生物柴油催化转移加氢改善其燃烧特性
2017-07-12袁银男戴鹏飞梅德清
袁银男,顾 萌,戴鹏飞,梅德清※
(1. 江苏大学汽车与交通工程学院,镇江 212013;2. 苏州大学能源学院,苏州 215006)
生物柴油催化转移加氢改善其燃烧特性
袁银男1,2,顾 萌1,戴鹏飞1,梅德清1※
(1. 江苏大学汽车与交通工程学院,镇江 212013;2. 苏州大学能源学院,苏州 215006)
采用催化转移加氢法对大豆油生物柴油(SME,soybean methyl ester)进行适度加氢制备部分加氢大豆油生物柴油(PHSME,partially hydrogenated soybean methyl ester),分析比较生物柴油加氢前后的组分、过氧化值、碘值、十六烷值与运动黏度等燃料特性参数,并基于热重-差示扫描量热法(TG-DSC,thermogravimetry-differential scanning calorimeter)研究加氢提质生物柴油的着火燃烧性能。结果表明:以异丙醇为供氢体、水为反应介质和Raney-Ni为催化剂,在温度85℃和常压下对SME进行催化转移加氢反应,经GC-MS(gas chromatography-mass spectrometry)检测发现高不饱和组分选择性转化为低不饱和或饱和组分,不饱和程度降低了46.2%。尽管PHSME的运动黏度略有增加,但其氧化安定性得到明显改善,且十六烷值也升高至合理的范围。在空气氛围下,由于PHSME相比于SME的分子结构变化及运动黏度增加,其在初始阶段不易挥发,但在高温阶段平均氧化速率更高,其终了失质量温度比SME提前7.2 ℃。由于PHSME的适度的高十六烷值属性,在DSC曲线可见其放热始点温度比SME提前10.7 ℃,说明提质生物柴油具有优越的着火性能。
燃烧;生物柴油;氢化;氧化;热重;差示扫描量热法
0 引 言
生物柴油作为一种清洁的可再生能源,已成为传统柴油的替代品[1-2]。但生物柴油含有不饱和碳链,特别是其中的高不饱和组分,在长期储放条件下易发生腐败,氧化安定性较差[3-7]。对生物柴油进行适度加氢是解决这一问题的有效手段[8-11]。按照供氢方式不同,催化加氢方法可分为两类,一是以氢气为氢源的氢气还原法[8-9];二是用含氢多原子分子(如醇、芳烃氢化物、甲酸及其盐等)作氢源的转移加氢法[10,12-14]。与氢气还原法相比,催化转移加氢反应可在较低的温度和压力下进行,反应条件较为温和。Basu等[15]研究发现以甲酸盐为氢源,Pd(OAc)2为催化剂时,可以较好地选择性催化加氢含有碳碳双键的化合物;Tike等[16]用甲酸铵为氢源,固载钯和铑为催化剂时,对大豆油进行催化转移氢化,发现催化选择性较好,硬脂酸生成速率也较低。可以得知,催化转移加氢法对反应物的选择性较高,能维持反应物中一定的双键数目。
近年来,适度加氢提质生物柴油的应用研究还较少报道。Wadumesthrige等[17]研究发现通过部分氢化来改变生物柴油的分子结构,有助于增加氧化安定性及改善着火性能。夏燕[18]比较了加氢前后生物柴油的氧化安定性和运动黏度等燃料特性的变化。由于生物柴油原料来源的多样化使得其所含脂肪酸酯组分差异较大[19-20],因而燃油品质参差不齐,也为其在发动机上优化燃烧应用设置了障碍。适度加氢后的生物柴油其碘值可控降低在一定数值范围,同时十六烷值得以提升,这既能收获高品质的燃油,又能使生物柴油油品品质更易于统一与标准化[9,20-21]。现有文献中,主要是采用氢气直接加氢法对生物柴油进行适度加氢提质的试验研究,而采用催化转移加氢法的报道较少。同时,对加氢后生物柴油燃料特性的研究还较少开展。
本文采用催化转移加氢法对大豆油生物柴油(soybean methyl ester,SME)进行适度加氢来制备部分加氢大豆油生物柴油(partially hydrogenated soybean methyl ester,PHSME),该部分氢化工艺同样可适用其他原料如餐饮废油制备的生物柴油。依据测量或计算出SME和PHSME的组分、过氧化值、碘值、十六烷值与运动黏度等燃料特性参数。同时,采用热重-差示扫描量热分析仪(thermogravimetry-differential scanning calorimeter,TG-DSC),对比分析加氢前后生物柴油的氧化与燃烧性能,以期为其在发动机上的应用提供重要的理论依据。
1 材料与方法
1.1 加氢提质生物柴油的制备工艺
以SME为原料,异丙醇为供氢体,水为反应介质,在雷尼镍(Raney-Ni)催化剂的作用下进行转移加氢反应。其中,溶剂水、异丙醇与SME的投料体积比设为100:32:7,催化剂用量为SME质量的13%左右。设定DF-101S集热式恒温加热磁力搅拌器的水浴温度为(85±1)℃,搅拌速度为600 r/min,加氢反应在带有回流冷凝器的三口烧瓶中进行[16,18]。
1.2 组分测定
为了对SME和PHSME的组分变化进行分析,采用美国Agilent公司的6890GC/5973NMSD气相色谱/质谱联用分析仪。色谱柱为DB-WAX(30 m×0.25 mm×0.25 μm);进样量1 μL;载气为氦气。程序升温:炉子起始温度50 ℃,以10 ℃/min升至200 ℃,保持9 min,再以6 ℃/min升至230 ℃,保持6 min。同时,2种燃油样品分别设定平行试验,且每种样品测定3次,取平均值。
1.3 理化特性测量与计算
生物柴油中含有的不饱和碳碳双键,在使用和储存过程中会与空气中的氧发生氧化聚合反应,形成聚合物、过氧化物等,影响其使用。通过测定燃油的过氧化值,可用来评价加氢前后生物柴油的氧化安定性。将360 mL试样装入氧化管中,通入O2,速率为(50±5)mL/min,在95 ℃下氧化8 h,每隔2 h取样1次,用饱和碘化钾溶液滴定测量燃油样品的过氧化值。为减小试验误差,重复测量3次,并取平均值。
燃油样品的碘值可间接判断样品反应前后饱和程度的变化。采用化学滴定法,用汉诺斯(Hanus)溶液(主要成分IBr)测定燃油样品的碘值。以CCI4为溶剂,在燃油样品中加入过量的汉诺斯溶液,反应30 min后,加入KI使剩余的IBr转化成碘,最后用Na2S2O3标准溶液进行滴定,平行测定3份,同时做空白对照试验。
加氢后的生物柴油,其分子结构发生变化,势必会影响其着火特性。依据式(1)及各脂肪酸甲酯的质量分数和十六烷值的大小,可计算出相应燃油的十六烷值[21-22]。

式中CN为燃油的十六烷值;Ai为各脂肪酸甲酯的质量分数;CNi为各脂肪酸甲酯的十六烷值。
运动黏度是影响燃油雾化质量的一个重要油品指标。生物柴油加氢后饱和度增加,必然导致其运动黏度增加。应用平氏毛细管黏度计对SME和PHSME进行运动黏度的测定。重复测定3次并取平均值,以降低试验误差。
1.4 燃烧试验
利用瑞士METTLER公司的TGA/DSC1型综合热分析仪,进行SME和PHSME氧化与燃烧行为热分析。以高纯空气(N2体积分数为78.9%,O2体积分数为21.1%)为工作气,流量为50 mL/min;保护气为高纯N2(99.99%),流量为50 mL/min;样品进样量为20 mg;温升区间为30~500 ℃,升温速率设为20 ℃/min。
1.5 数据处理与统计分析
相关数据采用Microsoft Excel 2013进行数据处理和绘图。同时,运用SPSS 17.0软件进行相关统计分析,且进行显著性检验时,显著水平设定为α=0.05。
2 结果与分析
2.1 加氢提质生物柴油的理化特性分析
经过多次试验,发现100 min后生物柴油的氢化程度已达最大,分离后的产物为PHSME。其催化转移加氢反应的机理可分为2步。其中,将SME记为底物A,异丙醇记为R2CHOH。第1步反应是供氢体R2CHOH和催化金属Ni的氧化加成,一个氢直接和Ni作用,而另一个氢仍在供氢体中;第2步反应是Ni和底物A的配位作用,底物A插入H-Ni键中,接着当第2个氢转移给底物A后就会生成还原产物H2A。此反应过程是供氢体和底物A分别与相邻的Ni原子位氧化加成,形成六元环过渡态[18,23],并不是发生在一个Ni原子位上,最终再进行氢转移和随后的产物脱除。同时,经GC-MS分析得到SME和PHSME各脂肪酸甲酯的含量,如表1所示。

表1 生物柴油样品组分Table 1 Compositions of biodiesel samples
图1为SME和PHSME总离子流色谱图。SME的各组分检出顺序分别为 C16:0、C18:0、C18:1、C18:2、C18:3、C20:0和C22:0,而PHSME中却未见C18:3组分。结合表1可见,SME中参与加氢反应的不饱和组分C18:3、C18:2及C18:1三者总量为70.9%,经适度加氢后,含2个和3个双键的高不饱和组分(C18:2和C18:3)优先被转化为C18:1和C18:0[16,18,24],低不饱和组分C18:1和饱和组分C18:0含量明显上升,加氢饱和或者部分饱和产物的增加总量为26.8%,由此可计算得到该加氢反应的转化率为37.8%。若以脂肪酸酯碳链的不饱和双键数目计,PHSME的不饱和程度比SME降低了46.2%。另外,采用分液、水洗、过滤、干燥和蒸馏等方式将脂肪酸甲酯从反应物和催化剂中分离出来,回收率可达98.7%。
由表2可见,在强制氧化条件下,在酯中不饱和碳碳双键上成功加氧,SME和PHSME的过氧化值随时间延长而增加。一般来说,过氧化值越高说明油品氧化变质越严重。PHSME的过氧化值比SME大幅降低,说明加氢后的产物PHSME的氧化安定性明显改善。在不同氧化时段下,SME和PHSME的过氧化值经显著性检验分析,均可见其差异(P<0.05)。

图1 SME和PHSME的总离子流色谱图Fig.1 Total ion chromatograms of SME and PHSME

表2 燃油样品加速氧化试验的过氧化值Table 2 Peroxide values of accelerated oxidation experiment for biodiesel samples
氢化前后生物柴油的碘值、十六烷值和运动黏度可见表3。如表3所示,氢化前后大豆油生物柴油(SME和PHSME)的碘值分别为93.5和50.5,加氢以后SME的碘值下降,差异显著(P<0.05)。同时,SME经适度加氢处理成为PHSME后,其十六烷值由57.4增至69.2,这预示着火品质的极大改善。但值得注意的是,若SME完全氢化,则燃油的十六烷值过高,不利于其燃烧应用。考虑到提质生物柴油一般不单独作为燃料使用,其适宜的高十六烷值品质可协调柴油-酯-醇等多元混合燃料的优化应用。另外,由表3也可得知PHSME的运动黏度比SME有所增加,提高了17.8%(P<0.05)。Moser等[25]研究也证实了部分加氢生物柴油的不饱和度得到降低,而其运动黏度要高于生物柴油。综合考虑生物柴油的过氧化值、碘值及运动黏度可知,SME和PHSME间的差异显著。
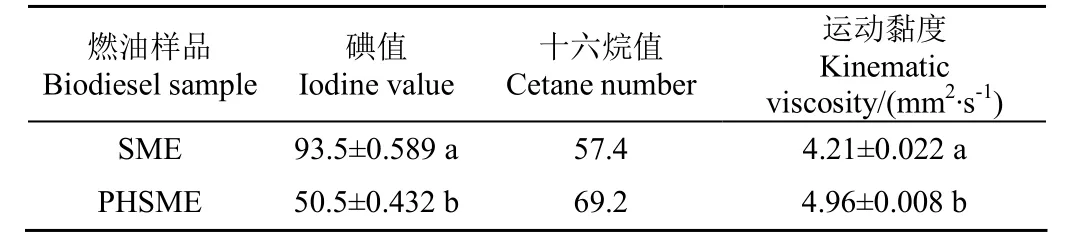
表3 燃油样品的碘值、十六烷值和运动黏度Table 3 Iodine value, cetane number and kinematic viscosity of biodiesel samples
2.2 加氢提质生物柴油的TG-DSC特性
按设定好的升温程序分别进行2种燃油样品的综合热分析试验,得到TG-DTG和DSC曲线如图3所示,燃烧特征参数见表4。

图2 空气氛围下SME和PHSME的TG-DTG和DSC曲线Fig.2 TG-DTG and DSC curves of SME and PHSME under air atmosphere

表4 空气氛围下SME和PHSME的氧化特征参数Table 4 Oxidation parameters of SME and PHSME under air atmosphere
从图2a与表4可见,在30~150 ℃范围内,2种测试样品的质量基本没有下降;随着温度上升,SME和PHSME分别在194.8和215.4 ℃时失质量率达5%,记为Ti;并于324.7和317.5 ℃时失质量率达95%,记为Tb。在330 ℃时2种燃油样品的氧化过程基本结束。由图2a可见,在120~250 ℃区间,与SME相比,PHSME的起始失质量温度滞后20.6 ℃,TG和DTG曲线均向高温区偏移。这主要归因于样品在低温阶段挥发的差异。物质由液态挥发成气态本质上可视为其吸收热量克服分子间作用力的过程[26]。SME部分加氢成为PHSME后,其分子量与分子空间结构变大,致使其分子间的作用力也增大,所以为了使燃油挥发则需要更多的能量,挥发难度变大。SME适度加氢后,PHSME饱和度提高,加之有反式脂肪酸酯的生成,在燃料属性表现为黏度增加[9,25]。尽管前文油品属性测试结果表明PHSME比SME的运动黏度略有增加,但却影响低温阶段燃油挥发特性[27]。
虽然PHSME失质量起点略晚,但在250 ℃之后,其氧化特性曲线已比SME向低温区偏移,且终了失质量温度还提前7.2 ℃。这说明PHSME更易氧化与燃烧,其平均氧化速率比SME更高。部分加氢后的生物柴油中高不饱和脂肪酸甲酯含量减少,总体而言碳链弯曲程度减小,更接近于正十六烷的直线“之”字形结构[28],在氧化氛围下,长碳链末端C更易脱氢形成活性OH·,诱发链锁反应,着火性能得到改善。Papadopoulos等[21]研究亦得出部分加氢生物柴油的十六烷值明显高于生物柴油,更有利于燃油自身的着火与燃烧。
DSC热分析技术常用来检测样品热流变化[26,29]。如图3b所示,在程序升温的低温阶段,SME和PHSME主要发生挥发,DSC曲线呈现吸热峰形;随着温度上升,SME和PHSME分别在278.1和267.4 ℃时达到着火条件并开始燃烧放出热量,DSC曲线呈现放热峰形。PHSME因十六烷值升高,在达到一定温度后会更易氧化与燃烧,因而其放热始点温度比SME提前10.7 ℃。此外,还发现PHSME的放热率峰值比SME大。这可归因于PHSME的运动黏度和十六烷值两方面油品属性综合作用的结果。在低温阶段时,PHSME因运动黏度较大,在一定的升温速率条件下,样品来不及挥发,滞留样品在较高温度下大量挥发,就会有更多的反应物参与燃烧,且PHSME因高十六烷值而着火性能更佳,因此会释放出更多的热量。而SME和PHSME的终了失质量温度与起始失质量温度之差分别为129.9和102.1 ℃,这也间接地说明了PHSME的燃烧持续期短于SME。综上所述,部分加氢后的生物柴油虽运动黏度略有增加,但其十六烷值也适度提升,使燃油的着火性能得到改善[30-31]。
3 结 论
1)以异丙醇为供氢体、水为反应介质和Raney-Ni为催化剂,在温度85 ℃和常压下对SME(soybean methyl ester)进行催化转移加氢反应。经GC-MS检测发现高不饱和组分选择性转化为低不饱和或饱和组分,PHSME(partially hydrogenated soybean methyl ester)的不饱和程度比SME降低了46.2%。
2)SME经适度加氢后,尽管其运动黏度略有增加,但氧化安定性得以明显改善,十六烷值也升高至合理的范围。适度加氢生物柴油的燃油品质得到提升。
3)由于SME适度加氢后的分子结构变化与运动黏度变大,因而PHSME的起始失质量温度比SME滞后20.6 ℃。尽管如此,PHSME的终了失质量温度比SME提前7.2 ℃,说明其平均氧化速率较高及更易氧化与燃烧。
4)在低温阶段,燃油样品主要发生挥发,在DSC曲线上呈现吸热峰形,而在较高温度下,燃油样品开始燃烧放热而呈现放热峰形。其中,因PHSME具有较高的十六烷值,着火性能优于SME,所以其放热始点温度比SME提前10.7 ℃。
[1] Lin C Y, Lin H A. Engine performance and emission characteristics of a three-phase emulsion of biodiesel produced by peroxidation[J]. Fuel Processing Technology, 2007, 88(1): 35-41.
[2] 梅帅,赵凤敏,曹有福,等. 三种小球藻生物柴油品质指标评价[J]. 农业工程学报,2013,29(15):229-235. Mei Shuai, Zhao Fengmin, Cao Youfu, et al. Evaluation of quality items for biodiesel made from three kinds of Chlorella vulgaris[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2013, 29(15): 229-235. (in Chinese with English abstract)
[3] 董芳,郑东前,张凤泉,等. 生物柴油储存过程中其质量性能的变化[J]. 石油学报:石油加工,2011,27(5):801-805. Dong Fang, Zheng Dongqian, Zhang Fengquan, et al. The variation of biodiesel quality during storage period[J]. Acta Petrolei Sinica: Petroleum Processing Section, 2011, 27(5): 801-805. (in Chinese with English abstract)
[4] Serrano M, Martínez M, Aracil J. Long term storage stability of biodiesel: Influence of feedstock, commercial additives and purification step[J]. Fuel Processing Technology, 2013, 116(6): 135-141.
[5] Knothe G. Some aspects of biodiesel oxidative stability[J]. Fuel Processing Technology, 2007, 88(7): 669-677.
[6] 李瑞娜,王忠,李铭迪,等. 过氧化改质对生物柴油及排放影响的研究[J]. 兵工学报,2012,33(12):1545-1548. Li Ruina, Wang Zhong, Li Mingdi, et al. Study on effect of peroxide properties for biodiesel and emissions[J]. Acta Armamentarii, 2012, 33(12): 1545-1548. (in Chinese with English abstract)
[7] 刘金胜,蔺建民,张建荣. 生物柴油氧化安定性研究的新进展[J]. 可再生能源,2010,28(4):145-150. Liu Jinsheng, Lin Jianmin, Zhang Jianrong. Progress in the study on oxidative stability of biodiesel[J]. Renewable Energy Resources, 2010, 28(4): 145-150. (in Chinese with English abstract)
[8] Hee Y S, Jae H R, Seong Y B, et al. Biodiesel production from highly unsaturated feedstock via simultaneous transesterification and partial hydrogenation in supercritical methanol[J]. The Journal of Supercritical Fluids, 2013, 82(1): 251-255.
[9] Souza B S, Pinho D M M, Leopoldino E C, et al. Selective partial biodiesel hydrogenation using highly active supported palladium nanoparticles in imidazolium-based ionic liquid[J]. Applied Catalysis A: General, 2012, 433/434(16): 109-114.
[10] 武文涛,支国. 碳碳双键催化加氢的研究进展[J]. 化学研究,2011,22(2):84-87. Wu Wentao, Zhi Guo. Research progress in catalytic hydrogenation of C=C bond[J]. Chemical Research, 2011, 22(2): 84-87. (in Chinese with English abstract)
[11] Thunyaratchatanon C, Luengnaruemitchai A, Chollacoop N, et al. Catalytic upgrading of soybean oil methyl esters bypartial hydrogenation using Pd catalysts[J]. Fuel, 2016, 163: 8-16.
[12] 夏燕,李菲,孙杨,等. Raney-Ni催化下菜籽油生物柴油催化转移加氢的研究[J]. 浙江工业大学学报,2012,40(3):261-264. Xia Yan, Li Fei, Sun Yang, et al. A study of catalytic transfer hydrogenation of biodiesel from rapeseed oil over Raney-Ni catalyst[J]. Journal of Zhejiang University of Technology, 2012, 40(3): 261-264. (in Chinese with English abstract)
[13] 龚灵,周少东,陈新志. 氢转移反应的研究概述[J]. 化工进展,2010,29(3):478-483. Gong Ling, Zhou Shaodong, Chen Xinzhi. Research progress in hydrogen transfer reaction[J]. Chemical Industry and Engineering Progress, 2010, 29(3): 478-483. (in Chinese with English abstract)
[14] Alonso F, Riente P, Yus M. Transfer hydrogenation of olefins catalysed by nickel nanoparticles[J]. Tetrahedron, 2009, 65(51): 10637-10643.
[15] Basu B, Bhuiyan M M H, Das P. Catalytic transfer reduction of conjugated alkenes and an imine using polymer-supported formats[J]. Tetrahedron Letters, 2004, 35(12): 8931-8934.
[16] Tike M A, Mahajani V V. Studies in catalytic transfer hydrogenation of soybean oil using ammonium formate as donor as donor over 5% Pd/C catalyst[J]. Chemical Engineering Journal, 2006, 123(1): 31-41.
[17] Wadumesthrige K, Salley S O, Ng K Y S. Effects of partial hydrogenation, epoxidation, and hydroxylation on the fuel properties of fatty acid methyl esters[J]. Fuel Processing Technology, 2009, 90(10): 1292-1299.
[18] 夏燕. 水环境下生物柴油催化转移加氢[D]. 杭州:浙江工业大学,2011. Xia Yan. Catalytic Transfer Hydrogenation of Biodiesel in Aqueous Media[D]. Hangzhou: Zhejiang University of Technology, 2011. (in Chinese with English abstract)
[19] 孟中磊,蒋剑春,李翔宇. 生物柴油的发展近况及趋势[J].农业工程学报,2006,22(增刊1):225-230. Meng Zhonglei, Jiang Jianchun, Li Xiangyu. Present situation and development prospects of biodiesel[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2006, 22(Supp.1): 225-230. (in Chinese with English abstract)
[20] 王利兵,于海燕,贺晓辉,等. 生物柴油树种油脂脂肪酸组成对燃料特性的影响[J]. 燃料化学学报,2012,40(4):397-404. Wang Libing, Yu Haiyan, He Xiaohui, et al. Influence of fatty acid composition of woody biodiesel plants on the fuel properties[J]. Journal of Chemistry and Technology, 2012, 40(4): 397-404. (in Chinese with English abstract)
[21] Papadopoulos C E, Lazzridou A, Koutsoumba A, et al. Optimization of cotton seed biodiesel quality (critical properties) through modification of its FAME composition by highly selective homogeneous hydrogenation[J]. Bioresource Technology, 2010, 101(6): 1812-1819.
[22] Knothe G. A comprehensive evaluation of the cetane numbers of fatty acid methyl esters[J]. Fuel, 2014, 119: 6-13.
[23] Johnstone R A W, Wilby A H, Entwistle I D. Heterogeneous catalytic transfer hydrogenation and its relation to other methods for reduction of organic compounds[J]. Chemical Reviews, 1985, 85(2): 129-170.
[24] Zaccheria F, Psaro R, Ravasio N. Selective hydrogenation of alternative oils: a useful tool for the production of biofuels[J]. Green Chemistry, 2009, 11(4): 462-465.
[25] Moser B R, Haas M J, Winkler J K, et al. Evaluation of partially hydrogenated methyl esters of soybean oil as biodiesel[J]. European Journal of Lipid Science and Technology, 2007, 109(1): 17-24.
[26] 梅德清,张永涛,谭文兵,等. 脂肪酸酯在TG-DSC下的挥发与氧化特性[J]. 林产化学与工业,2014,34(5):133-138. Mei Deqing, Zhang Yongtao, Tan Wenbing, et al. Volatilization and oxidation properties of fatty acid methyl esters based on TG-DSC[J]. Chemistry and Industry of Forest Products, 2014, 34(5): 133-138. (in Chinese with English abstract)
[27] Oliveira L, Giordani D, Paiva E, et al. Kinetic and thermodynamic parameters of volatilization of biodiesel from babassu, palm oil and mineral diesel by thermogravimetric analysis(TG)[J]. Journal of Thermal Analysis & Calorimetry, 2013, 111(1): 155-160.
[28] 陈秀,袁银男,孙平,等. 脂肪酸甲酯结构对生物柴油十六烷值的影响[J]. 石油与天然气化工,2007,36(6):481-484. Chen Xiu, Yuan Yinnan, Sun Ping, et al. Effects of structural features of the fatty acid methyl esters upon the cetane number of biodiesel[J]. Chemical Engineering of Oil & Gas, 2007, 36(6): 481-484. (in Chinese with English abstract)
[29] He F, Yi W M, Bai X Y. Investigation on caloric requirement of biomass pyrolysis using TG-DSC analyzer[J]. Energy Conversion & Management, 2006, 47(15/16): 2461-2469.
[30] Moser B R, Williams A, Haas M J, et al. Exhaust emissions and fuel properties of partially hydrogenated soybean oil methyl esters blended with ultra low sulfur diesel fuel[J]. Fuel Processing Technology, 2009, 90(9): 1122-1128.
[31] Tat M E. Cetane number effect on the energetic and exergetic efficiency of a diesel engine fuelled with biodiesel[J]. Fuel Processing Technology, 2011, 92(7): 1311-1321.
Biodiesel modified by catalytic transfer hydrogenation improving combustion performance
Yuan Yinnan1,2, Gu Meng1, Dai Pengfei1, Mei Deqing1※
(1. School of Automobile and Traffic Engineering, Jiangsu University, Zhenjiang 212013, China; 2. School of Energy, Soochow University, Suzhou 215006, China)
Biodiesel has
extensive attention as a kind of renewable and clean fuel. However, because of its intrinsic unsaturated composition, it is prone to auto-oxidation and corruption in long-term storage. Fortunately, the partial hydrogenation of biodiesel, in which the high unsaturated fatty acid esters are selectively converted to the low unsaturated or saturated fatty acid esters, has been an effective measure to improve the oxidation stability as well as the cetane number. In this study, the partially hydrogenated soybean methyl ester (PHSME) was produced from soybean methyl ester (SME) via the catalytic transfer hydrogenation. The catalytic transfer hydrogenation of SME was implemented using isopropanol as the hydrogen donor, water as the reaction medium and Raney-Ni as the catalyst. The ratio of solvent water, isopropanol and SEM was 100:32:7, and the catalyst loading accounted for 13% of SME approximately. The hydrogenation reaction was progressing under the water bath of (85±1) ℃ with a magnetic stirring speed of 600 r/min. After about 100 min, the degree of hydrogenation for biodiesel was found to reach the maximum, and the final product PHSME was collected by suitable separation. By the gas chromatography-mass spectrometry (GC-MS) analysis, methyl palmitate (C16:0), methyl stearate (C18:0), methyl oleate (C18:1), methyl linoleate (C18:2), methyl linolenate (C18:3), methyl eicosanoate (C20:0) and methyl docosanoate (C22:0) were detected out in sequence for SME sample, however, C18:3 did not exist in the PHSME. The total amount of unsaturated components C18:3, C18:2 and C18:1 in the SME was 70.9%. After moderate hydrogenation, the high unsaturated components C18:3 and C18:2 containing 3 and 2 double bonds were converted into C18:1 and C18:0 preferentially, and the conversion rate could reach 37.8%. In view of the number of unsaturated double bonds in carbon chain, the unsaturation degree of SME was reduced by 46.2%. Compared with SME, although the kinematic viscosity of PHSME increased slightly, its oxidation stability was improved significantly, and the cetane number of PHSME rose to a desirable level as well. In air atmosphere, the oxidation and combustion characteristics of SME and PHSME were comprehensively explored in a thermal analyzer. Due to the molecular structure change and increased kinematic viscosity, the start of weight loss for PHSME was a little late, whose TG (thermogravimetry) profile shifted to the high temperature region with respect to that for SME, however eventually the finish of weight loss advanced by 7.2 ℃, which affirmatively indicated that PHSME, owning a greater average oxidation rate than SME, was more prone to be oxidized and burned. Meanwhile, in DSC (differential scanning calorimeter) profiles, due to the desirable cetane number, the exothermic onset temperature of PHSME was 10.7 ℃ earlier than that of SME. In summary, the fuel properties including the oxidation stability, iodine value and cetane number of SME are beneficially upgraded by moderate hydrogenation. The better quality of partially hydrogenated biodiesel makes it more popular in the fuel blend market.
combustion; biodiesel; hydrogenation; oxidation; thermogravimetry; differential scanning calorimeter
10.11975/j.issn.1002-6819.2017.11.007
TK6
A
1002-6819(2017)-11-0054-06
袁银男,顾 萌,戴鹏飞,梅德清. 生物柴油催化转移加氢改善其燃烧特性[J]. 农业工程学报,2017,33(11):54-59.
10.11975/j.issn.1002-6819.2017.11.007 http://www.tcsae.org
Yuan Yinnan, Gu Meng, Dai Pengfei, Mei Deqing. Biodiesel modified by catalytic transfer hydrogenation improving combustion performance[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2017, 33(11): 54-59. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2017.11.007 http://www.tcsae.org
2016-10-09
2017-05-09
国家自然科学基金项目(51376095,51506101);江苏高校优势学科建设工程资助项目(苏政办发[2014]72号);江苏省科技厅重点研发计划项目(BE2016139)
袁银男,男,江苏常熟人,教授,博士生导师,从事发动机代用燃料及排放控制的研究。镇江 江苏大学汽车与交通工程学院,212013。Email:yuanyn@suda.edu.cn
※通信作者:梅德清,男,江苏仪征人,副教授,主要从事发动机排放控制与新能源研究。镇江 江苏大学汽车与交通工程学院,212013。
Email:meideqing@ujs.edu.cn
