微波辅助球磨还原含Cr(Ⅵ)废水的研究*
2017-07-18陈鼎熊维陈林陈振华
陈鼎,熊维,陈林,陈振华
(湖南大学 材料科学与工程学院,湖南 长沙 410082)
微波辅助球磨还原含Cr(Ⅵ)废水的研究*
陈鼎†,熊维,陈林,陈振华
(湖南大学 材料科学与工程学院,湖南 长沙 410082)
通过微波辅助球磨法还原含Cr(VI)废水及单因素法观察铁球质量的改变、还原铁粉量、pH值对还原Cr(VI)的影响.实验结果表明,球料比和还原铁粉量越大,pH值越小,Cr(VI)的还原速度越快.在中性溶液中,通过微波辅助球磨,能在55 min内,将六价铬质量浓度从50 mg/L还原到0.473 mg/L,产物经XRD和XPS检测为FeCr2O4;当pH=2时,将初始质量浓度为300 mg/L的含Cr(VI)废水还原到0.5 mg/L以下只需6 min,所以采用微波辅助球磨装置能够高效处理含Cr(Ⅵ)的工业废水.
微波;球磨;六价铬;还原
铬广泛运用于金属表面处理、皮革鞣制、电镀和印染等行业[1-4],这些行业含Cr(VI)的高浓度废水一旦排入公共水系,则严重影响人类的身体健康,因此必须在排放之前将Cr(VI)还原为Cr(Ⅲ),因为它们的化学性质、毒性和生物利用率是明显不同的[5].
传统的含铬工业废水处理方法有铁氧体法[6]、亚硫酸盐还原法[7]、离子交换法[8]、吸附法[9]、电化学法[10]、生物法[11]、滤膜法[12],铁还原法[13-14]等.物理化学法治理Cr(VI)污染所需费用过高,且对Cr(VI)的去除不具有选择性,只是发生了污染物的转移和浓缩;而采用生物修复技术去除Cr(VI)所需时间过长,并存在生物安全性问题;零价铁作为还原Cr(VI)的材料一直是科学家非常感兴趣的方向[13-14].可在还原过程中传统零价铁会在表面钝化使得还原速率降低并且造成铁的浪费.
本研究采用微波球磨法还原Cr(VI),能够在不同pH值环境的水溶液,通过微波的热效应和机械球磨的作用,不断将铁球表面形成的钝化层打击剥落形成新的反应层,并形成稳定的FeCr2O4沉淀,操作简便,为治理铬污染废水提供了一种新的思路.
1 实验
1.1 实验方法
微波辅助水溶液球磨还原六价铬是在特制的四氟乙烯球磨罐(直径140 mm,高度90 mm,球磨罐内容积为1.38 L)中加入一定量的铁球(Φ=1~1.5 mm)和一定浓度的Cr(VI)溶液,计入液体的高度(h),放入到特制的微波炉中,调整搅拌杆高度,在距球磨罐底部4 mm处时,同时开动搅拌球磨和微波炉,进行球磨,球磨搅拌速度300 r/min,微波的功率为400 W,在特定的时间内取3 mL样品,取得的样品离心进行固液分离,液体进行Cr(VI)浓度检测.取样后,加入去离子水使液体的高度与原高度(h)一致.将分离后的固体放置到真空干燥箱中干燥后,进行XRD和XPS检测.微波辅助球磨反应的装置如图1所示[15].
1.2 Cr(VI)浓度测定
Cr(VI)浓度测定方法为二苯碳酰二肼法(GB 7467-87[16]),选用10 mL的石英比色皿,用蒸馏水、酒精清洗多次,用待测液润洗三次,再将待测样本取至比色皿的2/3~3/4处,将比色皿四壁用擦镜纸擦干净,放入岛津UV-2550PC紫外分光光度计中测量.
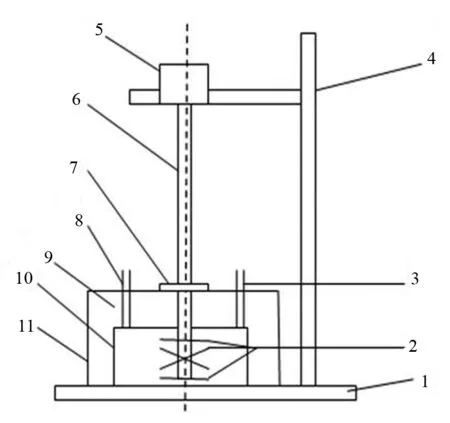
1.底座,2.搅拌杆,3.冷凝管,4.支架,5.电动机,6.万向节, 7.微波防漏盖,8.进水口,9.微波炉,10.球磨罐,11.微波炉盖图1 微波辅助球磨装置示意图Fig.1 Schematic illustration of microwave- assisted ball mill equipment.
2 实验结果与讨论
2.1 微波辅助球磨法还原Cr(Ⅵ)结果及机理分析
在铁球m=1 kg条件下,比较微波辅助球磨,球磨和微波辐射三种工艺对还原Cr(VI)的影响.从图2中可以看出无论是微波球磨法、球磨法还是微波法都能还原废水中的六价铬,使得废水中的六价铬含量随着时间不断减少直到达到国家标准.其中微波辅助球磨的速度最快,球磨次之,微波辐射最慢.在55 min时,微波球磨能将初始质量浓度为50 mg/L的Cr(VI)废水还原到0.473 mg/L.微波法和球磨法要将Cr(VI)废水质量浓度降到0.5 mg/L以下需要的时间分别为85 min和75 min.图3为反应产物干燥后的XRD分析图谱,图中结果表明产物为FeCr2O4,该产物具有软磁性.图4的XPS结果表明,特征峰全部为Cr(Ⅲ),并无Cr(VI)离子存在.

图2 微波辅助球磨,球磨,微波辐射还原Cr(Ⅵ)三者 对比(铁球m=1 kg,铁粉m=0 kg,pH=7)Fig.2 Degradation of Cr (VI) by microwave assisted ball milling,ball milling,microwave radiation (iron ball:m=1 kg ,iron powder:m=0 kg,pH=7)

图3 产物的XRDFig.3 XRD patterns of reaction products
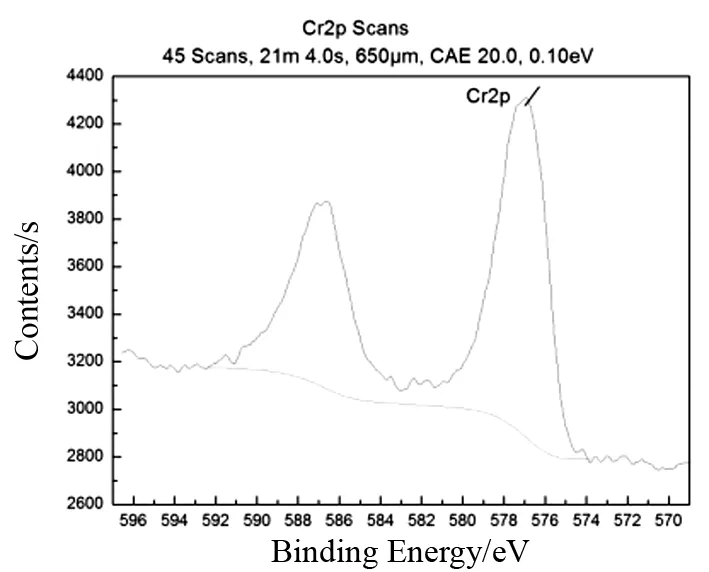
图4 产物的XPSFig.4 XPS patterns of reaction products
下面探讨微波辅助球磨还原六价铬的机理.
微波在水中传输过程中会产生热效应,使得反应物分子运动加剧,温度升高,提高了Fe和Cr6+之间的碰撞,从而改变了反应速率,另一方面,在微波场洛伦兹力作用下,反应体系的熵增加,有利于朝着反应产物的方向进行.同时在微波作用下,会使水中产生H· 和 OH· 自由基离子,H·能够促进零价铁和六价铬的还原反应[17],如反应(1)所示.
Cr2O72-(aq)+ 3Fe(s)+ 14 H· (aq)→ 2Cr3+(aq)+ 3Fe2+(aq)+7H2O
(1)
在球磨机械力的作用过程中,铁球与铁球之间会发生激烈的碰撞,摩擦和冲击使得铁球表面层脱落,产生新的表面层与六价铬进行反应,周而复始,直到反应结束[18-20].球磨可以使溶于水中的N2和O2发生反应生成HNO3和HNO2从而使溶液有H+生成[21],加快了六价铬的还原,其原理如 (2),(3) 和(4)所示.
N2+ 2O2+ H2O → HNO3+ HNO2
(2)
Cr2O72-(aq)+ 3Fe(s)+ 14H+(aq)→
2Cr3+(aq)+ 3Fe2+(aq)+7H2O
(3)
Cr2O72-(aq) + 6Fe2+(aq) + 14H+(aq) → 2Cr3+(aq) + 6Fe3+(aq) + 7H2O
(4)
微波球磨时微波的能量和球磨的机械力产生耦合作用,在微波的热效应下使得还原速度加快,在球磨作用下,因不断的打击剥落,铁球表面产生新的零价铁反应层.另外在二者的共同作用下会生产更多的H·[22]自由基离子,使得微波辅助球磨能够更快地还原六价铬.
2.2 微波辅助球磨中铁球质量对还原Cr(VI)离子影响
在微波球磨中,采用纯铁磨球还原Cr(VI)离子.图5结果表明,铁球为1 250 g的微波辅助球磨,初始废水溶液Cr(VI)质量浓度为75 mg/L,反应时间为75 min后,Cr(VI)质量浓度降至0.414 mg/L;可当铁球分别为1 000 g和750 g时,反应相同的时间,Cr(VI)质量浓度分别只降至1.117 5 mg/L和4.035 mg/L,达不到国家排放标准.究其原因,一方面纯铁球的质量增加,会增加铁球与六价铬离子的反应面积;另一方面,铁球在搅拌球磨的作用下,由于高能破碎及铁球与铁球之间的碰撞,使得在铁球表面形成的部分产物,通过打击剥落,产生新零价铁表面,加速了Cr(VI)的还原,并且这个过程周而复始,一直到反应完成为止.

图5 微波球磨下,球量改变对Cr(Ⅵ) 还原的影响(铁粉m=0 kg,pH=7)Fig.5 The effect of the balls’ mass on the degra- dation of Cr (Ⅳ)(iron powder:m=0 kg,pH=7)
2.3 微波辅助球磨中加入微细铁粉对还原高浓度Cr(VI)离子的影响
质量浓度为300 mg/L的Cr(Ⅵ)溶液,铁球质量为1 kg,从图6可知,加入铁粉100 g,反应时间是30 min,Cr(Ⅵ)质量浓度还原至0.4325 mg/L,达到我国污水处理的目标.当加入铁粉50 g时,要将Cr(Ⅵ)的质量浓度降到0.5 mg/L需要80 min.因为越多还原铁粉的加入,零价铁的比表面积增加,有利于增加零价铁和六价铬离子的反应面积,而且球磨的撞击剥落作用不但能够使得铁球产生新的零价铁表面,还使得还原铁粉在还原过程中不断被细化,从而再次产生新的反应层.另外随着微波的热效应,溶液温度升高,也促进反应的加速进行,从而促进了整个还原过程的加速进行.为了加快零价铁与Cr(Ⅵ)的反应速度,加入过量的铁粉,能促进六价铬的还原;没有反应完全的铁粉,可继续与溶液中新加入Cr(Ⅵ)反应.

图6 微波辅助球磨下,不同质量的微细铁粉 对还原Cr(Ⅵ)的影响(铁球m=1 kg,pH=7)Fig.6 The effects of different mass of fine iron powder on degradation Cr(Ⅵ) solution with microwave- assisted ball milling(iron ball:m=1 kg ,pH=7).
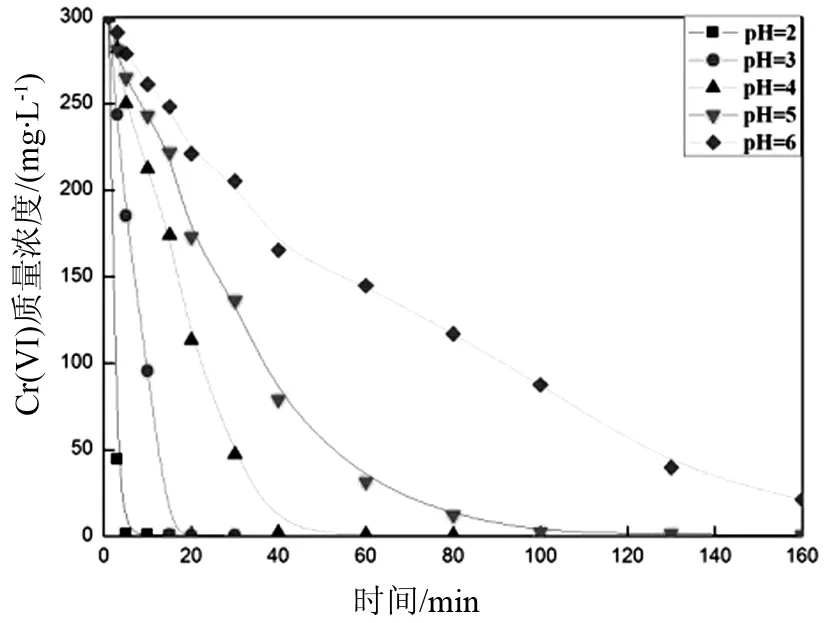
图7 溶液的pH值对Cr(Ⅵ)离子还原速度的影响 (铁球m=1 kg,铁粉m=0 kg)Fig.7 The effects of pH on the degradation of Cr (Ⅵ)
2.4 溶液pH值对微波辅助球磨还原Cr(Ⅵ)的影响
一般的电镀废水都偏酸性,为了模拟微波球磨法对不同酸性浓度下电镀废水中的六价铬的还原速率的影响,本研究配制了pH值为2,3,4,5,6,质量浓度为300 mg/L的六价铬废水模拟液.从图7中可以明显地看到pH值越低,越有利于Cr(Ⅵ)的还原.这是因为酸性越强,能够提供的H+越多,越能加快反应的进行.当pH=2,3时,分别只要15 min和30 min就能将300 mg/L的六价铬废水还原到低于0.5 mg/L,而pH=6时,在160 min时,溶液的Cr(Ⅵ)质量浓度还有10.4 mg/L.这说明微波球磨对酸性的高浓度六价铬废水修复效果是非常明显的.
3 结 论
1)微波球磨法能够高效处理含Cr(VI)的废水.
2)微波球磨法、球磨法、微波法都能对Cr(VI)起到还原作用,并且形成稳定的沉淀物FeCr2O4,其中微波球磨法还原的效率最高.
3)加入还原性的铁粉越多和增加球的质量均能够加快Cr(VI)的还原速度.
4)在微波球磨法中pH值越低,Cr(VI)的还原速度越快,当pH=2时,将初始质量浓度为300 mg/L的含Cr(VI)废水还原到0.5 mg/ L以下只要6 min.
[1] CHUNMING S,LUDWIG R D. Treatment of hexavalent chromium in chromite ore processing solid waste using a mixed reductant solution of ferrous sulfate and sodium dithionite[J]. Environmental Science & Technology,2005,39(16):6208-6216.
[2] AGRAWAL A,KUMAR V,PANDEY B D. Remediation options for the treatment of electroplating and leather tanning effluent containing chromium—a review[J]. Mineral Processing and Extractive Metallurgy Review,2006,27(2):99-130.
[3] FAHIM N F,BARSOUM B N,EID A E,etal. Removal of chromium(III) from tannery wastewater using activated carbon from sugar industrial waste[J].Journal of Hazardous Materials,2006,136(2):303-9.
[4] PASCHOAL F M M,ANDERSON M A,ZANONI M V B. Simultaneous removal of chromium and leather dye from simulated tannery effluent by photoelectron chemistry[J]. Journal of Hazardous Materials,2009,166(1):531-537.
[5] BERRY W J,BOOTHMAN W S,SERBST J R,etal. Predicting the toxicity of chromium in sediments[J]. Environmental Toxicology & Chemistry,2004,23(12):2981-2992
[6] ERDEM M,TUMEN F. Chromium removal from aqueous solution by the ferrite process[J]. Journal of Hazardous Materials, 2004,109(1):71-77.
[7] ALI S,FAROOQ M A,HUSSAIN S,etal. Alleviation of chromium toxicity by hydrogen sulfide in barley[J]. Environmental Toxicology & Chemistry,2013,32(10):2234-2239.
[8] RENGARAJ S,YEON K H,MOON S H. Removal of chromium from water and wastewater by ion exchange resins[J]. Journal of Hazardous Materials,2001,87(1/3):273-87.
[9] YONG J S,JIN W C,JANG H D,etal. Role of chemical hardness in the adsorption of hexavalent chromium species onto metal oxide nanoparticles[J]. Chemical Engineering Journal,2015,273:401-405.
[10]ZHANG Y,QIN L,RONG T,etal. Electrocatalytic reduction of chromium by poly(aniline- co-o-aminophenol):an efficient and recyclable way to remove Cr(VI) in wastewater[J]. Applied Catalysis B Environmental,2009,92(3/4):351-356.
[11]KUMAR R,BISHNOI N R,GARIMA,etal. Biosorption of chromium(VI) from aqueous solution and electroplating wastewater using fungal biomass[J]. Chemical Engineering Journal,2008,135(3):202-208.
[12]BHOWAL A,DATTA S. Studies on transport mechanism of Cr(VI) extraction from an acidic solution using liquid surfactant membranes[J]. Journal of Membrane Science,2001,188(188):1-8.
[13]GHEJU M,IOVI A. Kinetics of hexavalent chromium reduction by scrap iron[J]. Journal of Hazardous Materials,2006,135(1/3):66-73.
[14]CHEN S S,HSU B C,HUNG L W. Chromate reduction by waste iron from electroplating wastewater using plug flow reactor[J]. Journal of Hazardous Materials,2008,152(3):1092-1097.
[15]CHEN D,ZHANG Y Z. Synthesis of NiFe2O4nanoparticles by a low temperature microwave-assisted ball milling technique[J]. Science China Technological Sciences,2012,55(6):1535-1538.
[16]GB 7467-87 水质-六价铬测定-二苯碳酰二肼分光光度计法[S]. 北京:中国标准出版社,1987:1-4.
GB 7467-87 Water quality—determination of chromium(VI)-1,5 diphenylcarbohydrazide spectrophotometric method[S]. Beijing:Standards Press of China,1987:1-4.(In Chinese)
[17]COATA M. Toxicity and carcinogenicity of Cr (VI) in animal models and humans[J]. Critical Reviews in Toxicology,1997,27(5):431-442.
[18]FLURY B,EGGENBERGER U,MADER U. First results of operating and monitoring an innovative design of a permeable reactive barrier for the remediation of chromate contaminated groundwater[J]. Applied Geochemistry,2009,24(4):687-697.
[19]CHEN Z H,ZHOU Y,KANG Z T,etal. Synthesis of Mn-Zn ferrite nanoparticles by the coupling effect of ultrasonic irradiation and mechanical forces[J]. Journal of Alloys and Compounds,2014,609(19):21-24.
[20]CHEN D,LI D Y,ZHANG Y Z,etal. Preparation of magnesium ferrite nanoparticles by ultrasonic wave-assisted aqueous solution ball milling[J]. Ultrasonics Sonochemistry,2013,20(6):1337-1340.
[21]YUAN Z,CHEN Z H,CHEN D,etal. Analyses of factors affecting nickel ferrite nanoparticles synthesis in ultrasound-assisted aqueous solution ball milling[J]. Ultrasonics Sonochemistry,2015,22:188-197.
[22]CHEN D,ZHANG Y,CHEN B,etal. Coupling effect of microwave and mechanical forces during the synthesis of ferrite nanoparticles by microwave-assisted ball milling[J]. Industrial & Engineering Chemistry Research,2013,52(39):14179-14184.
Chromium Reduction from Cr (VI) Polluted Wastewater with Microwave-assisted Ball Milling
CHEN Ding†,XIONG Wei,CHEN Lin,CHEN Zhenhua
(College of Materials Science and Engineering,Hunan University,Changsha 410082,China)
Microwave-assisted ball milling was employed to reduce Cr (VI) in wastewater, and single factor method was used to observe the change of the iron ball quality, the amount of reduced iron powder and the effect of pH on the reduction of Cr (VI). These experimental results show that the greater the ball material ratio and the amount of reduced iron powder, the smaller the pH, the faster the reduction speed of Cr (VI). In the neutral solution, through microwave-assisted ball milling, the concentration of Cr (VI) can be reduced from 50 mg/L to 0.473 mg/L in 55 minutes, and the production is detected as FeCr2O4by XRD with XPS. When the pH is 2, the initial concentration of 300 mg/L Cr (VI) can be reduced to below 0.5 mg/L in 6 minutes. Therefore, the industrial wastewater containing Cr (VI) can be efficiently disposed by microwave assisted ball milling equipment.
microwave;ball milling; Cr (VI); reduction
1674-2974(2017)06-0052-05
10.16339/j.cnki.hdxbzkb.2017.06.009
2016-03-03
湖南省自然科学基金资助项目(14JJ1013),National Natural Science Foundation of Hunan Province(14JJ1013)
陈鼎(1975—),男,江苏溧阳人,湖南大学教授,博士生导师†通讯联系人,E-mail: chending@hnu.edu.cn
X756
A
