以胎儿心脏占位为首发表现的结节性硬化症1例报告并文献复习
2017-07-31朱融和孙媛媛梁雅琴陈斌殷薇薇钱
朱融和孙媛媛梁雅琴陈 斌殷薇薇钱 燕
温州医科大学附属第一医院 1.儿科,2.超声科,3.放射科(浙江温州 325000)
以胎儿心脏占位为首发表现的结节性硬化症1例报告并文献复习
朱融和1孙媛媛1梁雅琴1陈 斌2殷薇薇3钱 燕1
温州医科大学附属第一医院 1.儿科,2.超声科,3.放射科(浙江温州 325000)
目的探讨结节性硬化症的临床特点。方法收集1例结节性硬化症患儿的临床资料,分析其临床特征及基因突变结果。结果患儿,女,36日龄,胎儿及生后超声心动图发现异常回声结节,考虑为多发心脏横纹肌瘤;躯干部及双下肢有多处色素脱失斑;头颅磁共振成像示皮质结节、室管膜下结节和脑白质辐射状迁移线;高通量二代测序发现TSC2基因突变(c.4541-4544delCAAA),确诊为结节性硬化症。结论基因检测有助于早期确诊结节性硬化症。
结节性硬化;TSC2基因; 心脏肿瘤
结节性硬化症(tuberous sclerosis complex,TSC)是一种累及多系统的常染色体显性遗传性疾病,发病率约为1/10 000~1/6 000[1]。1862年Von Recklinghauson首次描述该病,1880年Bourneville首先命名,因此该病又称Bourneville病,以面部皮脂腺瘤、癫痫发作和智能减退为临床主要特征。致病基因为TSC1和TSC2,其中TSC1位于第9号染色体(9q34)[2],TSC2位于第16号染色体(16p13.3)[3]。现回顾性分析1例经基因检测确诊的TSC患儿的临床资料,以提高临床医师的认识。
1 临床资料

图1 患儿腹部
患儿,女,36日龄。母亲妊娠33周常规产检时发现胎儿心脏异常,随即行胎儿超声心动图示胎儿心室内异常回声结节,无其他不适主诉,为求进一步检查来温州医科大学附属第一医院就诊。患儿为G1P1,40+5周顺产,试管婴儿(其母双侧输卵管堵塞),出生体质量3 240 g,无窒息史,父母非近亲结婚,家族中无类似病史。入院体格检查:神志清,精神可,右下腹部有3 cm×2 cm色素缺失斑(图1),右下肢膝内侧1.0 cm×0.5 cm色素缺失斑,左侧大腿可及数块色素缺失斑,约绿豆大小,未见牛奶咖啡斑及鲨鱼皮样斑,面部未见异常,浅表淋巴结未及肿大,双侧瞳孔等大等圆,对光反射灵敏,口唇无发绀;两肺呼吸音清,未及干湿罗音;心律不齐,可闻及期前收缩,未闻及明显杂音;腹软,肝脾肋下未及;四肢活动可,神经系统检查阴性。实验室检查:血常规、肝肾功能、电解质、心肌酶谱、肌钙蛋白、脑钠肽未见明显异常。超声心动图示,左、右心室内异常回声结节(考虑心肌横纹肌瘤可能,图2)、卵圆孔未闭。头颅磁共振成像(MRI)示双侧室管膜下、皮质多发结节(图3)。腹部B超未见明显异常。24 h动态心电图示窦性心律,频发房性期前收缩(6.9%)、房性期前收缩未下传。视频脑电图示患儿自然睡眠,基本节律以弥漫性持续性低-高幅2~3 Hzδ节律、低-高幅4~7 Hzθ节律和低幅14~20 Hzβ节律交替出现为背景,调节条幅较差,未见明显的痫样放电。眼底检查正常。
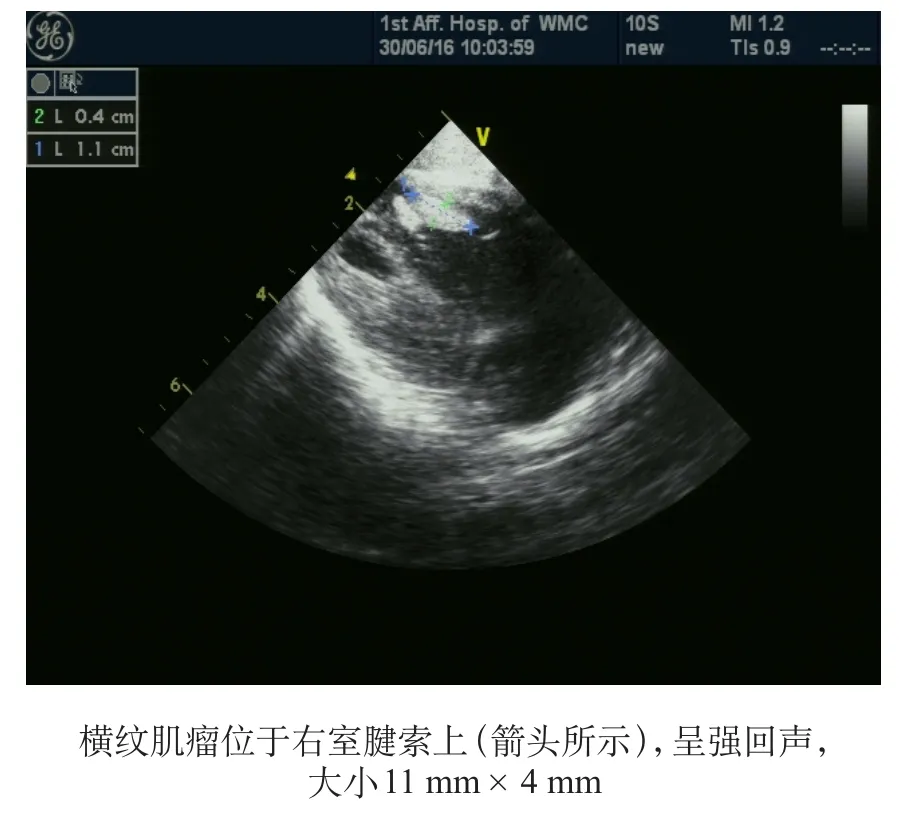
图2 彩色多普勒超声心动图
经医院伦理委员会审核,家长知情同意,采集患儿及父母血标本行基因检测。通过高通量二代测序方法检测,发现患儿TSC2基因突变(c.4541-4544delCAAA),采用Sanger测序方法对其父母就以上突变进行验证,未检测到突变基因 (图4) 。患儿确诊为结节性硬化症。现患儿7月余,随访至今,未有癫痫发作,一般情况可。
2 讨论
TSC是可依靠临床表现作出诊断的遗传性疾病之一。根据2012国际TSC联盟修订的最新诊断标准[1],该标准以临床特征为依据,其中主要特征有11个,次要特征有6个。具有2个主要特征,或1个主要特征加2个次要特征者就可确诊;具有1个主要特征,或1个主要特征加1个次要特征,或2个(或以上)次要特征者为疑似患者。本例患儿符合4个主要特征,可确诊为TSC。

图3 头颅MRI

图4 DNA测序结果
胎儿心脏肿瘤罕见,发生率约为0.14%,其中心脏横纹肌瘤占60%,且多与TSC有关[4]。有研究显示TSC患儿50%伴有心脏横纹肌瘤,而心脏横纹肌瘤患儿中80%伴有TSC,且多发心脏横纹肌瘤比单个心脏横纹肌瘤与TSC的关系更为密切[5]。心脏横纹肌瘤常发生于室壁或心腔内,多为多发、累及心室,而心房及心包很少受累;超声表现为圆形、均质的高回声。对TSC患儿心脏横纹肌瘤的随访研究发现,心脏横纹肌瘤常随年龄增长出现自然消退倾向[6]。Sciacca等[7]随访了33例心脏横纹肌瘤的转归,发现8例出现心律失常(2例为预激综合征),4例出现明显的流出道梗阻,其中1例婴儿因此死亡,存活的32例患儿中有31例患儿的肿瘤数目减少和体积缩小。本例患儿超声心动图示心室内多发肿瘤,超声表现与横纹肌瘤相符,24 h动态心电图提示有房性期前收缩,目前尚无心脏血流动力学改变。TSC相关致病基因为TSC1和TSC2,TSC1基因编码蛋白为错构瘤蛋白(hamartin),TSC2基因产物是马铃薯蛋白(tuberin),二者基因突变阳性率达75%~90%[8]。散发病例中,TSC2基因突变是TSC1基因突变的5倍,而有家族史的病例这两种基因突变率相近[9]。TSC1基因的突变多为小的截短损伤,故临床症状通常较TSC2轻;而TSC2基因的突变通常是大的缺失、错义突变,癫痫发作起始年龄更小、头颅MRI影像学表现更为明显、发生认知损害的概率更高,病情也更严重[10-12]。随着基因检测的普及,2012年国际TSC联盟会议把基因检测作为独立的诊断标准[1]。产前行基因检测对有高危因素的孕妇(如有TSC家族史或胎儿期发现心脏横纹肌瘤)非常有价值。Milunsky等[13]检测了50例胎儿的基因(所有孕妇均有高危因素),标本来源于羊水细胞、绒膜绒毛、流产胎儿的组织或血液,其中2例因标本质量差废弃,余48例中发现3例TSC1阳性,8例TSC2阳性。本例患儿无TSC家族史,但胎儿期发现心脏横纹肌瘤,属于高危人群,未行产前基因检测。运用高通量二代测序技术,在患儿TSC2基因上检测到突变c.4541-4544delCAAA(p.S1514fs),即第1514位氨基酸(丝氨酸)发生框移缺失突变,此后整个蛋白质的氨基酸结构发生改变,该变异位点为致病性变异。经登录TSC基因突变数据库和人类基因变异数据库网站,未发现TSC2中该变异方式报道,这是一种在TSC患儿中新发现的TSC2基因突变方式。其父母均未检测到致病基因,考虑患儿为自发突变,采用Sanger测序方法对以上突变进行验证,结果与高通量二代测序结果相符合,证明二代测序结果可信。
皮肤改变是TSC的主要表现之一,约90%患者伴有皮肤改变,包括面部血管纤维瘤、前额斑块、鲨革样斑、色素脱失斑、甲周纤维瘤等,且不同的年龄段有不同的皮肤改变[11]。国内外的临床报告[14,15]均显示皮肤损害中最常见、最早发生的是色素脱失斑。本例患儿躯干部及双下肢有多处色素脱失斑,未见其他皮肤改变,可能与年龄小有关。中枢神经系统受累在TSC患儿中较为常见,有报道癫痫发生率为93%~96%[16],近2/3患儿在1岁以内起病,约80%患儿在3岁内发病,可同时伴有神经系统发育迟缓、认知障碍和行为异常[17]。1岁内起病的患儿中婴儿痉挛占1/3,并可伴有其他发作类型或与其他发作类型相互转变[18,19]。在癫痫发作之前1~8天脑电图就可有痫样放电改变,因此监测脑电图动态变化对预测癫痫发生具有一定意义[20,21]。TSC特征性影像学表现包括皮质结节、皮质下异位结节、室管膜下结节和室管膜下巨细胞星形细胞瘤等[22]。本例患儿MRI上可见多种影像学改变,包括皮质结节、室管膜下结节和脑白质辐射状迁移线。到目前为止,TSC缺乏特异性的治疗方法。近年来,随着对TSC发病机制的进一步研究,发现哺乳动物雷帕霉素靶蛋白(mTOR) 抑制剂可改善疾病的预后。多项研究表明雷帕霉素对TSC合并室管膜下巨细胞星形细胞瘤、肾淋巴血管肌脂瘤、肺淋巴血管肌瘤病的有一定治疗效果[23-25],mTOR抑制剂(依维莫司)对控制TSC合并癫痫发作方面有一定的作用[26-28]。
总之,TSC是一种多脏器受累的系统性疾病,随着各项检查手段及分子遗传学检查的应用,早期诊断有助于对患儿加强随访,进行合理干预。
[1]Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference [J]. Pediatr Neurol, 2013, 49(4): 243-254.
[2]Van Slegtenhorst M, de Hoogt R, Hermans C, et al.Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34 [J]. Science, 1997, 277(5327): 805-808.
[3]European Chromosome 16 Tuberous Sclerosis Consortium.Identification and characterization of the tuberous sclerosis gene on chromosome 16 [J]. Cell, 1993, 75(7): 1305-1315.
[4]Bonnamy L, Perrotin F, Megier P, et al. Fetal intracardiac tumor(s): prenatal diagnosis and management. Three case reports [J]. Eur J Obstet Gynecol Reprod Biol, 2001,99(1):112-117.
[5]Tworetzky W, McElhinney DB, Margossian R, et al.Association between cardiac tumors and tuberous sclerosis in the fetus and neonate [J]. Am J Cardiol, 2003, 92(4): 487-489.
[6]Kocabas A, Ekici F, Cetin Iİ, et al. Cardiac rhabdomyomas associated with tuberous sclerosis complex in 11 children:presentation to outcome [J]. Pediatr Hematol Oncol, 2013,30(2): 71-79.
[7]Sciacca P, Giacchi V, Mattia C, et al. Rhabdomyomas and tuberous sclerosis complex: our experience in 33 cases [J].BMC Cardiovasc Disord, 2014, 14: 66.
[8]Northrup H, Koenig MK, Pearson DA, et al. Tuberous Sclerosis Complex [M]// Pagon RA, Adam MP, Ardinger HH,et al. Gene Reviews. Seattle (WA): University of Washington,Seattle, 2015.
[9]Kothare SV, Singh K, Chalifoux JR, et al. Severity of manifestations in tuberous sclerosis complex in relation to genotype [J]. Epilepsia, 2014, 55(7): 1025-1029.
[10]Overwater IE, Swenker R, van der Ende EL, et al. Genotype and brain pathology phenotype in children with tuberous sclerosis complex[J]. Eur J Hum Genet, 2016, 24(12):1688-1695.
[11]Camprubi M, Balaguer A, Azon Masoliver A, et al. Unilateral facial angiofibromas; a review of the literature [J]. Pediatr Dermatol, 2006, 23(3): 303-305.
[12]Van Eeghen AM, Black ME, Pulsifer MB, et al. Genotype and cognitive phenotype of patients with tuberous sclerosis complex [J]. Eur J Hum Genet, 2012, 20(5): 510-515.
[13]Milunsky A, Ito M, Maher TA, et al. Prenatal molecular diagnosis of tuberous sclerosis complex [J]. Am J Obstet Gynecol, 2009, 200(3): 321.
[14]Jozwiak S, Schwartz RA, Janniger CK, et al. Skin lesions in children with tuberous sclerosis complex: their prevalence,natural course, and diagnostic signifcance [J]. Int J Dermatol,1998, 37(12): 911-917.
[15]Kuhn CH, Casper KA, Green TR. Assessing Ohio grocery store patrons' perceptions of a comprehensive medication review [J]. J Am Pharm Assoc (2003), 2009, 49(6): 787-791.
[16]Devlin LA, Shepherd CH, Crawford H, et al. Tuberous sclerosis complex: clinical features, diagnosis, and prevalence within Northern Ireland [J]. Dev Med Child Neurol, 2006,48(6): 495-499.
[17]Curatolo P, Jozwiak S, Nabbout R, et al. Management of epilepsy associated with tuberous sclerosis complex (TSC):clinical recommendations [J]. Eur J Paediatr Neurol, 2012,16(6): 582-586.
[18]Holmes GL, Stafstrom CE, Tuberous Sclerosis Study Group. Tuberous sclerosis complex and epilepsy: recent developments and future challenges [J]. Epilepsia, 2007,48(4): 617-630.
[19]Hsieh DT, Jennesson MM, Thiele EA. Epileptic spasms in tuberous sclerosis complex [J]. Epilepsy Res, 2013, 106(1-2):200-210.
[20]Domanska-Pakiela, Kaczorowska M, Jurkiewicz E, et al.EEG abnormalities preceding the epilepsy onset in tuberous sclerosis complex patients - a prospective study of 5 patients[J]. Eur J Paediatr Neurol, 2014, 18(4): 458-468.
[21]Ikeno M, Okumura A, Abe S, et al. Clinically silent seizures in a neonate with tuberous sclerosis [J]. Pediatr Int, 2016,58(1): 58-61.
[22]Connolly MB, Hendson G, Steinbok P. Tuberous sclerosis complex: a review of the management of epilepsy with emphasis on surgical aspects [J]. Childs Nerv Syst, 2006,22(8): 896-908.
[23]Herry I, Neukirch C, Debray MP, et al. Dramatic effect of sirolimus on renal angiomyolipomas in a patient with tuberous sclerosis complex [J]. Eur J Intern Med, 2007, 18(1):76-77.
[24]Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex [J].Ann Neurol, 2006, 59(3): 490-498.
[25]Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis [J]. N Engl J Med, 2008, 358(2):140-151.
[26]Wiegand G, May TW, Ostertag P, et al. Everolimus in tuberous sclerosis patients with intractable epilepsy: a treatment option? [J]. Eur J Paediatr Neurol, 2013, 17(6):631-638.
[27]Krueger DA, Wilfong AA, Holland-Bouley K, et al.Everolimus treatment of refractory epilepsy in tuberous sclerosis complex [J]. Ann Neurol, 2013, 74(5): 679-687.
[28]Kotulska K, Chmielewski D, Borkowska J, et al. Longterm effect of everolimus on epilepsy and growth in children under 3 years of age treated for subependymal giant cell astrocytoma associated with tuberous sclerosis complex [J].Eur J Paediatr Neurol, 2013, 17(5): 479-485.
Tuberous sclerosis complex secondary to fetal heart occupying lesions: a case report and literature review
ZHU Ronghe1, SUN Yuanyuan1, LIANG Yaqin1, CHEN Bin2, YIN Weiwei3, QIAN Yan1(1. Department of Pediatrics, 2. Department of Ultrasonography, 3. Department of Radiology, The First Af fi liated Hospital of Wenzhou Medical University, Wenzhou 325000,Zhejiang, China)
ObjectiveTo explore the clinical characteristics of tuberous sclerosis complex (TSC).MethodsThe clinical data of one child with TSC were collected. The clinical features and gene mutation were analyzed.ResultsA 36-dayold girl had abnormal nodules found by echocardiography, which was considered multiple cardiac rhabdomyomas. There were multiple hypomelanotic macules distributed over the skin surface of the trunk and legs. Cranial MRI showed cortical nodules,subependymal nodules and cerebral white matter radial migration line. A mutation in theTSC2gene (c.4541-4544delCAAA)was found by second generation high-throughput sequencing technology and tuberous sclerosis complex was confirmed.ConclusionGene detection is helpful in the early diagnosis of tuberous sclerosis complex.
tuberous sclerosis complex;TSC2gene; cardiac tumor
2017-01-21)
(本文编辑:邹 强)
10.3969/j.issn.1000-3606.2017.07.001
钱燕 电子信箱:qianyan11@126.com
