Efficient Synthesis and Application in Heck Reaction of Pd/Fe3O4Magnetic Nanoparticles
2017-07-05SUNYuanXuGUODanDanZHUXiaoQingWANGChengCHENSheYunDAIJingTao
SUN Yuan-XuGUO Dan-DanZHU Xiao-QingWANG ChengCHENShe-YunDAI Jing-Tao*,
(1College of Chemical Engineering,Nanjing University of Technology,Nanjing 210009,China) (2College of Chemical and Environmental Engineering,Yancheng Teachers University,Yancheng,Jiangsu 224051,China)
Efficient Synthesis and Application in Heck Reaction of Pd/Fe3O4Magnetic Nanoparticles
SUN Yuan-Xu1GUO Dan-Dan1ZHU Xiao-Qing1WANG Cheng2CHENShe-Yun2DAI Jing-Tao*,2
(1College of Chemical Engineering,Nanjing University of Technology,Nanjing 210009,China) (2College of Chemical and Environmental Engineering,Yancheng Teachers University,Yancheng,Jiangsu 224051,China)
Pd/Fe3O4NPs have been successfully synthesized using polyvinyl pyrrolidone as a stabilizing agent. The resultant samples were characterized by X-ray diffraction,transmission electron microscopy,inductively coupled plasma,and magnetic studies.The Pd/Fe3O4NPs catalyst was also applied in the Heck reaction to evaluate the catalytic performance.The results show that the cubic phase of Pd coexists with the cubic phase of Fe3O4,and the catalysts have size less 20 nm and the excellent catalytic activity of Pd/Fe3O4NPs in the Heck reaction.In addition,the catalyst can be recovered via a magnet and reused several times without significant loss of its catalytic activity.
Pd/Fe3O4;magnetic nanoparticles;polyvinyl pyrrolidone;Heck reaction
0 Introduction
Palladium-catalyzed C-X(X includes C,N,O, etc.)cross-couplingreactionforformingaryl compounds has become a common bonding strategy andthesynthesismethodsinorganicsynthesis. Among them the formation of carbon-carbon bonds is one of the important processes of chemical change[1-3].
Palladium-catalyzedcarbon-carboncoupling reactionsincludeHeck-Mizoroki,Suzuki-Miyaura, Sonogashira reaction and so on[4-5].The Heck reaction, palladium-catalyzedcarbon-carbonbondformation between aryl halides and olefins,has become a most powerful synthetic tools[6]and has been proven to be arather versatile method in the syntheses of important building blocks in pharmaceuticals and bioactive compounds,naturalproducts,monomersand herbicides[7-8].
Ali et al.[9]employed palladium-phosphine complexes as catalyst and Jhous groups[10]have reported aboutpalladium(Ⅱ)complexesofN-heterocyclic carbine as catalyst in the Heck reaction.However,the catalysts above suffer from a series of drawbacks,such as intrinsic toxicity,poor thermal,air stability,harsh reaction conditions,and making it economically and environmentallymalignant[11-12].Moreover,mostof ligands are expensive and its poor recyclability[13].In order to overcome the disadvantages above,considerable efforts have been made[14-15],it seems that,the nanotechnology is a good candidate[16-18]which allows the surface area to increase dramatically among them and reactants in solution have easy access to the active sites on the surface of nanoparticles[19].Palladium nanoparticles(PNPs)supported on agarose as catalytic system has an highactivityinHeck reaction[13]. Preparation of PNPs under green conditions,the products were also obtained in highly short reaction times with excellent yields[20].However,the recyclability of PNPs using a simple filtration is a huge problem due to nanoparticles is dispersible in solution,forming emulsion[21].Moreover,palladium is an expensive metal that limits industrial applications[22-23].Thus under both economic and environmental concerns,it is essential that the isolation and recovery of PNPs[24-25].Methods have been focused on magnetic recyclable supports from solution[26-28].Catalyst on this supports can be separated from reaction condition using an external magnetic eld easily but also the reactivity could be improved[29].And magnetic nanoparticles(MNPs)have been applied in various fields such as the field of biotechnology[30-32].
Incontinuationofoureffortstodevelop environmentally friendly synthetic methodologies,we now report a protocol for the preparation of Pd/Fe3O4NPs by using polyvinyl pyrrolidone(PVP)as a stabilizing agent and the application of these MNPs as novel and stable heterogeneous catalysts in the Heck coupling reactions.
1 Experimental
1.1 Apparatus,materials and measurements
All reagents were purchased from the Sinopharm Chemical Reagent Co.,Ltd(Shanghai,China)and AladdinIndustrialCorporationandusedwithout further purification.Products were characterized by comparison of their physical and spectral data with thoseofauthenticsamplesandreportedinthe literature.
The analyses by GC were performed on an Agilent 7890B instrument equipped with a capillary (30 m×0.32 mm×0.25 μm film thicknesses).The GC parameters were as follows[33]:initial temperature, 120℃;initial time,1 min;solvent delay,3.70 min; temperature ramp 1,10℃·min-1;final temperature, 200℃;temperature ramp 2,20℃·min-1;final temperature,240℃;final time,14min;injector port temperature,260℃;detector temperature,300℃, injection volume,1.0 μL.The thermal properties of samples were examined by thermogravimetric analysis (TGA,model Q50,TA Instruments,USA),with the temperature increasing from RT to 700℃at a rate of 10℃·min-1under a flow of high-purity nitrogen at a rate of 60 mL·min-1.X-ray diffraction measurements (XRD)wereperformedwithaPhilipspowder diffractometer type PW 1373 goniometer(Cu Kα radiation,λ=0.154 06 nm,U=40 kV,I=80 mA)at room temperature.The scan rate was 2°·min-1in the 2θ range from 10°to 80°.Pd contents in the catalysts were determined by Inductively coupled plasma(ICP) analyzer(Varian,Vista-Pro)after dissolving each sample in the mixture of HNO3/HCl(1∶3,V/V). Transmission electron microscopy(TEM)analyses were performed on a Philips model CM 10 coupled with an energy dispersive X-ray spectrometer(EDX), with an accelerating voltage of 20 kV.The high resolution TEM(HRTEM)images were collected with Hitachi H9000NAR transmission electron microscope. The magnetic properties of the synthesized samples were measured by using Vibrating Sample Magneto-meter(VSM,M-155)at a maximum applied eld of 30 kOe.
1.2 Typical procedure for preparations of Fe3O4NPs,Pd NPs and Pd/Fe3O4NPs catalyst
Magneticnanoparticleswerepreparedvia hydrothermal method[34-35]in the presence of urea.In a canonical flask,a mixture of FeCl3·6H2O(0.79 mmol, 0.212 5 g)and PVP(1.50 mmol,0.166 3 g)with an average molecular weight of 40 000 Da and urea(2.42 mmol,0.145 3 g)was dissolved in 10 mL of 1,2-
propanediol.Thenthesolutionwasstirreduntil dissolved completely.Subsequently,the solution was transferred to the autoclave and the mixture was reacted at 190℃for the appropriate times.The magnetic nanoparticles as a dark solid were isolated from the solution by magnetic separation and washed with deionized water and absolute ethyl alcohol, respectively.Finally,the magnetic nanoparticles are dispersed in 10 mL ethanol.
PVP(0.71 mmol,0.078 9 g)with an average molecularweightof40000Dawasaddedto Palladium chloride solution(5.5 mL,0.011 28 mol·L-1) andstirreduntilthetotalsolubilizationofthe polymer.Then,a solution of sodium borohydride(0.99 mmol)was added dropwise to the mixture under constant magnetic stirring for 30 min.This solution of Pd(0)-PVP was directly used as the catalyst for the Heck reactions.
The prepared Pd NPs with Fe3O4NPs were mixed in a round-bottom flask at room temperature under vigorous stirring for 8 h.The value of Pd supported on Fe3O4was monitored by ICP analyzer.
1.3 TypicalprocedureforHeckcoupling reaction
To a stirred solution of 1.4 mmol of aryl halide in 3 mL of ethanol,1.7 mmol of alkene,2.0 mmol of K2CO3and the Pd/Fe3O4NPs catalyst was added and the mixture was heated on an water bath at 80℃for 7 h under constant stirring.After completion of the reaction,the reaction mixture was cooled to room temperature and the catalyst was separated using a magnetic separator and washed with diethyl ether(2× 10 mL)followed by deionized and oxygen-free water (2×10 mL).The reused catalyst was dried for the next run.The organic phase was analyzed by GC.All the products are known compounds and the spectral data and melting points were identical to those reported in the literature.
2 Results and discussion
2.1 XRD,TEM and magnetic analyses
The X-ray diffraction(XRD)results show that all the diffraction peaks in the pattern:Pd/Fe3O4NPs,as shown in Fig.1.The XRD pattern of the Pd/Fe3O4NPs catalyst shows that the cubic phase of Pd coexists with the cubic phase of Fe3O4.All the diffraction peaks could be readily indexed to(220),(311),(222),(400), (422),(511),(440)lattice planes of a face-centered cubic(fcc)FeO·Fe2O3(PDF No.89-0691)and(111) lattice planes of a face-centered cubic(fcc)Pd crvstal structure(PDF No.46-1043)[29,36].
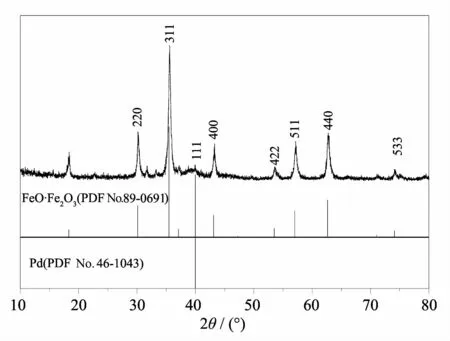
Fig.1X-ray diffraction patterns of Pd/Fe3O4NPs
The morphology and microstructure of the assynthesized Pd/Fe3O4NPs were characterized by TEM, and the results are shown in Fig.2.Fig.2a~b shows thatthefunctionalizedmagneticnanoparticles exhibited relatively good monodispersity.The inset of Fig.2b shows a typical HRTEM image of the Pd/Fe3O4NPs.ThisimageclearlyrevealsthatthePd nanoparticles are directly attached onto the Fe3O4nanocrystals.The lattice fringes of d=0.194 3 and 0.281 6 nm were ascribed to Pd(200)and Fe3O4(220), respectively[37].EDS spectrum in Fig.2c shows the presence of C,Fe,O,Cu and Pd elements,which confirms the co-exists of Pd and Fe3O4.In addition,itcan be seen from the histogram(Fig.2d),the size of Pd/Fe3O4NPs is in the range of 8~24 nm(mean diameter:(16.5±1.5)nm).
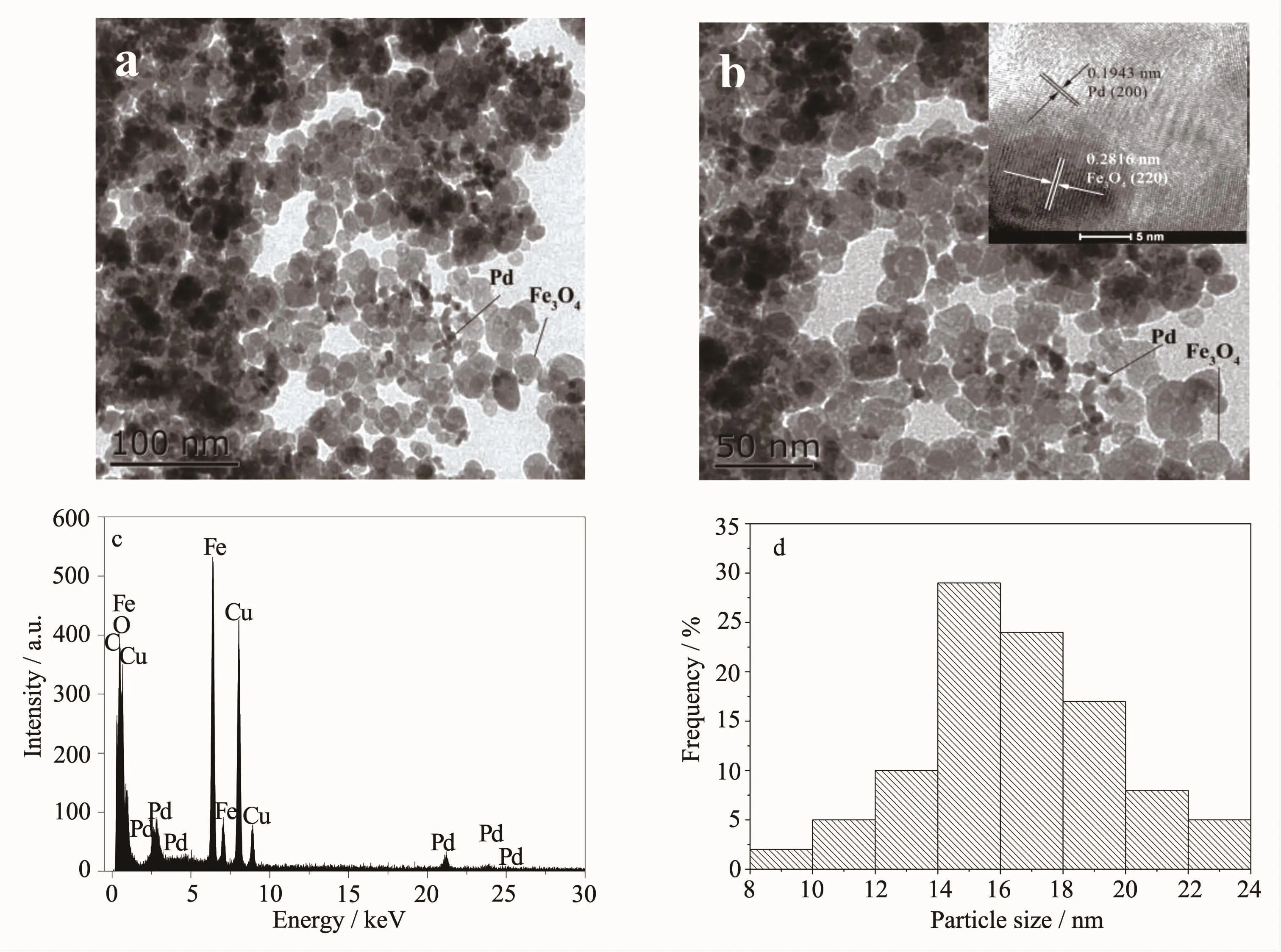
Fig.2TEM(a~b)and HRTEM(inset of b)image of Pd/Fe3O4NPs,TEM-EDS spectrum of Pd/Fe3O4NPs(c),and the histogram showing the particle size distribution of Pd/Fe3O4NPs(d)
Room temperature magnetic characteristics of the Fe3O4NPs,the Pd/Fe3O4NPs and Recycled Pd/Fe3O4NPs were investigated by recording the magnetization versus applied field curves(Fig.3)[38].At 298.15 K and 30 kOe,the hysteresis loops of the Fe3O4NPs and the Pd/Fe3O4NPs show that the magnetization decreases on palladium,which indicate the Pd NPs load on Fe3O4NPs.Furthermore,the magnetization of recycled Pd/Fe3O4NPs is higher than Pd/Fe3O4NPs,showing a loss of the Pd NPs during Heck reaction.
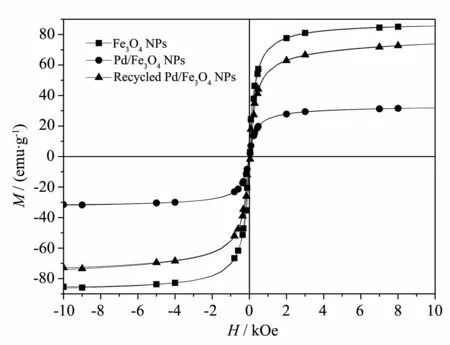
Fig.3Hysteresis loops of Fe3O4NPs,Pd/Fe3O4NPs and recycled Pd/Fe3O4NPs
2.2 Pd content
ThecontentofPdloadedonFe3O4was investigated via ICP,the results were shown in Fig.4. The initial content of Pd was 2.25%(n/n,the same below),whereas the Pd content decreased after the first run,the one recovered via magnetic separation, suggesting the Pd NPs may be leached out during reactionandrecovery.Althoughpalladium nanoparticles were lost,the loss is small after fiverecycling runs(from 2.25%to 1.28%).This finding revealed that the as-synthesized catalyst was Pd/Fe3O4and the catalyst can be used as a stable catalyst for Heck reactions.
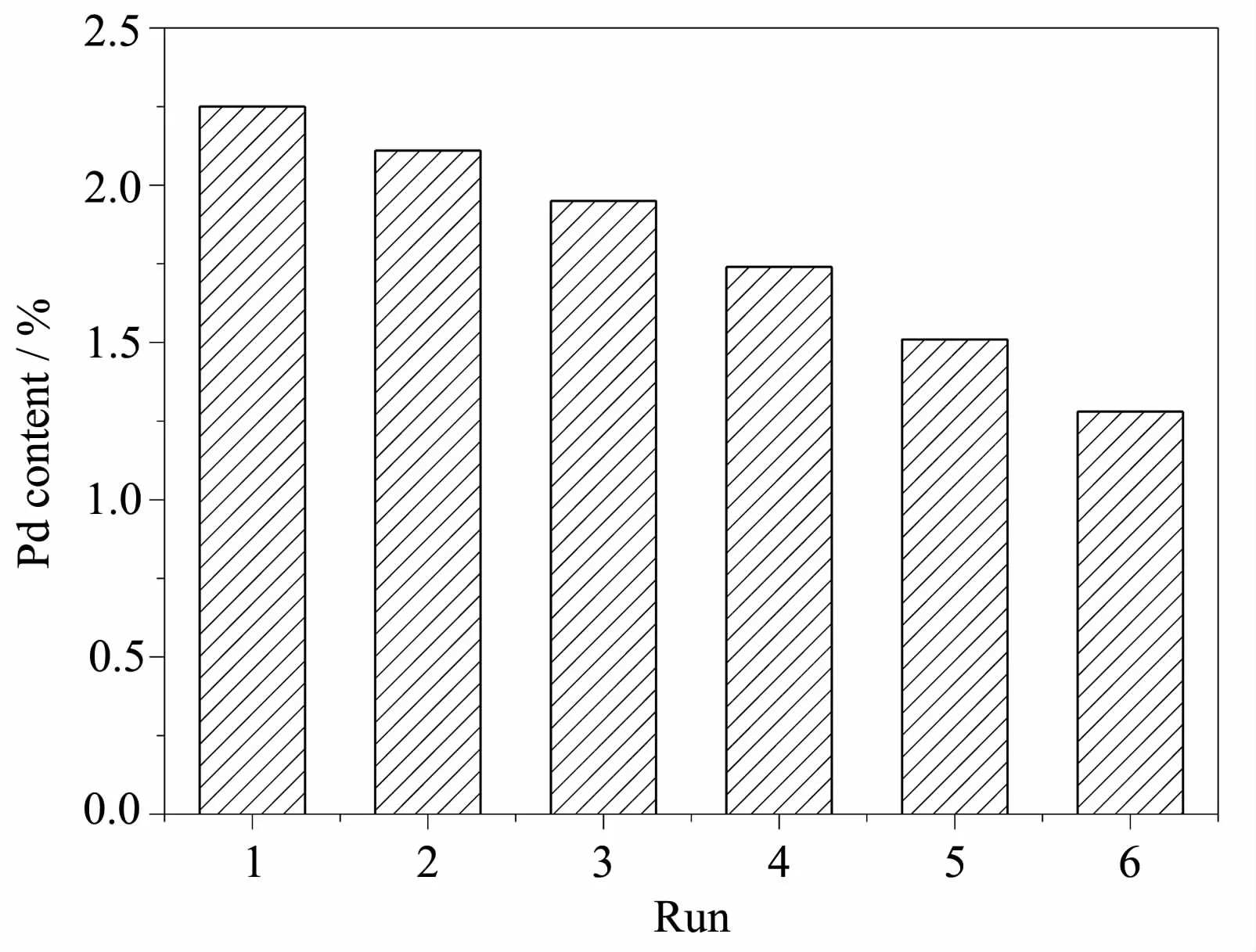
Fig.4ICP results for the content of Pd loaded onFe3O4for each run
2.3 TGA Analyses
TGA was used to determine the thermal stability. Fig.5 depicts the TGA result of Pd/Fe3O4NPs under a nitrogen atmosphere.The first weight loss at 162℃that is less weight loss is completely attributed to the removal of physically adsorbed solvent molecules and moisture.The other weight loss at 417℃corresponds to the degradation of PVP-Pd(0)complex.Thus,the catalyst was stable up to 162℃.In addition,the coated Fe3O4nanoparticles show an excellent thermal stability up to 800℃.
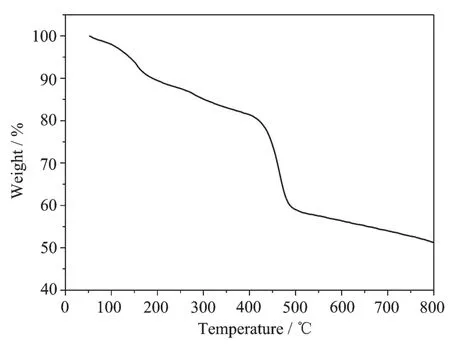
Fig.5TGA curve for Pd/Fe3O4catalyst
2.4 Catalytic activity for Heck coupling reaction
The Pd/Fe3O4NPs catalyst was applied in the Heck reaction to evaluate the catalytic performance (Scheme 1).Initially,the reaction was carried outbetweeniodobenzeneandstyreneasmodel substrates for the development of optimized conditions (Table 1).
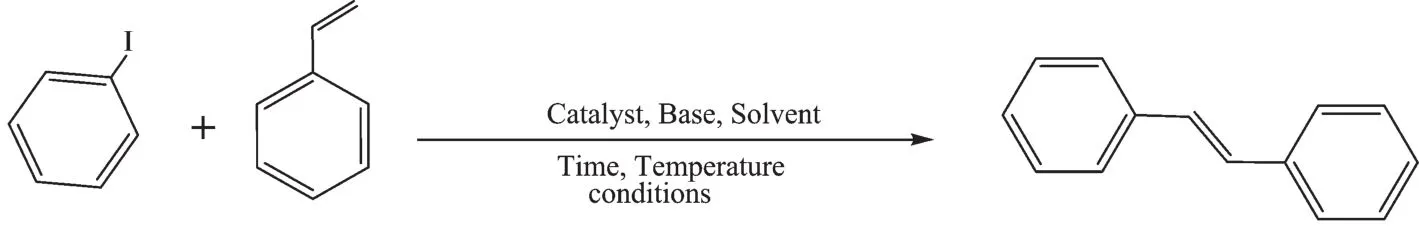
Scheme 1
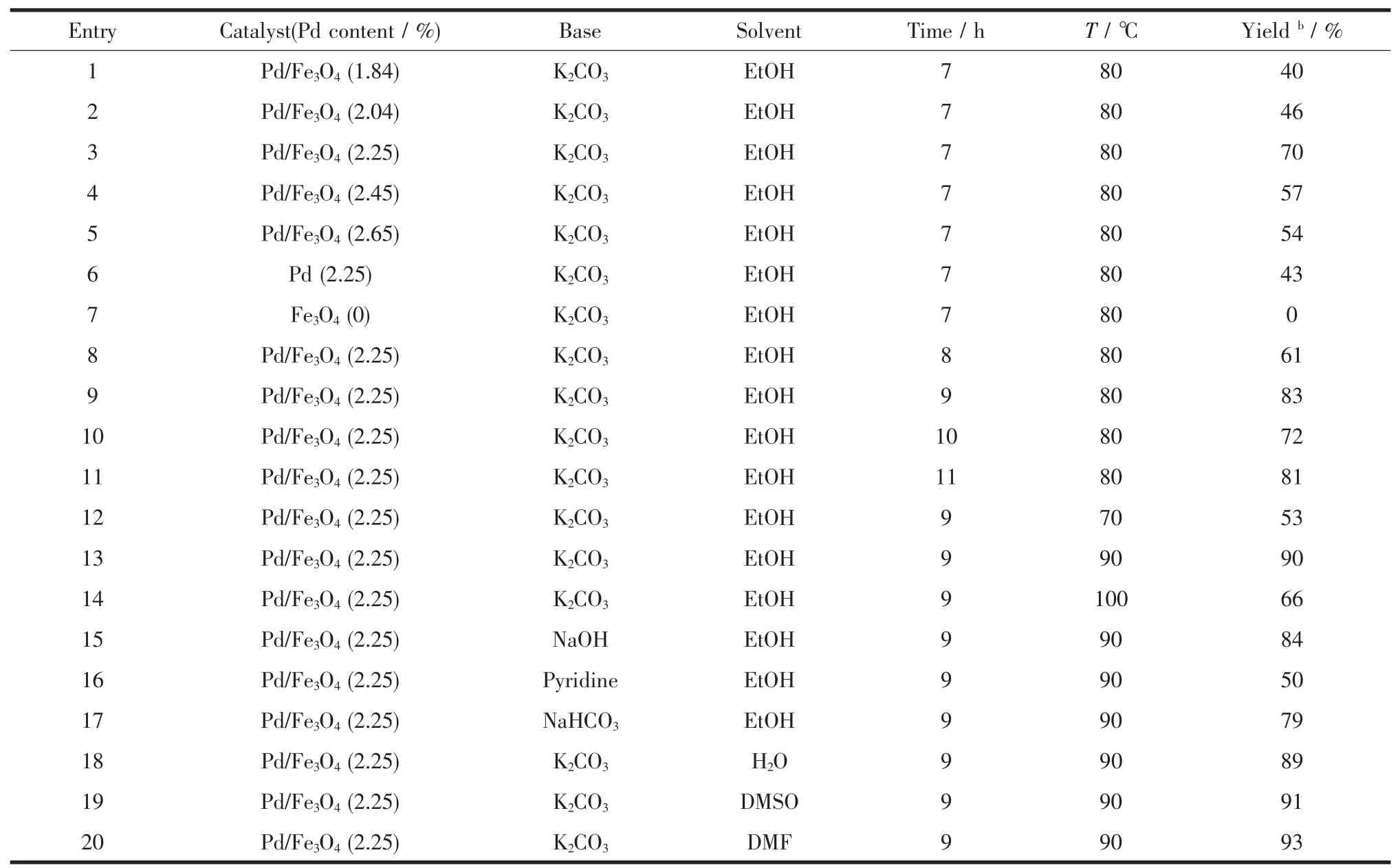
Table 1Various conditions for Heck reaction between iodobenzene and styrene using Pd/Fe3O4NPs catalysta
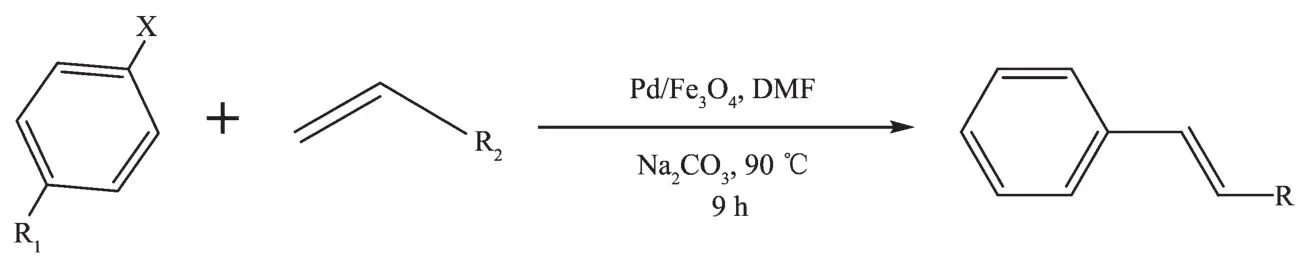
Scheme 2
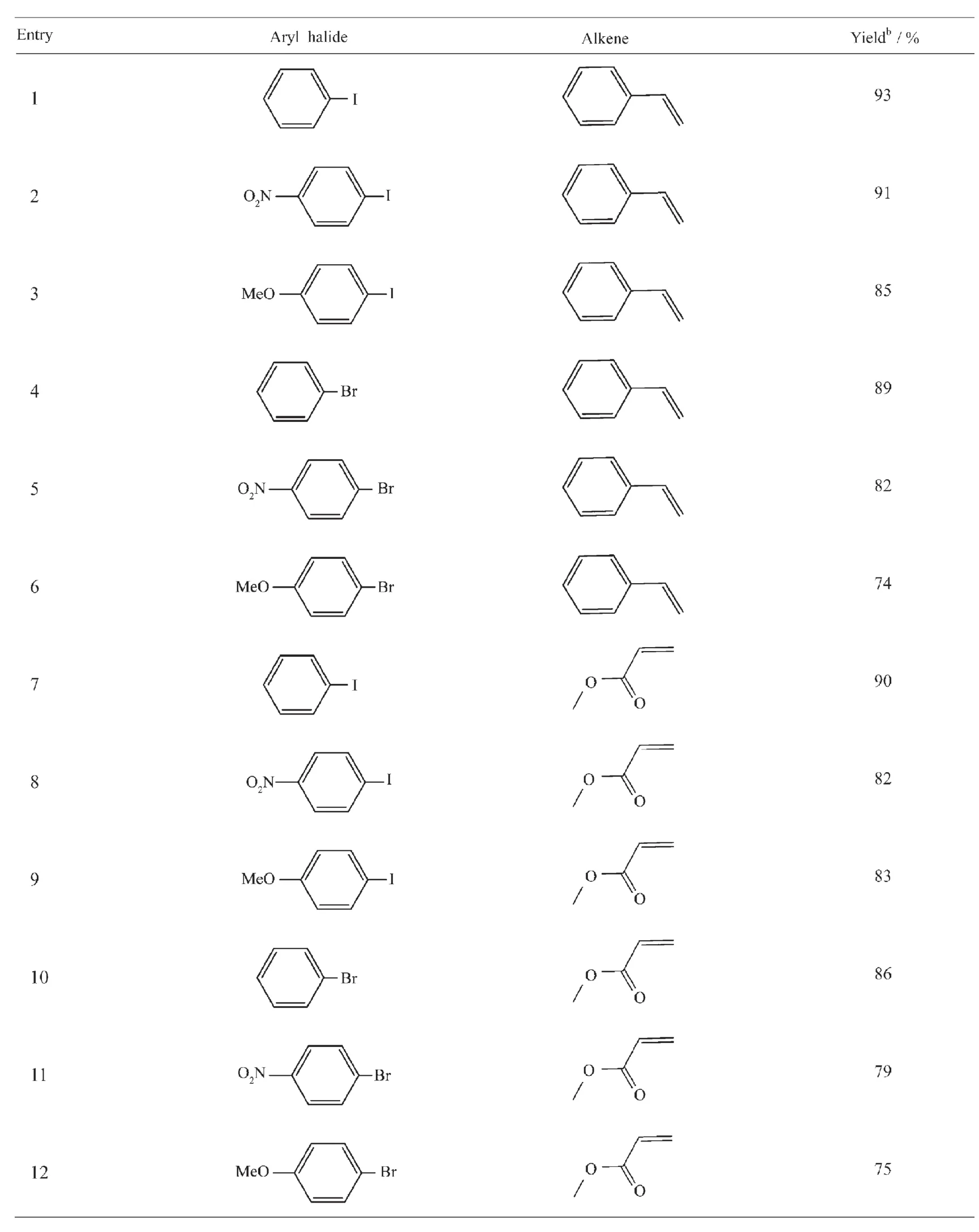
Table 2Heck reaction using different aryl halides with terminal alkenes catalyzed by Pd/Fe3O4NPsa
Control experiments show that there are different yieldsforthepreparedcatalystswithdifferent contents of Pd(Entries 1~5).A decrease in the catalyst loading from 2.25%to 1.84%afford the product in lower yield(Entries 1~3).No significant improvement on the yield was observed using the catalyst with higher amounts of Pd(Entries 3~5)and single Pd(Entry 6).And Fe3O4(Entry 7)have a lower yield.The catalyst with 2.25%of Pd was found to be optimum(Entry 3).The better yields were obtained when the time of the reaction was 9 h(Entry 9)and the temperature of the reaction was 90℃(Entry13). The reactivity of the catalyst in various solvents using both organic and inorganic bases was investigated to check the influence of nature solvent on the yield of the product(Entries 13,15~20).The results indicated that the nature of the base is of great importance in the Heck coupling.Among the tested bases,K2CO3was found to be superior(Entry 13).From cost, availability,and environmental impact,water(Entry 18)is the best solvent for reaction especially for potential industry application,even if a slightly higher yield(93%vs 90%)was achieved as DMF(Entry 20) was employed.But the cycle performance of the catalyst is poor,when water acts as a solvent for reaction.In addition,the use of polar solvents such as ethanol,DMSO(Entries 13,19)is not beneficial to the reaction as the yield of the product was same or very less than.
According to the data which were obtained from optimizing study,the Heck reaction can be properly carried out at 90℃in the presence of Pd/Fe3O4NPs (2.25%),using K2CO3as base,in DMF solvent for 9 h.
Next,using the optimized procedure,a variety of aryl halide and alkene possessing were employed (Scheme 2).As shown in Table2,a reaction of iodobenzene and styrene has a higher yield under the optimized conditions(Entry 1).
To show the advantages of Pd/Fe3O4NPs catalyst in comparison with other catalysts,we summarized some other reported homogeneous and heterogeneous palladium catalysts for Heck reaction between aryl halide and alkene.As shown in Table 3,the title catalyst is superior to some of the previously reported catalysts[33-37]with respect to reaction time,reaction temperature and yield.The notable features of this method are:the reaction system is simple;toxic reagents and homogeneous catalysts are eliminated; the Pd/Fe3O4NPs catalyst can be easily recovered.
2.5 Catalyst recyclability
The reusability of the catalysts is one of their most important advantages,which makes them useful for commercial applications.The reusability of the heterogeneousPd/Fe3O4NPscatalystwasalso evaluatedinHeckcouplingreactionunderthe optimized conditions.The recycled catalyst could be reused for five times without any treatment(Fig.6).
The reaction was found to yield products from 93%to 52%,and Pd leaching is the main cause for the activity drop(Fig.4).Thus,the magnetic catalyst is stable during the Heck coupling reaction.
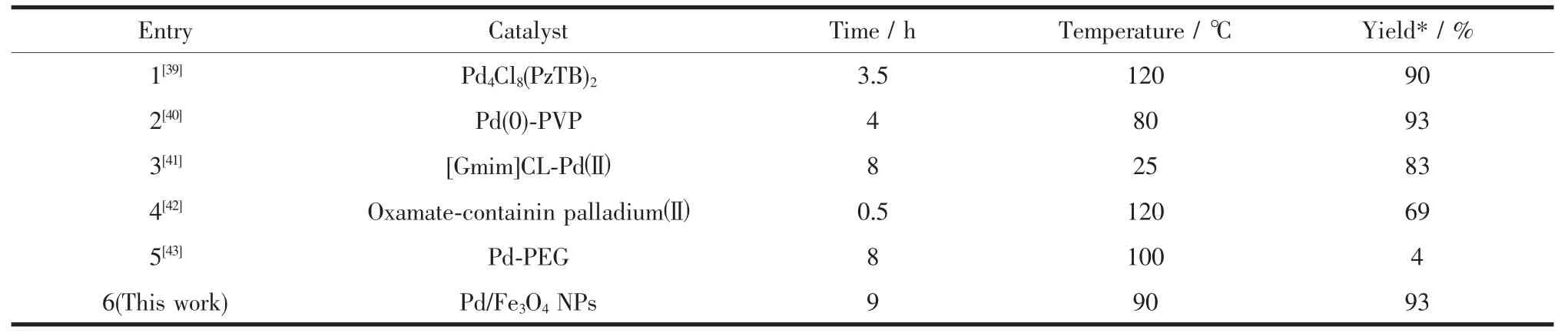
Table 3Comparison of present methodology with other reported methods for Heck reaction between iodobenzene with styrene
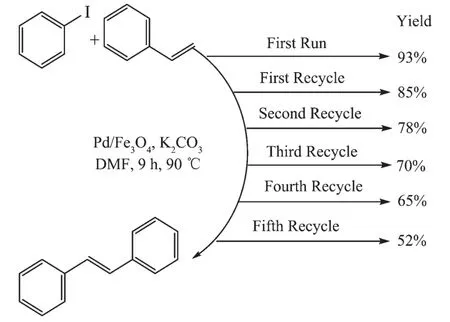
Fig.6Reusability of Pd/Fe3O4NPs for Heck coupling reaction
3 Conclusions
Palladium modied Fe3O4nanoparticles prepared by PVP as stabilizer is a highly efficient,magnetically recoverable and recyclable catalyst for the Heck couplingreactionunderoptimalconditions.Easy workup(usingexternalmagneticattraction)and environment are two other advantages of this catalyst system.Thus,the superior performance of Pd/Fe3O4NPsmakesitapromisingcatalystinorganic synthesis.
Acknowledgements:The work is financially supported by Jiangsu Province Prospective Science and Technology Guide Foundation(Grant No.BY2015058-04),the National Natural Science Foundation of China(Grant No.21307103),Yancheng City Agricultural Science and Technology Innovation Special Guide Capital Projects(Grant No.YK2015026)and Innovation and Entrepreneurship Training Program for College Students in Jiangsu Province(Grant No.201410324013Z).The authors expressed sincere gratitude to Jiangsu Provincial Key Laboratory of Coastal wetland Bioresources and Environmental Protection (Grant No.JLCBE10013)and Yancheng Teachers University (Grant No.14YSYJB0109).
[1]Ibrahim H,Bala M D.J.Organomet.Chem.,2015,794:301-310
[2]Majumder A,Gupta R,Mandal M,et al.J.Org.Chem., 2015,781:23-34
[3]Moussa S,Siamaki A R,Gupton B F,et al.ACS Catal., 2012,2(1):145-154
[4]Lee Y,Hong M C,Ahn H,et al.J.Org.Chem.,2014,769:, 80-93
[5]Kogan V,Aizenshtat Z,Popovitz B R,et al.Org.Lett.,2002, 4:3529-3532
[6]Zhang Z,Zha Z,Gan C,et al.J.Org.Chem.,2006,71:4339-4342
[7]Chang Y C,Chang C H,Wang L W,et al.Polyhedron, 2015,100:382-391
[8]LI Xue-Ting(李雪亭),ZANG Peng-Yuan(臧鹏远),YE Qiu-Ming(叶秋明),et al.Chinese J.Inorg.Chem.(无机化学学报),2011,27(8):1550-1554
[9]Nezhad A K,Panahi F.J.Organomet.Chem.,2013,741-742: 7-14
[10]Jhou Y M,Nandi D J,Lee Y,et al.Polyhedron,2015,100: 28-35
[11]Nowrouzi N,Zarei M.Tetrahedron,2015,71:7847-7852
[12]Zhang J,Li T,Zhao X,et al.J.Colloid Interface Sci., 2016,463:13-21
[13]Firouzabadi H,Iranpoor N,Kazemi F,et al.J.Mol.Catal. A:Chem.,2012,357:154-161
[14]Astruc D.Inorg.Chem.,2007,46:1884-1894
[15]Fernández G L,Blanco M,Blanco C,et al.J.Mol.Catal.A: Chem.,2016,416:140-146
[16]Andujar P,Lanone S,Brochard P,et al.Rev.Mal.Respir., 2011,28:e66-e75
[17]Schmid G.Chem.Rev.,1992,92:1709-1727
[18]Szucs A,Berger F,Dekany I.Colloids Surf.A,2000,174: 387-402
[19]Deraedt C,Astruc D.Acc.Chem.Res.,2014,47:494-503
[20]Khazaei A,Khazaei M,Rahmati S.J.Mol.Catal.A:Chem., 2015,398:241-247
[21]Ojala J,Sirviö J A,Liimatainen H.Chem.Eng.J.,2016, 288:312-320
[22]Magdesieva T V,Nikitin O M,Zolotukhina E V,et al. Electrochim.Acta,2014,122:289-295
[23]Petrucci C,Cappelletti M,Piermatti O,et al.J.Mol.Catal. A:Chem.,2015,401:27-34
[24]Gulcan M,Zahmakiran M,Özkar S.Appl.Catal.B:Environ., 2014,147:394-401
[25]Yuan D,Huang B.Catal.Commun.,2012,18:126-131
[26]Carosio M G A,Bernardes D F,Andrade F D,et al.J.Food Eng.,2016,173:143-149
[27]Petrov A.Y,Chase J G,Sellier M,et al.Math.Biosci.,2013, 246:191-201
[28]XIAO Wang-Chuan(肖旺钏),WANG Ye-Min(王叶敏), WANG Ren-Zhang(王仁章),et al.Chinese J.Inorg.Chem. (无机化学学报),2014,30(11):2559-2563
[29]Li S.Z,Zhang W,Chen F X,et al.Mater.Res.Bull.,2015, 66:186-191
[30]Li H,Lu Z,Cheng G,et al.RSC Adv.,2015,5:5059-5067
[31]Agiotis L,Theodorakos I,Samothrakitis S,et al.J.Magn. Magn.Mater.,2016,401:956-964
[32]Haracz S,Hilgendorff M,Rybka J D,et al.Nucl.Instrum. Methods Phys.Res.,Sect.B,2015,364:120-126
[33]Feng J,Cai C.J.Fluorine Chem.,2013,146,6-10
[34]Liu E T,Zhao H P,Li H,et al.New J.Chem.,2014,38: 2911-2916
[35]NIU Yi-Fan(牛一凡),YANG Ying(杨赢),YANG Wen-Tao (杨文韬).Chinese J.Inorg.Chem.(无机化学学报),2016,32 (12):2129-2135
[36]Li S Z,Zhang W,So M H,et al.J.Mol.Catal.A:Chem., 2012,359:81-87
[37]Sanchez-Padilla N M,Montemayor S M,Torres L A,et al. Int.J.Hydrogen Energ,2013,38:12681-12688
[38]Bhowmik R N,Poddar A,Saravanan A.J.Magn.Magn. Mater.,2010,322:2340-2349
[39]Cuenú F,Abonia R,Bolaños A,et al.J.Organomet.Chem., 2011,696:1834-1839
[40]Martins D D L,Alvarez H M,Aguiar L C S,et al.Appl. Catal.A:Gen.,2011,408:47-53
[41]Karthikeyan P,Muskawar P N,Aswar S A,et al.J.Mol. Catal.A:Chem.,2012,358:112-120
[42]Fortea-Pérez F R,Schlegel I,Julve M.J.Organomet.Chem., 2013,743:102-108
[43]Karami K,Moghadam Z K,Hosseini-Kharat M.Catal. Commun.,2014,43:25-28
磁性纳米Pd/Fe3O4催化剂的制备及其在Heck反应中的应用
孙远旭1郭丹丹1朱啸庆1王成2陈社云2戴兢陶*,2
(1南京工业大学化工学院,南京210009) (2盐城师范学院化学与环境工程学院,盐城224051)
通过使用聚乙烯吡咯烷酮作为稳定剂,合成了磁性Pd/Fe3O4纳米颗粒催化剂。对该催化剂进行粉末X射线衍射、透射电子显微镜、感应耦合等离子体和磁性表征。将Pd/Fe3O4催化剂用于Heck反应,检测其催化性能。测试结果表明Pd纳米颗粒负载在Fe3O4纳米颗粒上,而且催化剂的尺寸<20 nm,并在Heck反应中表现了极好的催化性能。此外,催化剂可以通过磁场回收利用,且催化活性没有显著的降低。
Pd/Fe3O4;磁性纳米颗粒;聚乙烯吡咯烷酮;Heck反应
TQ426.6
A
1001-4861(2017)06-1081-09
2017-02-16。收修改稿日期:2017-04-26。
10.11862/CJIC.2017.132
国家自然科学基金(No.21307103)资助项目。
*通信联系人。E-mail:ycjtdai@163.com
猜你喜欢
杂志排行
无机化学学报的其它文章
- CoAl2O4/蜂窝陶瓷催化剂的制备及其催化臭氧化性能
- Photocatalytic Hydrogen Production Based on Cobalt-Thiosemicarbazone Complex with the Xanthene Dye Moiety
- 两种金属-有机钙钛矿材料的负热膨胀性质
- Pyrazolate-Based Dipalladium(Ⅱ,Ⅱ)Complexes:Syntheses,Characterization and Catalytical Performance in Suzuki-Coupling Reaction
- 以滤纸为模板合成新型介孔生物活性玻璃微管材料
- Br-掺杂Bi2WO6的水热法合成及其可见光催化性能
