo reactivation and dark repair took place when ciprofloxacin-resistant bacteria were not completely inactivated. The reactivation ratio reached -3.9~123.4% after photo reactivation of 48h and dark repair. During light irradiation, the ciprofloxacin resistance of the strain was only affected by 1000W visible light with UVA irradiation. By irradiation for 60min, its inhibition diameter decreased from 11.0mm to 8.0mm.
ciprofloxacin-resistant bacteria;visible light;UVA;inactivation ratio;ciprofloxacin resistance
X703.1,X172
A
1000-6923(2017)07-2599-08
石 娜(1988-),女,辽宁辽阳人,硕士,主要研究方向为水污染控制理论与技术.
2016-11-28
北京市属高等学校高层次人才引进与培养计划项目(CIT&TCD201304032)
* 责任作者, 副教授, sunyx@th.btbu.edu.cn
